ABSTRACT
The objective of this study was to investigate the utilization of Caralluma fimbriata an edible succulent cactus as natural antioxidants for retarding acrylamide formation, lipid oxidation, and the degradation of bioactive compounds in French fries due to frying at 190°C. The fresh Caralluma fimbriata extract exhibited the highest phenolic content of 96.4 ± 0.1 mg gallic acid equivalent/g when the raw potato extract showed significantly 27.4 ± 0.3 mg GAE/g before frying. The Caralluma fimbriata extract had the higher flavonoid content of 54.4 ± 0.1 mg of Quercetin equivalent/g, while the raw potato had 38.8 ± 0.2 mg of Quercetin equivalent/g. The total flavonols and flavanols in Caralluma fimbriata were significantly high, and those were found to be 27.6 ±0.8 mg Quercetin Equivalent/g and 19.1 ±0.6 mg Catechin equivalent/g, respectively, and, eventually, higher than potato extract. These bioactive compounds are easily degraded due to frying at high temperatures. The Caralluma fimbriata extract retards the formation of acrylamide precursors in potato, primary and secondary oxidation products, and the degradation of polyphenols after the immersion treatment. The Caralluma fimbriata extract was found to be more effective against acrylamide level (42.5 μg/kg) in French fries. The Caralluma fimbriata treated sample exhibited comparatively better oxidative stability during holding time with highest overall acceptability than the sample treated with butylated hydroxyanisole, distilled water (control) and raw potato (without treatment). The results will provide scientific basis in the use of Caralluma fimbriata as natural antioxidant against acrylamide formation and the oxidative deterioration of bioactive compounds.
Introduction
Acrylamide, a “probable carcinogen to humans” is formed in carbohydrate-rich foods that are processed at high temperatures. The concern about improving health involving natural antioxidants with high potential benefits has enhanced advanced research on the inhibition of lipid oxidation and acrylamide formation.[Citation1,Citation2] The frying process is used worldwide generating products with unique organoleptic and sensory attributes.[Citation3] The non-volatile, degraded products of used frying fats and oils can remain in the oil, and they can be retained in the food, and subsequently ingested.[Citation4] Non-volatile products include polymeric triglycerides and monomeric triglycerides that contain cyclic fatty acyls and various breakdown products.[Citation5] Potatoes possess a high content of asparagine and reducing sugars and are subject to the formation of acrylamide during frying.[Citation6] According to the European Commission, processed potatoes with snack-based products are the main sources of exposure to acrylamide in the diet.[Citation7] Acrylamide is mainly formed through the Maillard reaction from free asparagine and a carbonyl source.[Citation3] The carbonyl compounds in foods can arise from lipid oxidation during heating.[Citation8,Citation9] Lipid oxidation has been proposed as a minor pathway of acrylamide formation, with acrylic acid as a direct precursor that can be formed by way of acrolein, by oxidative degradation of lipids.[Citation10] The growing concern over the potential hazards of synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) there is renewed interest in the use of natural antioxidants.[Citation11] The synthetic antioxidants, such as BHT and BHA, are associated with possible carcinogenic effects and come under the scrutiny of consumers. These preservatives include benzoates, nitrates, sorbates, formaldehyde, and others, they possess life-threatening adverse effects and the symptoms such as diarrhea, abdominal pain, asthma, and anaphylactic shock resulting in death have been recorded. Caralluma fimbriata (CF), an edible succulent cactus in the family of Apocynaceae (Kallimudayan in Tamil), is a perennial herb growing in dry parts of Tamil Nadu, India. The extract of CF can be used as a natural antioxidant[Citation12] and its polyphenols are playing a big role in the inhibition of lipid oxidation by neutralizing free radicals and quenching singlet and triplet oxygen.[Citation13,Citation14] Natural antioxidants are another important way to reduce the acrlyamide content in foods[Citation5,Citation15] by preventing the generation of a “reactive carbonyl pool,” trapping key Maillard reaction intermediates and eliminating the formation of acrylamide as their oxidized forms.[Citation16–Citation19] The advantages of natural antioxidants include their relatively unquestioned safety, according to their generally recognized as safe (GRAS) status, the higher concentrations allowed, their world wide acceptance, and their lower volatility in heated foods.[Citation3,Citation20] CF can be used for retarding lipid oxidation and particularly active at the high temperatures of frying fats and enhance their shelf life.[Citation17,Citation21,Citation22] The overall objectives of our study were (1) to obtain a better understanding of the inhibitory effect of bioactive compounds in CF on acrylamide formation in French fries, (2) to evaluate the degradation of bioactive compounds and antioxidant properties in French fries after frying, and (3) to examine the effectiveness of CF on the oxidative deterioration of French fries over a holding time of 60 min.
Materials and methods
Chemicals
All the chemicals were bought from local supplier for Merck Millipore (Merck Specialities Pvt. Ltd.) and Sigma-Aldrich. Purity: EMPARTA ACS grade for analysis. The sunflower oil and potatoes were purchased from Reliance super market in Chennai, Tamilnadu. The CF plant was collected from Thiruthuraipoondi, in the Thiruvarur District, Tamilnadu, India. Prior to the study, the CF plant was identified as edible succulent cactus and authenticated by experts at Tamilnadu Agricultural University, Tamilnadu, India.
Extraction of polyphenols: CF, raw potatoes, and French fries
The plant was cleaned and cut in to small pieces and dried in a fluidized bed drier (Sherwood Model 501, England) at 45°C for 60 min and dried sample was homogenized using food processor (Preethi Chefro Model, India make). Fifty grams of dried powder was mixed with 200 mL of hot water (at 65°C) and kept for 3 h at 400 × g in a temperature-controlled incubator shaker (Labortechnik Gmbh, Ilmenau, Germany). The mixture was cooled to room temperature and filtered through a No. 1 sinter glass funnel. After centrifugation, the extracts were lyophilized, protected from light, and stored at –20°C until analysis.[Citation12]
A batch of 10 g of raw potato pieces were crushed and extracted using 50 mL of 60% methanol. In order to prevent sample oxidation during the extraction process, a reduced environment was created using nitrogen flushing. Flasks were kept in a shaker incubator (Innova 42, Mason Technology, Dublin, Ireland) at 40°C with 400 × g for 3 h. The residues were re-extracted with same conditions and the lyophilized extracts were protected from light and stored at –20°C until used for analysis.[Citation6]
Immersion analysis and preparation of French fries
Fresh potatoes were washed cleanly and disinfected. The skin was peeled off and cut into uniform pieces (50.8 mm in length and 15 mm in thickness). Before frying, a batch of 100 g of potato pieces were subjected to immersion with 200 mL of CF extract (200 ppm) and BHA (200 ppm) separately according to the Codex Alimentarius commission[Citation7,Citation23] for 15 min at 25°C. A similar procedure was done for control with distilled water. After immersion, the potato pieces were air dried (at 30°C for 20 min, 60–65% relative humidity). The control and treated potato pieces were taken and fried at 190 ± 5°C for 3 min in a 2 L domestic deep-fat fryer in the laboratory (at 25°C, 60–65% relative humidity). The fried samples were cooled on an absorbing paper and a batch of French fries was used for acrylamide analysis using high-performance liquid chromatography (HPLC), and another batch of the French fries samples are kept for the estimation of lipid oxidation in a dark colored air-tight sealed glass container at 25°C and protected from light and moisture.[Citation24]
The extraction of polyphenols from potato pieces immersed with distilled water (control), CF extract, and BHA before frying, and all of the control, raw potato, CF-, and BHA-treated French fries samples (after frying) was done with 50 mL of methanol (60%) and shaken by hand for 30 min. After centrifugation for 30 min at 9000 × g and the fat-free part was transferred to a 200 mL separating funnel, the aqueous layer was collected and used for polyphenols and antioxidant analysis[Citation15,Citation23] for the evaluation of degradation of bioactive compounds and antioxidants due to frying at 190°C.[Citation15,Citation25,Citation26]
Analyses of polyphenols—antioxidant activities/radical scavenging activities
The total phenolic content (TPC) and total flavonoid content (TFC) were determined by Singleton et al.[Citation13] and Samec et al.,[Citation1] respectively. The TPCs and TFCs were estimated by the methods of Oomah et al.[Citation2] and Samec et al.,[Citation1] respectively. The contents of ascorbic acid were measured by 2,6-dichlorophenolindophenol titration according to the method of Zhou et al.[Citation21] The radical scavenging properties of CF and raw potato were determined by using 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; ABTS), and nitric oxide radical scavenging according to the methods Farhadi et al.,[Citation14] Yang et al.,[Citation29] and Samec et al.,[Citation1] respectively. BHA was used as positive control for all the analysis.
Estimation of quercetin, catechin, β-carotene, and ascorbic acid
The quercetin content was determined by the method Pejic et al.[Citation19] The ethanolic extract was collected in 2-mL Eppendorf tubes and the pH was adjusted to 4.2 and placed into water bath at 34°C. Then the extracts were vortexed, and diluted 10:1 with 80% ethanol to a total of 5 mL. Absorbance readings were made in at 362 nm using a Spectronic Genesys 5 (Waltham, MA) spectrophotometer. The standard was prepared by accurately weighing about 10 mg quercetin in 10 mL of methanol in a volumetric flask. It was then sonicated for 10–15 min. The contents of flask were filtered through Whatman no. 41 paper (Merck, Mumbai, India) to attain the stock solution of 1 mg/mL.
The catechin was estimated according to Singh et al.[Citation20] A highly specific diazotized sulfanilamide reagent was prepared for determination of catechins. The reagent was employed for their spectrophotometric quantification in the crude extracts of polyphenols isolated from CF and potato samples. The formation of yellow color (lambda [max] = 425 nm) between catechins and diazotized sulfanilamide was determined by a spectrophotometric method. The β-carotene content was determined by the method of Barrros et al.[Citation21] The sample (0.6 g) was weighed into 250 mL amber screw-top vials (Fisher, #03-391-8F) that contained 5 mL of 0.05% (w/v) BHT in acetone, 5 mL of 95% ethanol, and 10 mL of hexane. Samples were stirred with magnetic stirring and kept on an orbital shaker at 180 × g for 15 min on ice. After shaking, 3 mL of deionized water was added to each vial and the samples were shaken for an additional 5 min on ice. The vials were left at room temperature for 5 min to allow for phase separation. The absorbance of the upper hexane layer was measured at 479 nm blanked with hexane. The data were plotted against β-carotene content and appeared to obey Beer’s law with respect to β-carotene content of the samples. The contents of ascorbic acid were measured by 2,6-dichlorophenolindophenol titration according to the method of Zhou et al.[Citation21]
Analyses of peroxide, p-anisidine, and Iodine values (IVs)
The oxidative stability of all French fries samples stored for 60 min at 25°C after frying was determined by the formation of peroxide, p-anisidine, and IVs according to the previous method of Aladedunye and Matthaus.[Citation10] Five grams of French fries were weighed, the fat was extracted by 3.6 mL chloroform/methanol (2:1, v/v), and 1.2 mL water was added to the samples and then homogenized with a mixer (Preethi Chefro Model, India make) for 10 min. The mixture was separated by centrifugation at 3000 × g for 20 min. The resultant lower layer of chloroform was withdrawn and transferred to another tube. The upper layer was similarly extracted with 3.6 mL chloroform/methanol (2/1, v/v). The rest was similarly extracted with 2.4 mL chloroform. These two chloroform layers were combined with the initial chloroform extract. The combined chloroform extract was washed with 3 mL 0.88% potassium chloride and dried under a stream of argon gas. The residue was then weighed exactly, after being washed twice with 3.0 mL hexane and the residue from the extraction was taken as the weight of the sample for all the analysis according to the method of the Association of Official Analytical Chemists (AOAC).[Citation29]
Sample preparation and extraction of acrylamide from French fries
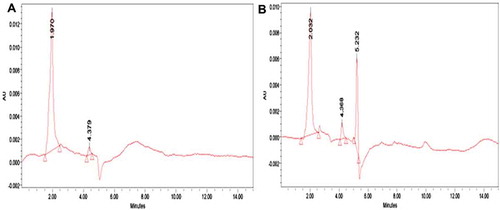
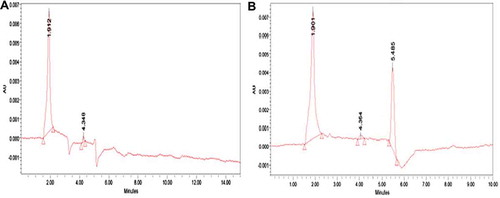
Finely ground and homogenized sample (4.0 g) were weighed into a closed flask, defatted twice by adding 10 mL hexane and shaking for 5 min. The mixture was dried under a vacuum after decantation. For the extraction of acrylamide, 20 mL of acetone and 100 μL of water were added to the defatted sample. The flask was placed in an ultrasonic bath at 40°C for about 20 min. The acetone was filtered through a filter paper. Gently, 10 mL of the filtrate was evaporated under a vacuum until it reached dryness. Then, 2 mL of water was added and shaken thoroughly to dissolve the residue. The aqueous solution was filtered through a 0.2 Polyvinylidene Fluoride syringe filter and injected to the column using a 20 μL injection loop. The acrylamide content in the samples was analyzed using HPLC (Hewlett Packard 1050) with a C18 SPE (250 × 4.6 nm, 5 μ purity, Reverse phase column (Waters, Milford MA 0175). The injected volume was 20 µL with a flow rate of 1 mL/min and the effluent of the column was continuously monitored with the ultraviolet (UV) detector at 254 nm.[Citation14] AA concentrations in treated samples were determined from the standard curve, using peak area for quantization.
The standard solutions of acrylamide were prepared according to a method described by El-Desouky et al.[Citation24] A stock solution of acrylamide (1000 μg/mL) was prepared by dissolving 0.1 g of acrylamide in deionized water and diluted to 100 mL in a volumetric flask. The successive 100 and 2 μg/mL stock solutions of acrylamide were prepared from this solution. Working standard solutions of acrylamide (20, 40, 80, 160, 240, 320, and 400 ng/mL) were prepared by appropriate dilution of 2 μg/mL stock solution with water. All standard solutions were protected from the light and stored in a refrigerator at 4ºC.[Citation23]
Sensory evaluation
The sensory evaluation was conducted with 30 trained panellists (15 men and 15 women) whose ages ranged from 20–40-years-old in an air-ventilated room under white light (at 25°C for 30 min, 60–65% relative humidity). The panellists were the students, technical staff, and faculty members of the Centre for Biotechnology at Anna University. On each sampling, with the serving volume of 4–5 pieces of samples, the coded samples were randomly presented to each panellist, with a rest period between sample presentations to minimize sensory fatigue. Although, they were regular consumers of fried potatoes, we had explained the procedure and the conditions of the tasting. Important parameters such as appearance, color, texture, taste, flavor, mouthfeel, and overall acceptability of the French fry samples were scored on a 9-point hedonic scale (10 = very good, 9 = good, 8 = acceptable, 5 = poor and 1 = very poor).[Citation23] The average grade was obtained from the reported data.[Citation6,Citation8]
Statistical analysis
Data was analyzed using one-way analysis of variance (ANOVA) tests with SIGMA STAT software package. All data are presented as mean ± standard error mean. Data with a p ≤ 0.05 was considered as statistically significant with a post hoc Tukey’s test were applied to perform a statistical analysis.
Result and discussion
Analysis of polyphenols and antioxidants
The phenolic compounds play an important role in adsorbing and neutralizing free radicals, quenching singlet triplet oxygen, or decomposing peroxides.[Citation13] Hydrogen donating characteristics of the phenolic compounds is responsible for the inhibition of free radical induced lipid oxidation.[Citation1] The fresh CF extract exhibited maximum phenolic content of 96.4 ± 0.1 mg gallic acid equivalent (GAE)/g when the raw potato extract showed significantly 27.4 ± 0.3 mg GAE/g before frying (). The potent free radical-scavenging and antioxidant activity of CF extract may be due to its high contents of flavonoids. Flavonoids mediate their antioxidant effects by scavenging free radicals by chelating metal ions.[Citation14] The treatment of a flavonoid-rich CF could effectively reduce acrylamide levels in French fries and the mechanism by which flavonoids mitigate the production of acrylamide can be attributed to their antioxidant properties, which may allow them to react with acrylamide precursors or key intermediates during acrylamide formation.[Citation16] Nems et al.[Citation11] showed that the flavonoids could scavenge the amino source asparagine-derived intermediates at positions 6 and 8 of the A-ring in its flavonoid structure and divert them from the pathways leading to acrylamide formation. Moreover, flavonoids reduce free radicals by quenching, up-regulating, or protecting antioxidant defenses and chelating radical intermediate compounds.[Citation2] The fresh CF extract had a higher flavonoid content than the raw potato extract. The results revealed that the total flavonols and flavanols in CF were significantly high (p < 0.05), and eventually higher than potato extract. Samec et al.[Citation1] reported that the quercetin and catechin were the main compounds with respect to flavonols and flavanols and they were dominating the antioxidant and radical scavenging properties. Quercetin and catechin in CF were quantified specifically () and comparatively higher than potato extract. Moreover, the mechanism by which flavonoids mitigate the production of acrylamide can be attributed to their antioxidant properties, which may allow them to react with acrylamide, acrylamide precursors, or key intermediates during acrylamide formation.[Citation23] Our results suggested that polyphenolic compounds and antioxidants may inhibit acrylamide formation in a similar way to when they inhibit Maillard reactions. The carotenoids, with their highly reactive conjugated double bonds, act as free radical traps or antioxidants and might be played an important role in quenching of toxic radicals.[Citation20] The β-carotene content in CF was high () while raw potato extract was exhibited the lowest content of 3.4 ± 0.4 μg/100 g. The β-carotene was attributed to the close association to antioxidant and radical scavenging properties and the current study agrees with a previous study by Cheng et al.[Citation16] Ascorbic acid has drawn increasing attention due to their potent antioxidant properties and their marked effects in the prevention of oxidative deterioration.[Citation21] The fresh extract of CF exhibited a higher content of ascorbic acid in comparison with raw potato and it was found to be 28.3 ± 0.7 and 19 ± 0.2 mg/g, respectively. The antioxidant level was found in CF was higher than those reported in earlier study Dutt et al.[Citation12]
Table 1. Effect of frying on degradation of polyphenols and antioxidant property in French fries.
Analysis of radical scavenging activities
The effect of antioxidants on DPPH radical scavenging is generally due to their hydrogen donating ability.[Citation13] The DPPH radical scavenging method is widely accepted for screening antioxidants, as well as determining comparative effects of different antioxidants based on reduction of absorbance at 517 nm.[Citation2] Othman et al.[Citation14] was reported that the DPPH radical was considered to be a model of lipophilic radicals, and the chains of lipophilic radicals are initiated by lipid autoxidation. These radicals react with hydrogen donors, such as polyphenols, to form stable diamagnetic molecules and induce color fading of the assay solutions. Our results were in agreement with the previous findings of Yang et al.,[Citation3] as shown in . These results pointed out that CF has a potential to reduce acrylamide during the heat treatment probably due to their high antioxidant properties and reduces the oxidative deterioration in the French fries. The ABTS radical scavenging method is based on the direct production of blue/green ABTS•+ chromophore through the reaction between ABTS and potassium persulfate.[Citation14] The antioxidant activity was evaluated by the capacity of the antioxidant compounds to reduce the radical cation ABTS•+ to ABTS, a protonated radical, has characteristic absorbance maxima at 734 nm which decrease with the scavenging of the proton radicals.[Citation3] This method was used to measure the antioxidant activity of both water-soluble and lipid-soluble antioxidants as well as extracts from natural sources.[Citation13] The highest free ABTS radical scavenging percentage of fresh CF extract was found to be 91.3 ± 0.5% () when the fresh raw potato exhibited 81.3 ± 0.9% inhibition. Samec et al.[Citation1] revealed that the polyphenols in CF have more ability to quench the ABTS radical and they are capable of donating a hydrogen atom were more effective in scavenging ABTS radicals. Nitric oxide plays an important role in various types of inflammatory processes in the animal body. The nitric oxide generated from sodium nitroprusside reacts with oxygen to form nitrite and the CF extract inhibits nitrite formation by directly competing with oxygen in the reaction with nitric oxide.[Citation13] Further, the scavenging activity may also help to arrest the chain of reactions initiated by excess generation of nitric oxide that are detrimental to the health of humans.[Citation2,Citation20] The extract of the CF was exhibited maximum (p ≤ 0.05) inhibitory effect on nitric oxide radicals formation (92.9 ± 0.4%; ).
Effect of CF on degradation of bioactive compounds and antioxidants in French fries after frying
The effect of frying on the degradation of polyphenols and antioxidant property of the French fries samples after frying was evaluated (). The frying process relies on high temperatures and can change the structure of labile nutrients, such as polyphenols, vitamins, and antioxidants in French fries.[Citation9] The heating process degrades the polyphenols and other bioactive compounds immediately after frying. The results showed that a significant (p ≤ 0.05) reduction was observed in the retention level of polyphenols and antioxidant properties in CF treated samples. Chammam et al.[Citation23] reported that, when the food is immersed in hot oil in the presence of oxygen, the oil is exposed to three agents that cause changes in its composition such as, water from the food (which causes hydrolytic changes) and oxygen (that gets in contact with the oil and causes oxidative changes from the surface to inside of the food).[Citation5] Predominant classes of flavonoids reported in the raw potatoes were catechin.[Citation23] At 190°C, the retention level of polyphenols in French fries obtained from the raw potato was comparatively lower than the BHA-treated sample. El-Desouky et al.[Citation26] reported that the browning process occurred in the fresh potatoes during peeling, cutting, or grating due to the oxidation and dehydrogenation of polyphenols, which was caused by polyphenol oxidase. Our results agreed with the previous findings of Yang et al.[Citation28] .Interestingly, the immersion process leads to a higher leaching of an important acrylamide precursor, such as glucose, that finally results in lower acrylamide formation.[Citation17] Similarly, some water soluble bioactive compounds might be leached out in the distilled water during immersion.[Citation15] The browning phenomenon induces the significant polyphenolic degradation in French fries sample before frying.[Citation28] Polyphenolic concentration of fresh potato did not considerably vary in comparison with sample immersed with BHA before frying. Moreover, a maximum reduction of polyphenolic concentration was observed in control due to the leaching of polyphenols in to water. Our results were in agreement with the previous study.[Citation12,Citation15] The high content of flavonols and flavanols such as quercetin and catechin in CF were effectively stabilizing the lipid oxidation.[Citation15] The CF treated sample showed maximum protection against degradation on radical scavenging property significantly (p ≤ 0.05) and comparatively higher than French fries samples from raw potato, control, and BHA-treated after frying. Among the bioactive components, the β-carotene and ascorbic acid seemed to be more heat sensitive.[Citation20] Zhou et al.[Citation21] reported that the ascorbic acid is very susceptible to oxidation under heat, presence of oxygen, heavy metal ions, and alkaline pH degrading their biological activity. Hence, the reduction in ascorbic acid was found to be significantly low when the sample was subjected to CF treatment ().
Effect of CF on acrylamide inhibition in French fries
In the oxidation of lipids, during the degradation of these macro-molecules, acrolein is formed, which reacts by generating acrylic acid or an intermediary acrylic radical through oxidation. These intermediaries finally generate acrylamide by interacting with nitrogen sources.[Citation4] The polyphenols may block the oxidation of acrolein, thereby reducing the formation of acrylamide.[Citation3] The results showed that the French fries sample immersed with CF extract significantly reduce the acrylamide level at 190°C during frying. Nems et al.[Citation9] found that the enhanced potency of CF could be attributed to its higher solubility in a lipidic food system such as French fries. CF may be more accessible to scavenge reactive carbonyls formed in Maillard reactions (which are responsible for the formation of acrylamide in food systems), and this property might result in reduced acrylamide levels.[Citation5] The acrylamide was formed in French fries obtained from control within the range of 260.36 ppb. Its formation in the raw potato French fry sample showed more than the level noted in the control, it was found to be 288.96 ppb (). The reducing sugars are one of the important components that interference in the formation of acrylamide.[Citation15] Our results, confirmed by Abboudi et al.,[Citation8] reported that immersion of potato pieces in distilled water causes a significant reduction in the glucose level and resulted the minimum acrylamide in the control and was comparatively lower than the fresh potato. However, CF treated French fries sample show the most efficient in acrylamide mitigation strategy with the lowest concentration of acrylamide and it was found to be 42.5 ppb when the BHA-treated sample showed in the range of 83.96 ppb (). According to the International Agency for Research on Cancer (IARC) reports, the maximum permissible limit of WHO (2005) is at the range of 21/140 μg/70 kg/day for the general population. The reduction of acrylamide in CF-treated samples may be due to flavonoids reacting with acrylamide precursors or key intermediates during acrylamide formation.[Citation16] Piedrahita et al.[Citation10] reported that the real possibility of minimizing the acrylamide contents in fried food by the polyphenols efficiently may be ascribed to combined mechanisms such as (1) radical scavenging activity, (2) carbonyl trapping effect, and (3) limitation of sugar degradation through the Maillard reaction.[Citation5,Citation6] Acrylamide formation in fried potatoes is related to raw material (potato variety and field site) and the production process.[Citation15,Citation16] The Maillard reaction is responsible for the development of undesirable dark colored compounds melanoidins with bitter taste.[Citation8,Citation27] Recently, it was discovered that the potential carcinogenic compound acrylamide is also formed in potatoes at high temperatures and that the precursors for melanoidins and acrylamide might be the same. Fried potato color is the result of Maillard, non-enzymatic browning reactions that depend on the superficial reducing sugar content, and the temperature and frying period.[Citation5,Citation8] In many cases, there is a good correlation between the acrylamide content of the fried potatoes and their color since acrylamide formation seems to be associated with the Maillard reaction.[Citation16]
Effect of CF on peroxide value (POV) and p-anisidine value (p-AV)
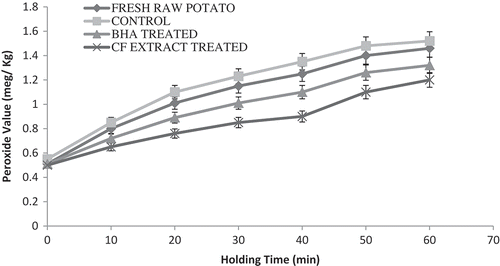
The POV is an indicator of the initial stage of autoxidation or oxidative rancidity in fried oil and thus determines the extent of lipid oxidation.[Citation24] This parameter was determined because the antioxidative treatment applied to fried French fries cannot only contribute to lowering the concentration of acrylamide, but can also prevent the oxidation of lipids.[Citation10] A significant difference (p ≤ 0.05) in POV was observed between the control, raw potato, and BHA- and CF-treated French fry samples. The results revealed that the polyphenols retard the hydroperoxide formation significantly (p ≤ 0.05) in the CF-treated sample (), and the results also confirmed that the POV increased gradually in the control, raw potato, and BHA-treated French fry samples of throughout the holding time at 25ºC from 0 to 60 min. Liu et al.[Citation5] reported that the polyphenols were interrupting the chain reaction of lipid oxidation and influence the superior antioxidant activity in French fries. The increasing POV indicated that the oxidation was gradually occurred in the absorbed oil in French fries.[Citation17] The POV of the CF-treated sample had the maximum influence of natural antioxidants to liberate minimum level of peroxides comparatively better than the BHA-treated sample at 60 min. The interactions between frying oil and fried food had great relevance on nutritional quality of the final product during holding time.[Citation13] The results seems to be attributed to a higher content of antioxidants which were effectively involved in the retardation of lipid oxidation. Our results were in agreement with the previous findings Chammem et al.[Citation24]
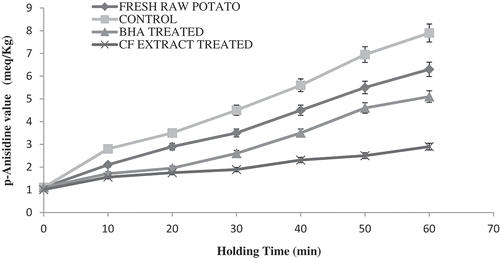
Thermal decomposition of lipid hydroperoxides during frying generates a number of secondary oxidation products, with carbonyl compounds being the most prominent.[Citation11] Although some of the aldehydes produced were volatile and lost by evaporation during frying, a significant amount remains and was assessed by p-AnV.[Citation5] The p-AnV was used to determine the later stages of oxidation products in sample and these values significantly (p ≤ 0.05) increased as the holding time period progressed after frying up until 60 min.[Citation3] Secondary oxidation products generated by hydroperoxide decomposition were found as early as time 0 of holding time. A potential source of these initial secondary oxidation products can be caused by the extraction of oil from the French fries. During the blending of the sample, exposure to air and mixing of the extract may have resulted in the initial oxidation in the oil. The sample treated with CF exhibited the highest stability during frying compared to the control and BHA and assessed by the amount of non-volatile carbonyl secondary oxidation products formed (), our results agreed with Bassama et al.[Citation17] The results revealed that the antioxidant activity may be accounted for because polyphenolic content and the stability of phenoxy radicals reduces the rate of propagation and further reactions and thus increases the oxidative stability of French fries.
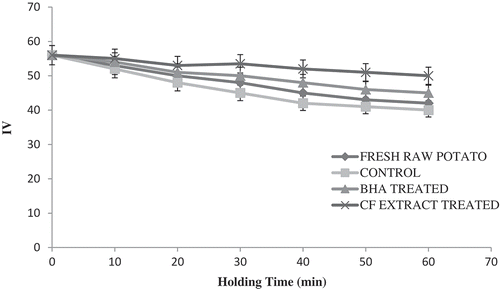
Effect of CF on IV
The IV is a measure of the unsaturation of the oils and it is one of the parameters used to measure the oil quality. A high IV denotes high degree of unsaturation of the oil caused by the extent of oxidation.[Citation11] A decrease in IV indicated that lipid oxidation might be due to metallic ions present, among other factors, which promotes oxidation after the formation of hydroperoxides.[Citation10] This unsaturation was in the form of double bonds that react with iodine compounds. The reduction of IV is shown in . The IV decreased significantly (p ≤0.05) with the time of storage for all samples. The CF extract could significantly (p ≤0.05) protect the oil from further oxidation.[Citation5] The IV results revealed that the antioxidant effect of CF could minimize the oxidation of the stored sample.
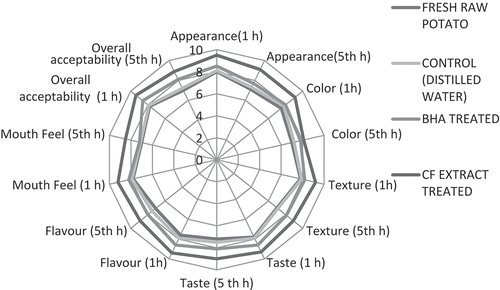
Sensory analysis
Sensory attributes of French fries were evaluated and presented in . When the potato products are fried, the changes in starch are very important. Starch granules are rapidly gelatinized upon contact with hot oil.[Citation12] Changes in the food surface may be caused by caramelization and/or the Maillard reaction as well as evaporation of surface water, which characterizes crust formation, responsible for the texture of fried food.[Citation5]. The evaluation of all these attributes showed that samples treated with CF were the most favorable French fries evaluated by panellists and had the highest score throughout the holding time. The control and raw potato French fry samples were assessed as the most unfavorable by panellists, which seems to be due to the breakdown of triglycerides, the extracted oil had an unpleasant taste and odor. The control and raw potato samples had a pungent taste when compared to BHA- and CF-treated samples, due to the oxidation of absorbed oil. The CF-treated samples were capable of maintaining its crispiness, there was a significant difference (p ≤0.05) observed with increasing holding time, unlike the control, fresh potato, and BHA, where the score decreased significantly (p ≤ 0.05) with the storage time. Natural antioxidants present in CF could influence the texture and flavor of the treated sample against lipid oxidation. Previous results revealed that the polyphenols are capable of maintaining not only the quality, but are also involved in the inhibition of lipid oxidation throughout the holding time, the results were in agreement with Ismial et al.[Citation12,Citation24,Citation27]
Conclusion
There have been many articles published demonstrating how acrylamide and lipid oxidation can be reduced in fried potato products by the modification of processes. However, in this work, we have systematically investigated the formation of acrylamide, lipid oxidation, and the degradation level of bioactive compounds in the French fries. The present study showed that the CF extract was capable of maintaining not only the quality, but highly influenced the overall acceptability of the product. The results showed that the effectiveness of polyphenols and antioxidants in French fries was eventually corroborated, thus suggesting its great potential for application in food processing to decrease acrylamide formation and lipid oxidation. Our results could be utilized in the development of a new, health-beneficial, functional potato products with enhanced antioxidant activity. This method can be applied in pilot plants, as well as at the industrial level since it does not require any specialized equipment and involves neither a significant increase in preparation time nor elevated costs.
Acknowledgment
The first author, Packirisamy Azhagu Saravana Babu, expresses his greatest gratitude to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the award of Senior Research Fellowship (SRF).
References
- Samec, D.; Bogovic, M.; Vincek, D.; Martincic, J.; Salopek Sondi, B. Assessing the Authenticity of the White Cabbage (Brassica Oleracea var. Capitata F. Alba) cv. Varazdinski by Molecular and Phytochemical Markers. Food Research International 2014, 60, 266–272.
- Oomah, B. D.; Mazza, G. Flavonoids and Antioxidative Activities in Buckwheat. Journal of Agricultural and Food Chemistry 1996, 44(7), 1746–1750.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology & Medicine 1999, 26, 1231–1237.
- Kita, A.; Barczak, A.B.; Lisinska, G.; Hamouz, K.; Kulakowska, K. Antioxidant Activity and Quality of Red and Purple Flesh Potato Chips. LWT–Food Science and Technology 2015, 62, 525–531.
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of Plant Polyphenols in Acrylamide Formation and Elimination. Food Chemistry 2015, 186, 46–53.
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of Cooking Methods on Polyphenols, Pigments and Antioxidant Activity in Potato Tubers. LWT–Food Science and Technology 2012, 45, 161–171.
- European Commission (EC). Commission Recommendation of November 8, 2013 on investigations into the levels of acrylamide in food. DOUE 315–317, 12-11-13. L 301:15–17. European commission. Brussels.
- Abboudi, M.; Al-Bachir, M.; Koudsi, Y.; Jouhara, H. Combined Effects of Gamma Irradiation and Blanching Process on Acrylamide Content in Fried Potato Strips. International Journal of Food Properties 2016, 19, 1447–1454.
- Nems, A.; Peksa, A.; Kucharska, A.Z.; Letowska, A.S.; Kita, A.; Drozdz, W.; Hamouz, K. Anthocyanin and Antioxidant Activity of Snacks with Coloured Potato. Food Chemistry 2015, 172, 175–182.
- Piedrahita, A.M.; Penaloza, J.; Cogollo, A.; Rojano, B.A. Kinetic Study of the Oxidative Degradation of Choibá Oil (Dipteryx Oleifera Benth.) with Addition of Rosemary Extract (Rosmarinus Officinalis L.). Food and Nutrition Sciences 2015, 6, 466–479.
- Aladedunye, F.; Matthaus, B. Phenolic Extracts from Sorbus Aucuparia (L.) and Malus Baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil During Frying and Storage. Food Chemistry 2014, 159, 273–281.
- Dutt, H.C.; Singh, S.; Avula, B.; Khan, I.A.; Bedi, Y.S. Pharmacological Review of Caralluma R. Br. with Special Reference to Appetite Suppression and Anti-Obesity. Journal of Medicinal Food 2012, 15, 108–119.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic–Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Farhadi, K.; Esmaeilzadeh, F.; Hatam, M.; Forough, M.; Molaie, R. Determination of Phenolic Compounds Content and Antioxidant Active in Skin, Pulp, Seed, Cane and Leaf of Five Native Grape Cultivars in We Azerbaijan Province, Iran. Food Chemistry 2016, 199, 847–855.
- Othman, A.; Ismail, A.; Hassan, F.A.; Yusof, B.N.M.; Khatib, A. Comparative Evaluation of Nutritional Compositions, Antioxidant Capacities, and Phenolic Compounds of Red and Green Sessile Joyweed (Alternanthera Sessilis). Journal of Functional Foods 2016, 21, 263–271.
- Ismial, S.M.A.; Ali, R.F.M.; Askar, M.; Samy, W.M. Impact of Pre-Treatments on the Acrylamide Formation and Organoleptic Evolution of Fried Potato Chips. American Journal of Biochemistry and Biotechnology 2013, 9, 90–101.
- Cheng, J.; Chen, X.; Zhao, S.; Zhang, Y. Antioxidant-Capacity-Based Models for the Prediction of Acrylamide Reduction by Flavonoids. Food Chemistry 2015, 168, 90–99.
- Afify, A.E.M.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant Enzyme Activities and Lipid Peroxidation as Biomarker Compounds for Potato Tuber Stored by Gamma Radiation. Asian Pacific Journal of Tropical Biomedicine 2012, S1548–S1555.
- Pejic, N.; Kuntic, V.; Vujic, Z; Micic, S. Direct Spectrophotometric Determination of Quercetin in the Presence of Ascorbic Acid. II Farmaco 2004, 59, 21–24.
- Singh, H.P.; Ravindranath, S.D.; Singh, C. Analysis of Tea Shoot Catechins: Spectrophotometric Quantitation and Selective Visualization on Two-Dimensional Paper Chromatograms Using Diazotized Sulphanilamide. Journal of Agricultural and Food Chemistry 1999, 47(3), 104–115.
- Barros, L.; Ferreira, M.J.; Queiros, B.; Ferreira, I.C.F.; Baptista, P. Total Phenols, Ascorbic Acid, B-Carotene and Lycopene in Portuguese Wild Edible Mushrooms and Their Antioxidant Activities. Food Chemistry 2007, 103, 413–419.
- Zhou, C.L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.Y.; Li, Q.H. The Effect of High Hydrostatic Pressure on Themicrobiological Quality and Physical–Chemical Characteristics of Pumpkin (Cucurbita Maxima Duch.) During Refrigerated Storage. Innovative Food Science and Emerging Technologies 2014, 21, 24–34.
- Codex Alimentarius Commission. Codex General Standard for Food Additives, CX-STAN. FAO/WHO: Rome, 2001; 192–1995.
- Chammem, N.; Saoudi, S.; Sifaoui, I.; Sifi, S.; Person, M.D.; Abderraba, M.; Moussa, F.; Hamdi, M. Improvement of Vegetable Oils Quality in Frying Conditions by Adding Rosemary Extract. Industrial Crops and Products 2015, 74, 592–599.
- AOAC, Official Methods of Analysis. Association of Official Analytical Chemist International: Arlington, VA, 1998.
- Deuber, H.; Guignard, C.; Hoffmann, L.; Evers, D.; Polyphenol and Glycoalkaloid Contents in Potato Cultivars Grown in Luxembourg, Food Chemistry 2012, 135, 2814–2824.
- El-Desouky, T.A.; Amer, M.M.; EL-Sedeek, L. Reduction of Acrylamide Formation in Potato Chips by Aqueous Extract of Roselle. Journal of Drug Delivery & Therapeutics 2015, 5(5), 26–32.
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Hoyos, L.C. Physical Features, Phenolic Compounds, Betalains and Total Antioxidant Capacity of Coloured Quinoa Seeds (Chenopodium Quinoa Willd.) from Peruvian Altiplano. Food Chemistry 2015, 183, 83–90.
- Yang, Y.; Achaerandio, I.; Pujola, M. Influence of the Frying Process and Potato Cultivar On Acrylamide Formation in French Fries. Food Control 2016, 62, 216–223