ABSTRACT
Atherosclerosis is an impairment of the artery walls made up of two membrane layers, intima and media. Oxidative stress, hypertension, and hypercholesterolemia are the three main factors that cause atherosclerosis. These conditions are frequently found together and may cause atherogenesis to rapidly occur. The edible genus Pleurotus is commonly known as oyster mushrooms. Pleurotus spp. has been proven to have valuable medicinal attributes. Hence, they have been listed among “mushroom nutriceuticals” and categorized as both functional foods and medicinal mushrooms. In this review, we report the benefits of Pleurotus spp. for the prevention and treatment of atherosclerosis via reduction of oxidative stress, hypertension, and hypercholesterolemia in terms of the therapeutic compounds responsible. This review revealed that at least ten different types of Pleurotus spp. have been reported to have anti-atherogenic capabilities, with six of them possessing high levels of anti-atherogenic compounds such as ACE inhibitor peptide, ergothioneine, chrysin, and lovastatin. Hence, it has been demonstrated that Pleurotus spp. has great potential for use as food or extracts from fruiting bodies or mycelium in an alternative therapy for atherosclerosis, through prevention and treatment of oxidative stress, hypertension, and hypercholesterolemia.
Atherosclerosis
Atherosclerosis is the most prevalent life-threatening disease in modern society. Atherosclerosis is an impairment of the artery walls, which are made up of two membrane layers, intima and media.[Citation3] It is the underlying cause for most cases of cardiovascular disease (CVD) such as coronary heart disease and stroke.[Citation2] Oxidative stress, hypertension, and hypercholesterolemia are the main risk factors for CVD.[Citation2,Citation3] They are linked together in many ways to cause atherosclerosis. Thus, compounds able to reduce these factors may prevent and/or treat atherosclerosis.[Citation3–Citation5] Oxidative stress and hypertension could initiate the atherogenesis by causing endothelial cell damage.[Citation4,Citation5] This may cause the membrane to become permeable to low-density lipoprotein (LDL) leakages, leading to the formation of atherosclerotic plaque.[Citation3–Citation5] High levels of cholesterol (hypercholesterolemia) lead to high levels of LDL and increase the risk of LDL leakages. Hypercholesterolemia also induces the expression of LDL receptor (LDL-R) gene in macrophage cells, eventually triggering the formation of macrophages-LDL or macrophages-cholesterol accumulation.[Citation6]
Oxidative stress in atherogenesis
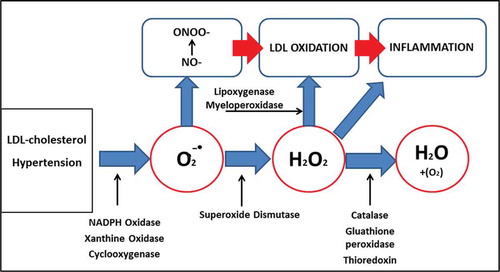
CVD risk factors, such as hypercholesterolemia, hypertension, diabetes, excessive alcohol use, and smoking lead to the increase of reactive oxidative species (ROS) which eventually lead to oxidative stress.[Citation2,Citation3] The reactions involved are catalyzed by oxidant enzymes such as Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase, xanthine oxidase, and cyclooxygenase.[Citation2,Citation3] The formation of superoxide may contribute to the reduction of nitric oxide (NO-) bioactivity and an increase in peroxynitrite (ONOO-) formation. NO- is known to have anti-atherogenic properties by causing vasodilation and offering protection to the vascular membrane.[Citation2,Citation3] On the other hand, the formation of peroxynitrite ONOO- will cause the lipid oxidation process to occur. Enzymes like lipoxygenase (LO) and myeloperoxidase (MPO) are oxidant enzymes that may be involved in lipid oxidation, either via ONOO- or hydrogen peroxide (H2O2), as a tool of oxidant.[Citation5] The link between the ROS and enzyme (oxidant and antioxidant) systems in the production and detoxification of ROS is summarized in . This oxidative stress mechanism eventually activates the inflammation signal. Oxidation may then trigger the expression of pro-inflammatory signals, which then induce the expression of adhesion molecule such as vascular cell adhesion molecule (VCAM-1), intracellular adhesion molecule (ICAM-1), and endothelial-leukocyte adhesion molecule-1 (E-selectin).[Citation3] The expression attracts more monocytes to differentiate into macrophages and adhere to the site of an injured endothelial membrane.[Citation7] It is known that oxidized LDL (ox-LDL) is an important initiator for many atherosclerotic pathways.[Citation8] The fusion of many macrophages and ox-LDL will cause the formation of foam cells.[Citation9] This progresses to a fatty streak and aortic lesions, which in turn may lead to severe endothelial injury and development of fibrous plaque.[Citation10]
Oxidation of LDL in atherogenesis
LDL is thought to be pro-atherogenic once structurally modified by oxidation, enzymatic modification, aggregation, or other mechanisms which can convert the LDL to a form identifiable by macrophages scavenger receptors as a foreign substance.[Citation3] The modified LDL would induce the formation of foam cell and then progresses to the development of fibrous plaque or atherosclerotic plaque.[Citation3] The contribution of modified LDL to the formation of foam cell and development of atherosclerotic plaque was initially demonstrated by Goldstein and colleagues in 1979. They found that there was no cholesterol aggregation during incubation of monocytes with high doses of native LDL as opposed to the incubation of monocytes with modified LDL, through which high cholesterol aggregation was observed.[Citation11] Modified LDL, especially ox-LDL, or the LDL modified by oxidation may damage the endothelial membrane, for example, by weakening endothelial NO- bioavailability and its vascular-protective function. ROS like ONOO- and hydrogen peroxide (H2O2) trigger the LDL to oxidize. These reactions may be mediated by oxidant enzymes such as LO and MPO. Napoli et al.[Citation12,Citation13] identified the presence of ox-LDL in the fatty streaks of hypercholesterolemic-human fetal aortas. The evidence is numerous regarding the role of LO and MPO enzymes in the LDL oxidation. For example, Cyrus et al.[Citation14] noted decreasing lipid peroxidation and the occurrence of murine atherogenesis by the absence of LO gene expression. Besides that, Bocan et al.[Citation15] successfully attenuated the development of atherosclerosis in hypercholesterolemic rabbits by treating them with the known inhibitors of LO. The expression of MPO in human atherosclerotic lesions was identified by Daugherty and colleagues in 1994. MPO is an enzyme generated by neutrophils and monocytes that secrete hypochlorous acid (HOCl),[Citation11] and also nitrating oxidants which cause oxidation and as well as inflammation.[Citation4]
Hypertension and atherosclerosis
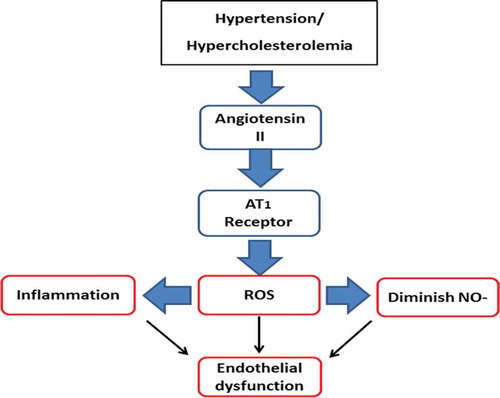
The renin-angiotensin and renin-aldosterone systems are linked together in the regulation of cardiovascular system via monitoring sodium balance, extracellular fluid volume, and structural, functional cardiac, and vascular effects. The combination of these two systems is known as the renin-angiotensin-aldosteron system (RAAS). Over-activity of RAAS is associated with the progression of hypertension, stroke, atherosclerosis, and other CVDs. The most important effector in RAAS, Angiotensin II, not only causes contraction of blood vessels, but through its binding to AT1 receptor in the vascular system, may activate several pathways that initiate atherogenesis via inflammation, LDL oxidation, and endothelial damage.[Citation16] The activation of AT1 initially triggers NADPH oxidase (NOX) to generate reactive oxygen species (ROS). This causes the activation of inflammatory signals, diminishes NO- bioavailability, and finally endothelial dysfunction, as described in .[Citation16,Citation17] Endothelial dysfunction causes LDL leakages and eventually leads to the formation of foam cells.[Citation18] The accumulation of foam cell in the blood vessels initiates the development of atherosclerotic plaque.[Citation18]
Regarding the role of angiotensin converting enzyme (ACE) in blood pressure homeostasis, it is an enzyme involved with blood pressure homeostasis. ACE is a dipeptidyl carboxypeptidase in the renin-angiotensin system (RAS) of mammals. ACE is widely distributed in the human body, for example, in the kidneys, gastrointestinal tract, lungs, liver, brain, testis, ovaries, and prostate.[Citation19,Citation20] It is called ACE because of its involvement in the conversion of angiotensin I to angiotensin II by breaking the C-terminal dipeptide His9-Leu10 from angiotensin I.[Citation21] Over expression of angiotensin II causes rapid constriction of blood vessels resulting in hypertension. Other than causing constriction by stimulating the formation of angiotensin II, ACE also has the ability to deteriorate bradykinin (potent vasodilator) and other vasoactive peptides. ACE acts by cleaving the active bradykinin into inactive bradykinin(1-7) and bradykinin(1-5) in kallikrein-kini cascade system.[Citation22] Bradykinin is a potent vasodilator that dilates the arteries, resulting in a drop in blood pressure. It also reduces the blood pressure by inducing other vasodilators such as NO-, prostacyclin, and endothelium-derived hyperpolarizing factor.[Citation19]
Hypercholesteromia
Hypercholesteromia is a condition in which levels of cholesterol in the blood are elevated.[Citation23] High levels of cholesterol leads to the high level of LDL, and hence, increase the risk of atherosclerotic plaque formation. Cholesterol homeostasis may be interfered with by a high intake of exogenous cholesterol. It induces disturbances of several regulation pathways such as the cholesterol biosynthetic pathway and expression of LDL-R genes in macrophage cells. The expression of LDL-R genes in macrophage is one of the main keys leading to the formation of macrophage-LDL/macrophage-oxLDL or macrophage-cholesterol accumulation.[Citation6] Besides that, the elevation in the level of cholesterol induces the formation of angiotensin II that up-regulate the expression of AT1 receptors. The activation of AT1 receptor and formation of angiotensin II would then lead to inflammation, vasoconstriction, endothelial dysfunction, and eventually atherosclerosis ().[Citation16,Citation17]
However, it must be noted that improvement of the serum high density lipoprotein (HDL) is essential in ameliorating atherogenesis, and has been a new target for atherosclerosis therapies.[Citation24–Citation26] HDL is also known as “reverse cholesterol transport” due to its involvement in shipping surplus stored cholesterol to the liver for metabolism and modification.[Citation24] On top of that, HDL has other anti-atherogenic properties such as anti-inflammation, antioxidant, endothelial fixation, and anti-thrombosis.[Citation24,Citation27–Citation30] It is believed that the quality of HDL is more important for prevention and treatment of atherosclerosis than quantity.[Citation25]
Role of 3-hydroxy-3-methylglutaryl-coA (HMG-CoA) reductase in cholesterol metabolism
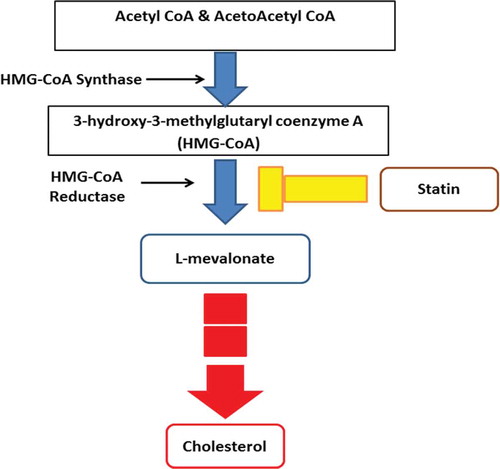
HMG-CoA reductase belongs to a class of proteins located in the endoplasmic reticulum.[Citation31] It has eight transmembrane domains with the active site along the carboxyl terminal in the cytosol.[Citation32] HMG-CoA reductase is a rate-limiting enzyme in the endogenous cholesterol metabolism. It is responsible for catalyzing the conversion of HMG-CoA to mevalonic acid in the mevalonate pathway,[Citation33] as shown in the . The mevalonate pathway consists of 28 steps. The interference of statin occurs in the second step of the pathway. Statin works by retarding the activity of HMG-CoA reductase from converting the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) into L-mevalonate. In some cases, statin has been criticized as having myopathy side effects,[Citation34] leading to an increase in demands for natural product alternative therapy.
Edible mushrooms
The first reliable evidence of mushrooms being consumed as food was recorded to several hundred years BC in China. The Chinese have used mushrooms in their traditional medicine.[Citation35] Currently, people consume mushrooms for texture, taste, and also for their nutritional and medicinal properties. Mushrooms are considered valuable resources in food production as well as a source of lead compounds in the production of drugs.[Citation36] Mushrooms are categorized as a healthy food because they are low in calories and fat, but rich in proteins and minerals.[Citation37] Their benefits to health include immunomodulatory, hypocholesterolemic effects, and anti-tumor effects.[Citation38] Mushrooms are currently considered a natural source in prevention and treatment of CVD via various mechanisms like anti-hypertension, cholesterol lowering, and anti-atherosclerotic. Lau et al.[Citation39] reported that protein fraction of fruiting bodies from various types of mushrooms like Agaricus bisporus (button mushroom), Flammulina velutipes (golden needle), Lentinula edodes (shiitake), Hericium erinaceus (monkey’s head mushroom), Pleurotus citrinopileatus (yellow oyster mushroom), P. cystidiosus (abalone mushroom), P. flabellatus (pink oyster mushroom), P. florida (white oyster mushroom), and P. pulmonarius (grey oyster mushroom) possess anti-hypertensive properties through inhibition of ACE. Gil-Ramirez et al.[Citation40] has proven that edible-medicinal mushrooms like Cantharellus cibarius, Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes, Boletus edulis, Amanita caesarea, Lactarius deliciosus, Lyophyllum shimeji, Agrocybe aegerita, Craterellus cornucopioides, Marasmius oreades, Pleurotus eryngii, Lepiota procera, Agaricus blazei, Grifola frondosa, and Flammulina velutipes are good alternative sources for hypocholesterolemic activity through the ability to retard the activity of HMG-CoA reductase enzyme. Abdullah et al.[Citation41] reported that 14 species of culinary-medicinal mushrooms (Agrocybe sp., Auricularia auricular-judae, Flammulina velutipes, Ganoderma lucidum, Hericium erinaceus, Lentinula edodes, Pleurotus cystidiosus, P. eryngii, P. flabellatus, P. florida, P. sajor-caju, Schizophyllum commune, Termitomyces heimii, and Volvariella volvaceae) have anti-oxidant and anti-hypertensive properties. In other work, Pleurotus eryngii, Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) are three species of edible mushrooms that have displayed anti-atherosclerotic effects in apolipoprotein E–deficient mice by lowering of serum total cholesterol concentrations.[Citation42]
Pleurotus spp. as a functional food
Pleurotus is a genus of edible-mushroom commonly known as Oyster mushrooms. This species is widely cultivated especially due to its flavor and texture.[Citation43] They are a good source of protein and carbohydrates. They are rich in minerals, low in fat, and have a short life cycle.[Citation44,Citation45] Mushrooms cultivation of the genus Pleurotus is widely carried out in several countries because of their high adaptability and health benefits. There are many different species in the genus Pleurotus with pharmacological properties, such as P. florida, P. tuber-regium, P. eryngii, P. ostreatus, and P. pulmonarius.[Citation46]
Pleurotus spp. has been demonstrated in many studies to have valuable medicinal attributes, such as immunomodulatory, anti-oxidant, anti-inflammatory, hypocholesterolemic, anti-genotoxic, anti-hyperglycaemic, anti-viral, anti-tumour, anti-Human Immunodeficiency Virus (HIV), anti-mutagenic, hepatoprotective, anti-ageing, and anti-allergic effects, from a diverse sort of extracted compounds.[Citation47,Citation48] They also have been listed among “mushroom nutriceuticals” and hence following categorized as functional foods and medicinal mushrooms.[Citation48,Citation49] In this review, we will present evidence to support the utilization of oyster mushrooms (Pleurotus spp.) for the prevention and treatment of atherosclerosis via oxidative stress, hypertension, and hypercholesterolemia ().
Table 1. Pleurotus spp. with reported anti-atherogenic properties and compounds.
Anti-atherogenic properties of pleurotus spp
Alternative therapy for oxidative stress
Previous studies have shown that methanol extracts of P. pulmonarius are effective in scavenging hydroxyl peroxide (OH−) and inhibiting lipid peroxidation with half maximal inhibitory concentration (IC50) 0.48 and 0.96 mg/mL, respectively.[Citation50] Water and alkali-extracted polysaccharide of P. tuber-regium have been shown to possess strong antioxidant capabilities in scavenging superoxide radical (O2−) and hydroxyl radical (OH−). Water-extracted polysaccharide was better than alkali-extracted polysaccharide in scavenging O2−, while alkali-extracted polysaccharide was better than water-extracted polysaccharide in scavenging OH−.[Citation51] Adebayo et al.,[Citation52] showed that the polysaccharide produced from the mycelium of P. pulmonarius exhibited good antioxidant activity by acting as hydrogen donor. Ergothioneine was proven to be able to be absorbed into endothelial cells membrane via organic cation transporter (OCTN) and hence protect the cells from oxidative stress.[Citation53,Citation54] In addition to that, a review by Paul and Snyder emphasized that a lack of ergothioneine in cells may lead to DNA damage and protein and lipid oxidation induced by oxidative stress.[Citation55] Ergothioneine was identified in large amount in Pleurotus spp. like P. ostreatus with 118.91 mg/kg wet weight and 97.35/123.42 mg/L which was higher than portabella mushroom (Agaricus bisporus, brown strain), button mushroom (Agaricus bisporus, white strain), chanterelle (Cantharellus cibarius), black turtle bean, red kidney bean, garlic, broccoli, onion, and spinach.[Citation54,Citation56] However, in vivo and clinical studies on the effect of ergothioneine on oxidative stress remain limited.[Citation57–Citation60] Thus, future work can be performed for the validation in vivo and clinical studies by investigating the effect of ergothioneine isolated or extracted from Pleurotus spp. for anti-atherogenic activities, especially in terms of the protective effect against oxidative damage.
Alternative therapy for hypertension
Lau et al.[Citation39] discovered several types of protein fractions from nine different types of mushrooms, including Pleurotus spp., with high inhibitory activity toward ACE. They then suggested that active proteins from mushrooms for the anti-hypertensive properties are those proteins with 3 to 10 kDa molecular masses. Abdullah et al.[Citation41] investigated ACE activity from hot water extract of selected medicinal mushrooms and demonstrated that Pleurotus spp. possessed the best ACE inhibitory activity with low IC50 values (P. cystidiosus: 0.054 mg/mL; P. eryngii: 0.067 mg/mL; P. flabellatus: 0.058 mg/mL; P. florida: 0.050 mg/mL; P. pulmonarius: 0.056 mg/mL) compared to other mushroom species. Jang et al.[Citation61] previously characterized a new ACE inhibitory peptide from the fruiting bodies of P. cornucopiae. Besides that, D-mannitol (sugar alcohol) extracted via hot water extraction of P. cornucopiae showed anti-hypertensive effect by inhibiting ACE activity and reducing the blood pressure of hypertensive rats.[Citation62] D-mannitol has been reported to be able to protect heart and myocardial from damage by previous works on Cordyceps sinensis.[Citation63,Citation64] A hot water extract, protein fraction, polysaccharide fraction and 6% dry powder of Pleurotus nebrodensis were shown to prevent and also ameliorate hypertension on hypertensive rats.[Citation65] A preparation of ethyl acetate fraction from Pleurotus eryngii done by Wang et al.[Citation66] has been patented for the prevention and treatment of hypertension in animal model. The fraction is believed to contain antihypertensive agents; however, the identified compounds were not revealed in that publication.
Alternative therapy for hypercholesterolemia
Lovastatin is a natural statin first discovered in the fermentation broth of Aspergillus terreus in 1978 by Albert and colleagues with name mevinolin. Later it was officially recognized as lovastatin.[Citation67,Citation68] Lovastatin has been clinically accepted as a great compound to reduce LDL cholesterol via inhibiting the activity of HMG-CoA reductase.[Citation68] Pleurotus spp. has earlier been reported to produce large amount of lovastatin/ mevinolin in the fruiting bodies ranged from 50–5991 µg/g dry weight.[Citation69,Citation70] Mevinolin produced from Pleurotus spp. such as P. sapidus, P. saca, and P. ostreatus have displayed good inhibitory of HMG-CoA reductase activity.[Citation70] The extraction of lovastatin from Pleurotus spp. done by Gunde-Cimerman and his co-workers were via 12 h incubation of the fruiting bodies with water: methanol [1:1] at 30ºC.[Citation40,Citation70] Alarcon et al.[Citation71] successfully identified the presence of lovastatin ranged from 5 to 70 mg/L in mycelium liquid culture from four different strains of P. ostreatus and in the fruiting bodies was ranged from 0.40 to 2.07%.[Citation71]
Recent studies have shown that Pleurotus ostreatus exhibited a very convincing ability to improve the lipid profile of high-fat diet fed on par to simvastatin (synthetic hypolipidemic) at 10% (w/w) biomass dosage, by reducing the amount of triglyceride and LDL, the bad cholesterol, but elevating the level of HDL, the good cholesterol.[Citation72] Pleurotus ostreatus also showed a significant reduction in plasma β-lipoprotein (lipoprotein for LDL) and pre-β-lipoprotein (lipoprotein for VLDL) and increment in α-lipoprotein (lipoprotein for HDL) in hypercholesterolemic rat supplemented with 5% (w/w) dry powder of P. ostreatus fruiting bodies.[Citation73,Citation74] Anandhi et al.[Citation75] suggested that chrysin (5,7-dihydroxyflavone) is the compound responsible for hypocholesterolemic activities from Pleurotus ostreatus. Chrysin was identified as a major component in ethanolic extract of Pleurotus ostreatus.[Citation76] Therefore, further study is required to validate the effect of chrysin in lowering cholesterol level and hence to be a new therapeutic compound for hypercholesterolemia besides lovastatin.
In other research on Pleurotus eryngii, the excreted polysaccharides from submersion culture of P. eryngii displayed inhibition of macrophage and ox-LDL (foam cells) accumulation via down regulation of the ox-LDL scavenger receptor on macrophage CD36.[Citation77] In hypercholesterolemic rats with diets supplemented with 5% (w/w) dry powder of Pleurotus eryngii fruiting bodies showed improvement in the atherogenic lipid profile due to lowered plasma β and pre-β-lipoprotein, while increasing α-lipoprotein and also reduced the level of LDL, triglyceride, plasma total cholesterol, phospholipid, total lipid, and LDL/ HDL (High-density lipoprotein) ratio.[Citation78] Fruiting bodies of P. florida have been reported to have hypocholesterolemic capability in elevating HDL/LDL ratio and decreasing total cholesterol, lipid and glyceride levels of liver and plasma.[Citation79] Diets supplemented with ethyl acetate and methanol extract of P. citrinopileatus (0.5 g/kg body weight daily) showed reduction in total cholesterol and triglycerides levels, and increments of HDL levels in hyperlipidemic hamsters.[Citation80]
The first ever research on the benefit of oyster mushroom (P. ostreatus) diet in humans was conducted by Schneider et al.[Citation81] Based on that study, a daily intake of oyster mushroom soup (30 g dried oyster mushroom per soup) by 20 subjects (9 male, 11 female; age 20–34 years) led to a significant reduction in triacylglycerol concentration, oxidized-LDL rates, and total cholesterol levels with –0.44 mmol/L, –7.2 U/mL, and –0.47 mmol/L, respectively. However, the presence of mevinolin compound was not detected in this study.[Citation81]
Conclusion
Based on the review, Pleurotus spp. has great potential as part of an alternative therapy for atherosclerosis through the prevention and treatment of oxidative stress, hypertension, and hypercholesterolemia, either directly as food or extracts from fruiting bodies or mycelium. However, a comparison study about how Pleurotus spp. may be best used is needed for further investigation. Among the Pleurotus species, P. ostreatus exhibited the best candidate for prevention and treatment of atherosclerosis due to the fact that it has been proven to contain a large amount of anti-atherosclerotic agents such as ergothioneine, lovastatin, and chrysin. Further investigations are also essential to elucidate its mechanisms and determine the compounds responsible for the anti-atherogenic properties. Further evaluation and clinical trials are also needed to validate the effectiveness of anti-atherogenic agent from Pleurotus spp. in human metabolism conditions.
Funding
Special thanks to the Mushroom Research Centre, University of Malaya and the High Impact Research (HIR) grant (UM.C/625/1/HIR/MoE/SC/02), Ministry of Education, Malaysia, University Malaya Research Grant (RP014A-13AFR) and PPP grant No PG142-2012B.
Additional information
Funding
References
- Chang, S.T. World Production of Cultivated and Medicinal Mushrooms in 1997 with Emphasis on Lentinus Edodes (Berk.) Sing, China. Internationl Journal of Medicinal Mushroom 1999, 1, 291–300.
- St. Clair, R. Pathogenesis of Atherosclerosis. Cardiology in Review 1997, 5, 14–71.
- Landmesser, U.; Drexler, H. Antioxidant and Cardiovascular Disese. In General Concept about Oxidative Stress; Bourassa, M.G.; Tardif, J.; Eds.; New York, United States of America, 2006.
- Gaut, J.P.; Byun, J.; Tran, H.D. Myeloperoxidase Produces Nitrating Oxidants in Vivo. The Journal of Clinical Investigation 2002, 109, 1311–1319.
- Claudio Borghi, G.G.; Taddei, S. Interactions Between Hypertension and Hypercholesterolemia: From Epidemiology to Therapeutic Implications. Current Hypertension Reviews 2008, 4, 1–7.
- Wilson, M.D.; Rudel, L.L. Review of Cholesterol Absorption with Emphasis on Dietary and Biliary Cholesterol. The Journal of Lipid Research 1994, 35, 943–955.
- George, S.J.; Johnson, J. Atherosclerosis: Molecular and Cellular Mechanisms. John Wiley & Sons: New Jersey, United States of America, 2010.
- Bourassa, M.G.; Tardif, J.-C. Antioxidants and Cardiovascular Disease. Springer: New York, United States of America, 2006.
- Thilakarathna, S.H.; Rupasinghe, H.V. Anti-Atherosclerotic Effects of Fruit Bioactive Compounds: A Review of Current Scientific Evidence. Canadian Journal of Plant Science 2012, 92(3), 407–419.
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and Oxidative Stress, 2008.
- Tsimikas, S. Antioxidant and Cardiovascular Disease. In Lipoprotein and Oxidation; Bourassa, M.G.; Tardif, J.; Eds.; Springer: New York, United States of America, 2006, 23(3), 381–390.
- Napoli, C.; D’armiento, F.; Mancini, F.; Postiglione, A.; Witztum, J.; Palumbo, G.; Palinski, W. Fatty Streak Formation Occurs in Human Fetal Aortas and is Greatly Enhanced by Maternal Hypercholesterolemia. Intimal Accumulation of Low Density Lipoprotein and Its Oxidation Precede Monocyte Recruitment into Early Atherosclerotic Lesions. Journal of Clinical Investigation 1997, 100(11), 2680.
- Napoli, C.; Glass, C.K.; Witztum, J.L.; Deutsch, R.; D’Armiento, F.P.; Palinski, W. Influence of Maternal Hypercholesterolaemia During Pregnancy on Progression of Early Atherosclerotic Lesions in Childhood: Fate of Early Lesions in Children (FELIC) Study. The Lancet 1999, 354(9186), 1234–1241.
- Cyrus, T.; Pratico, D.; Zhao, L. Absence of 12/15-lipoxygenase Expression Decreases Lipid Peroxidation and Atherogenesis in Apolipoprotein E-Deficient Mice. Circulation 2001, 103, 2277–2282.
- Bocan, T.M.; Rosebury, W.S.; Mueller, S.B. A Specific 15-Lipoxygenase Inhibitor Limits the Progression and Monocyte-Macrophage Enrichment of Hypercholesterolemia induced Atherosclerosis in the Rabbit. Atheroslerosis 1998, 136, 203–216.
- Borghi, C.; Grassi, G.; Taddei, S. Interactions Between Hypertension and Hypercholesterolemia: From Epidemiology to Therapeutic Implications. Current Hypertension Reviews 2008, 4(1), 1–7.
- Nickenig, G.; Harrison, D.G. The AT1-Type Angiotensin Receptor in Oxidative Stress and Atherogenesis, Part I: Oxidative Stress and Atherogenesis. Circulation 2002, 105, 393–396.
- Falk, E. Pathogenesis of Atherosclerosis. Journal of the American College of Cardiology 2006, 47(8s1), C7–C12.
- Imig, J.D. ACE Inhibition and Bradykinin-Mediated Renal Vascular Responses EDHF Involvement. Hypertension 2004, 43(3), 533–535.
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-Converting Enzyme: Cloning and Functional Expression as a Captopril-Insensitive Carboxypeptidase. The Journal of Biological Chemistry 2000, 275, 33238–33243.
- Grote, K.; Drexler, H.; Schieffer, B. Renin-Angiotensin System and Atherosclerosis. Nephrology Dialysis Transplant 2004, 19, 770–773.
- Schmaier, A.H. The Plasma Kallikrein-Kinin System Counterbalances The Renin-Angiotensin System. Review. Journal of Clinical Investigation 2002, 109, 1007–1009.
- Durrington, P. Dyslipidaemia. The Lancet 2003, 362(9385), 717–731.
- Barter, P. HDL-C: Role as a Risk Modifier. Atherosclerosis Supplements 2011, 12(3), 267–270. DOI:10.1016/s1567-5688(11)70885-6
- Badimon, L.; Vilahur, G. LDL-Cholesterol Versus HDL-Cholesterol in the Atherosclerotic Plaque: Inflammatory Resolution Versus Thrombotic Chaos. Annals of the New York Academy of Sciences 2012, 1254, 18–32. DOI:10.1111/j.1749-6632.2012.06480.x
- Tani, S.; Nagao, K.; Hirayama, A. HMG-Coa Reductase Inhibitor (Statin) Therapy and Coronary Atherosclerosis in Japanese Subjects: Role of High-Density Lipoprotein Cholesterol. American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions 2011, 11(6), 411–417. DOI:10.2165/11594620-000000000-00000
- Navab, M.; Ananthramaiah, G.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N. Thematic Review Series: The Pathogenesis of Atherosclerosis the Oxidation Hypothesis of Atherogenesis: The Role of Oxidized Phospholipids and HDL. Journal of Lipid Research 2004, 45(6), 993–1007.
- Barterm, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circulation Research 2004, 95(8), 764–772.
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and Antithrombotic Actions of HDL. Circulation Research 2006, 98(11), 1352–1364.
- Tso, C.; Martinic, G.; Fan, W.-H.; Rogers, C.; Rye, K.-A.; Barter, P.J. High-Density Lipoproteins Enhance Progenitor-Mediated Endothelium Repair in Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 2006, 26(5), 1144–1149.
- Bonifacino, J.S.; Lippincott-Schwartz, J. Degradation of Proteins within the Endoplasmic Reticulum. Current Opinion in Cell Biology 1991, 3(4), 592–600.
- Roitelman, J.; Olender, E.H.; Bar-Nun, S.; Dunn, W.A.; Simoni, R.D. Immunological Evidence for Eight Spans in the Membrane Domain of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase: Implications for Enzyme Degradation in the Endoplasmic Reticulum. The Journal of Cell Biology 1992, 117(5), 959–973.
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430.
- Rallidis, L.S.; Fountoulaki, K.; Anastasiou-Nana, M. Managing the Underestimated Risk of Statin-Associated Myopathy. International Journal of Cardiology 2012, 159(3), 169–176.
- Boa, E.R. Wild Edible Fungi: A Global Overview of Their Use and Importance to People. Food & Agriculture Org 2004, 17.
- Mizuno, T. The Extraction and Development of Antitumor-Active Polysaccharides from Medicinal Mushrooms in Japan (Review). International Journal of Medicinal Mushrooms 1999, 1(1) , 9–29.
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in Edible Mushrooms: An Inter-Species Comparative Study. Food Chemistry 1999, 65(4), 477–482. DOI:http://Dx.Doi.Org/10.1016/S0308-8146(98)00212-X
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An Aqueous Polysaccharide Extract from the Edible Mushroom Pleurotus Ostreatus Induces Anti-Proliferative and Pro-Apoptotic Effects on HT-29 Colon Cancer Cells. Cancer Letters 2006, 244(1), 61–70. DOI:10.1016/J.Canlet.2005.12.007
- Lau, C.-C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Proteomic Analysis of Antihypertensive Proteins in Edible Mushrooms. Journal of Agricultural Food Chemistry 2012, 60(50), 12341–12348. DOI:10.1021/Jf3042159
- Gil-Ramirez, A.; Cristina, C.; Palanisamy, M.; Reglero, G.; Perez, M. Edible Mushrooms as Potential Sources of New Hypocholesterolemic Compounds. Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), 2011; 110–119 pp.
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. (2012) Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid-Based Compl Alt 2012, 2012 (2012),1–12. DOI:10.1155/2012/464238
- Mori, K.; Kobayashi, C.; Tomita, T.; Inatomi, S.; Ikeda, M. Antiatherosclerotic Effect of The Edible Mushrooms Pleurotus Eryngii (Eringi), Grifola Frondosa (Maitake), and Hypsizygus Marmoreus (Bunashimeji) in Apolipoprotein E-Deficient Mice. Nutrition Research 2008, 28(5), 335–342. DOI:10.1016/J.Nutres.2008.03.010
- Ro, H.-S.; Kim, S.S.; Ryu, J.S.; Jeon, C.-O.; Lee, T.S.; Lee, H.-S. Comparative Studies on The Diversity of the Edible Mushroom Pleurotus Eryngii: ITS Sequence Analysis, RAPD Fingerprinting, and Physiological Characteristics. Mycological Research 2007, 111(6), 710–715.
- Yildiz, A.; Karakaplan, M.; Aydin, F. Studies on Pleurotus Ostreatus (Jacq. Ex Fr.) Kum. Var. Salignus (Pers. Ex Fr.) Konr. Et Maubl.: Cultivation, Proximate Composition, Organic and Mineral Composition of Carpophores. Food Chemistry 1998, 61(1), 127–130.
- Yildiz, S.; Yildiz, Ü.C.; Gezer, E.D.; Temiz, A. Some Lignocellulosic Wastes Used as Raw Material in Cultivation of the Pleurotus Ostreatus Culture Mushroom. Process Biochemistry 2002, 38(3), 301–306.
- Ragunathan, R.; Gurusamy, R.; Palaniswamy, M.; Swaminathan, K. Cultivation of Pleurotus spp. on Various Agro-Residues. Food Chemistry 1996, 55(2), 139–144.
- Gregori, A.; Švagelj, M.; Pohleven, J. Cultivation Techniques and Medicinal Properties of Pleurotus spp. Food Technology and Biotechnology 2007, 45(3), 238–249.
- Patel, Y.; Naraian, R.; Singh, V. Medicinal Properties of Pleurotus Species (Oyster Mushroom): A Review. World Journal of Fungal and Plant Biology 2012, 3(1), 1–12.
- Chang, S.; Buswell, J. Mushroom Nutriceuticals. World Journal of Microbiology and Biotechnology 1996, 12(5), 473–476.
- Ajith, T.A.; Janardhanan, K.K. Indian Medicinal Mushrooms as a Source of Antioxidant and Antitumor Agents. Journal of Clinical Biochemistry and Nutrition 2007, 40(3), 157.
- Wu, G.-H.; Hu, T.; Li, Z.-Y.; Huang, Z.-L.; Jiang, J.-G. In Vitro Antioxidant Activities of the Polysaccharides from Pleurotus tuber-regium (Fr.) Sing. Food Chemistry 2014, 148(0), 351–356. DOI:http://Dx.Doi.Org/10.1016/J.Foodchem.2013.10.029
- Adebayo, E.A.; Oloke, J.K.; Ayandele, A.A.; Adegunlola, C.O. Phytochemical, Antioxidant and Antimicrobial Assay of Mushroom Metabolite from Pleurotus pulmonarius—LAU 09 (JF736658) Journal of Microbiology and Biotechnology Research 2012, 2(2), 366–374.
- Li, R.; Kwan, Y.; Sit, A.; Ho, E.; Man, R.; Vanhoutte, P.; Leung, G. Ergothioneine Shows Protective Effect on Endothelial Cells in Oxidative Stress. FASEB 2011, 23, 630–633.
- Li, R.W.; Yang, C.; Sit, A.S.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P.; Chan, S.W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P. Uptake and Protective Effects of Ergothioneine in Human Endothelial Cells. The Journal of Pharmacology and Experimental Therapeutics 2014, 350(3), 691–700. DOI:10.1124/Jpet.114.214049
- Paul, B.D.; Snyder, S.H. The Unusual Amino Acid L-Ergothioneine is a Physiologic Cytoprotectant. Cell Death Differ 2010, 17(7), 1134–1140.
- Ey, J.; Schömig, E.; Taubert, D. Dietary Sources and Antioxidant Effects of Ergothioneine. Journal of Agricultural Food Chemistry 2007, 55(16), 6466–6474.
- Halliwell, B.; Lee, C.Y. Using Isoprostanes as Biomarkers of Oxidative Stress: Some Rarely Considered Issues. Antioxidants & Redox Signaling 2010, 13(2), 145–156. DOI:10.1089/Ars.2009.2934
- Gruber, J.; Tang, S.Y.; Jenner, A.M.; Mudway, I.; Blomberg, A.; Behndig, A.; Kasiman, K.; Lee, C.Y.; Seet, R.C.; Zhang, W.; Chen, C.; Kelly, F.J.; Halliwell, B. Allantoin in Human Plasma, Serum, and Nasal-Lining Fluids Aas A Biomarker of Oxidative Stress: Avoiding Artifacts and Establishing Real in Vivo Concentrations. Antioxidants & Redox Signaling 2009, 11(8), 1767–1776. DOI:10.1089/Ars.2008.2364
- Seet, R.C.; Lee, C.Y.; Chan, B.P.; Sharma, V.K.; Teoh, H.L.; Venketasubramanian, N.; Lim, E.C.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Ong, B.K.; Halliwell, B. Oxidative Damage in Ischemic Stroke Revealed Using Multiple Biomarkers. Stroke; A Journal of Cerebral Circulation 2011, 42(8), 2326–2329. DOI:10.1161/Strokeaha.111.618835
- Cheah, I.K.; Halliwell, B. Ergothioneine; Antioxidant Potential, Physiological Function and Role in Disease. Biochimica et Biophysica Acta 2012, 1822(5), 784–793. DOI:10.1016/J.Bbadis.2011.09.017
- Jang, J.-H.; Jeong, S.-C.; Kim, J.-H.; Lee, Y.-H.; Ju, Y.-C.; Lee, J.-S. Characterisation of a New Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from Pleurotus Cornucopiae. Food Chemistry 2011, 127, 412–418.
- Hagiwara, S.Y.; Takahashi, M.; Shen, Y.; Kaihou, S.; Tomiyama, T.; Yazawa, M.; Tamai, Y.; Sin, Y.; Kazusaka, A.; Terazawa, M. A Phytochemical in the Edible Tamogi-Take Mushroom (Pleurotus cornucopiae), D-Mannitol, Inhibits ACE Activity and Lowers the Blood Pressure of Spontaneously Hypertensive Rats. Bioscience, Biotechnology, and Biochemistry 2005, 69(8), 1603–1605. DOI:10.1271/Bbb.69.1603
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps Fungi: Natural Products, Pharmacological Functions and Developmental Products. Journal of Pharmacy and Pharmacology 2009, 61(3), 279–291.
- Xu, H.; Zheng, X.; Xu, C.; Zhao, Y.; Liu, F. The Protective Effect of Cordyceps Sinensis on Adriamycin-Induced Myocardial Damage. Acta Chin Med Pharmacol 2000, 28, 64–65.
- Miyazawa, N.; Okazaki, M.; Ohga, S. Antihypertensive Effect of Pleurotus Nebrodensis in Spontaneously Hypertensive Rats. Journal of Oleo Science 2008, 57(12), 675–681.
- Wang, R.; Huang, Y.; Zhao, W. Pleurotus Extract and Use in Treating Hypertension. U.S. Patent: 20,030,161,842, 2003
- Alberts, A.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E. Mevinolin: A Highly Potent Competitive Inhibitor of Hydroxymethylglutaryl-Coenzyme A Reductase and a Cholesterol-Lowering Agent. Proceedings of the National Academy of Sciences 1980, 77( 7), 3957–3961.
- Tobert, J.A. Lovastatin and Beyond: The History of the HMG-Coa Reductase Inhibitors. Nature Reviews Drug Discovery 2003, 2(7), 517–526.
- Gunde-Cimerman, N.; Cimerman, A. Pleurotus Fruiting Body Bodies Contain the Inhibitor of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase-Lovastatin. Experimental Mycology 1995, 19, 1–6.
- Gunde-Cimerman, N.; Plemenitas, A.; Cimerman, A. Pleurotus Fungi Produce Mevinolin, an Inhibitor of HMG Coa Reductase. FEMS Microbiology Letters 1993, 113(3), 333–337.
- Alarcon, J.; Aguila, S.; Arancibia-Avila, P.; Fuentes, O.; Zamorano-Ponce, E.; Hernandez, M. Production and Purification of Statins from Pleurotus Ostreatus (Basidiomycetes) Strains. Zeitschrift Fur Naturforschung C 2003, 58(1/2), 62–64.
- Dos Santos, L.F.; Zanatta, A.L.; Soccol, V.T.; Torres, M.F.; Bonatto, S.J.R.; Rubel, R.; Soccol, C.R. (2013) Hypolipidemic and Antiatherosclerotic Potential of Pleurotus ostreatus, Cultived By Submerged Fermentation in The High-Fat Diet Fed Rats. Biotechnology and Bioprocess Engineering 2013, 18(1),201–208.
- Alam, N.; Amin, R.; Khan, A.; Ara, I.; Shim, M.J.; Lee, M.W.; Lee, U.Y.; Lee, T.S. Comparative Effects of Oyster Mushrooms on Lipid Profile, Liver and Kidney Function in Hypercholesterolemic Rats. Mycobiology 2009, 37, 37–42.
- Alam, N.; Yoon, K.N.; Lee, T.S.; Lee, U.Y. Hypolipidemic Activities of Dietary Pleurotus Ostreatus in Hypercholesterolemic Rats. Mycobiology 2011, 39(1), 45–51.
- Anandhi, R.; Annadurai, T.; Anitha, T.S.; Muralidharan, A.R.; Najmunnisha, K.; Nachiappan, V.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and Antioxidative Effects of an Extract of the Oyster Mushroom, Pleurotus Ostreatus, and Its Major Constituent, Chrysin, in Triton WR-1339-Induced Hypercholesterolemic Rats. Journal of Physiology and Biochemistry 2013, 69(2), 313–323.
- Jayakumar, T.; Thomas, P.; Geraldine, P. In-Vitro Antioxidant Activities of an Ethanolic Extract of the Oyster Mushroom, Pleurotus Ostreatus. Innovative Food Science & Emerging Technologies 2009, 10(2), 228–234.
- Chen, J.; Yong, Y.; Xia, X.; Wang, Z.; Liang, Y.; Zhang, S.; Lu, L. The Excreted Polysaccharide of Pleurotus Eryngii Inhibits the Foam-Cell Formation via Down-Regulation of CD36. Carbohydrate Polymers 2014 , 114(4), 16–23.
- Alam, N.; Yoon, K.N.; Lee, J.S.; Cho, H.J.; Shim, M.J.; Lee, T.S. Dietary Effect of Pleurotus Eryngii on Biochemical Function and Histology in Hypercholesterolemic Rats. Saudi Journal of Biological Sciences 2011, 18(4), 403–409.
- Bajaj, M.; Vadhera, S.; Brar, A.; Soni, G. Role of Oyster Mushroom (Pleurotus Florida) as Hypocholesterolemic/Antiatherogenic Agent. Indian Journal of Experimental Biology 1997, 35(10), 1070–1075.
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and Antioxidant Effects of Extracts from Pleurotus citrinopileatus. Journal of Agricultural Food Chemistry 2006, 54(6), 2103–2110.
- Schneider, I.; Kressel, G.; Meyer, A.; Krings, U.; Berger, R.G.; Hahn, A. Lipid Lowering Effects of Oyster Mushroom (Pleurotus Ostreatus) in Humans. Journal of Functional Foods 2011, 3(1), 17–24. DOI:http://Dx.Doi.Org/10.1016/J.Jff.2010.11.004
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible Mushrooms: Role in the Prevention of Cardiovascular Diseases Filoterapia 2010, 81, 715–723.