ABSTRACT
Traditional kokorec, which is one of the products of offal (edible by-products) and consumed enthusiastically in Turkey, is produced from fresh and washed lamb and calf small intestines. Some health risks can occur unless hygiene and sanitation rules are followed and proper cooking procedures are applied during the production process of kokorec. One of the most important among these risks is the microbial origin hazard and another is the formation of polycyclic aromatic hydrocarbons. In this study, the aim was to determine the concentrations of eight polycyclic aromatic hydrocarbons formed during the cooking of kokorec produced from beef and lamb small intestines by adding various animal fats. Polycyclic aromatic hydrocarbonformation was specified in ready-to-consume kokorec samples in which eight polycyclic aromatic hydrocarbon concentrations varied between 3.07 and 40.11 µg/kg. The consumption of lamb kokorec could be recommended to consumers because its average total eight polycyclic aromatic hydrocarbon concentration was lower than that of beef kokorec.
Introduction
Kokorec is one of the most widely consumed traditional Turkish meat products.[Citation1] Omurtag et al.[Citation2] described kokorec as grilled sheep intestine. It’s also widely consumed in Greece and some Adriatic countries.[Citation3] Kokorec is made from animal fats, small intestines, and chitterlings, which include pathogenic bacteria, so it has some potential risks as regards foodborne illness for human health. On the other hand, kokorec is served grilled, so it has some carcinogenic risks such as polycyclic aromatic hydrocarbons (PAHs) due to the grilling process.
PAHs are compounds containing several aromatic rings consisting of only carbon and hydrogen atoms. These compounds are formed during incomplete combustion or pyrolysis of organic materials, and have a high persistence in the environment.[Citation4] Some of them have low biodegradability and high lipophilicity, and are potentially highly carcinogenic, mutagenic, and genotoxic. The PAHs that comprise fused aromatic rings containing up to four fused rings are referred to as light PAHs, and those containing more than four benzene rings are referred to as heavy PAHs. Heavy PAHs are more stable and more toxic than the light ones.[Citation5]
IARC[Citation6] reported that a significant source of PAH exposure in the general population is the consumption of particular foods, notably toasted cereals and grilled meats.[Citation7] These foods contain measurable levels of benzo[a]pyrene (BaP) and other PAHs that are carcinogenic, probably carcinogenic, or possibly carcinogenic to humans, and there is strong evidence that some of these compounds, including BaP, induce digestive-tract tumors in experimental animals when administered by ingestion.
One of the main sources of exposure to PAHs is food.[Citation7] Grilling (broiling) meat, fish or other foods with intense heat over a direct flame results in fat dripping on the hot fire and yielding flames containing a number of PAHs.[Citation8–Citation10] In this study, it was observed that the animal fats were dripping directly onto the flames and causing smoke during charcoal grilling of kokorec samples. According to the European Food Safety Authority,[Citation11] four specific PAHs (BaP, benz[a]anthracene (BaA), benzo[b]fluoranthene (BbF), and chrysene [Chry]) can be used as a marker for the occurrence and impact of carcinogenic PAHs in food. Since August 2011, the European Union (EU) maximum level for the four marker PAHs in grilled meat has been 12 μg kg−1[Citation12].
Tfouni et al.[Citation13] indicated that some studies were carried out on the isolation and determination of PAHs in thermally treated high-protein food samples (fried, roasted, grilled, smoked). The first report found that BaP and related PAHs were present in charcoal broiled beef in a study by Lijinsky and Shubik.[Citation14]
Since then, studies have provided much information on the levels of carcinogens found in grilled meat products.[Citation15] PAH contamination by intense thermal processing, such as charcoal grilling, is due to the direct pyrolysis of food nutrients and the direct deposition of PAHs from smoke produced through incomplete combustion of different thermal agents.[Citation16,Citation17] Charcoal grilling is one of the most intense thermal sources. In the food industry, this intense thermal source can be applied to foods directly or indirectly. Traditionally produced kokorec is exposed directly to charcoal flame in the pre-cooking process and indirectly in the final cooking process. It has been observed that if the meat contacts the grill flame directly, various carcinogenic PAHs can be detected.[Citation18]
It is well known that the charcoal grilling cooking method creates a risk for human health via carcinogenic compounds formed due to PAHs, but the consumption of grilled foods is increasing day by day.[Citation15] In general, tallow fats are used in the production of kokorec. While other animal fats (tail fats and subcutaneous fats) have not been used in kokorec production until now, they are occasionally used in other meat products. Yılmaz and Karakaya[Citation19] reported that sheep subcutaneous fats and sheep tail fats had a higher total unsaturated fatty acid content than sheep tallow fats. However, tallow fats had the highest total saturated fatty acid content. It was determined that the total saturated fatty acid contents of sheep subcutaneous fats, tail fats, and tallow fats were 47.77, 45.12, and 56.00%, respectively.[Citation19] Thus, subcutaneous and tail fats were more appropriate for human nutrition. The fat content of meat products is one of the determinant factors in PAH concentration,[Citation11,Citation20,Citation21] but there are almost no studies on the unsaturation degree of animal fats that affects PAH contamination. On the other hand, there have been very few studies on PAH determination while some microbiological properties have been studied for kokorec.[Citation22–Citation24]
In this study, different animal fats (lamb subcutaneous fat and lamb tail fat) substituted for lamb tallow fat were used in an attempt to improve the organoleptic features of kokorec and determine the effect of various animal fats on concentrations of PAHs formed during the cooking of kokorec produced from beef and lamb small intestines.
Materials and methods
Materials
PAH standards, BaA, Chry, BbF, benzo[k]fluoranthene (BkF), BaP, dibenz[a,h]anthracene (DhA), benz[g,h,i]perylene (BgP), and indo[1,2,3-cd]pyrene (IcP), were purchased from Sigma-Aldrich (Chemie GmbH, Riedstrasse, Steinheim). Stock standard solutions of 1 µg ml−1 in acetonitrile were prepared and used for further dilution. Water was distilled and purified with activated carbon (Millipore, Bedford, MA, USA). Sodium hydroxide was obtained from Fluka (Buchs, Switzerland). High-performance liquid chromatography (HPLC)-grade solvents consisting of acetonitrile, dichloromethane, and n-hexane were purchased from Merck (Darmstadt, Germany). Diatomaceous earth extraction cartridges (Extrelut, 20 mL) and refill material were obtained from Merck (Darmstadt, Germany). Bond Elut Propylsulfonic acid/silica based (PRS) cartridges were obtained from Agilent (Santa Clara, CA, USA) for solid-phase extraction. PRS cartridges were preconditioned with dichloromethane (2 mL). For column chromatography, silica gel (70–230 mesh) was purchased from Merck (Darmstadt, Germany). It was activated at 200°C for 18 h and preconditioned with n-hexane (25 mL).
Preparation of “kokorec”
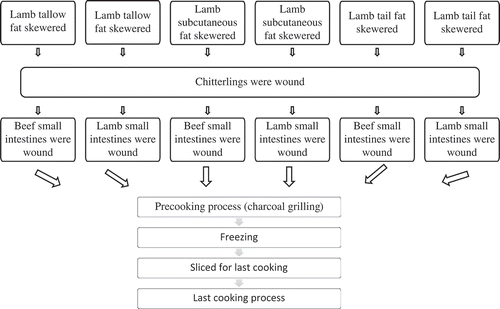
The beef and lamb small intestines, animal fats (lamb tallow fat, lamb subcutaneous fat, and lamb tail fat), and chitterlings were obtained from a local slaughterhouse in Konya. Production of kokorec is described step by step in the below: (1) Skewering of animal fats to spits; (2) Covering of skewered animal fat by chitterlings and small intestines, respectively, which is called as “raw kokorec;” (3) Pre-cooking of the raw kokorec on charcoal grilling (50 min pre-cooking time and 10 cm distance from the heat source); (4) Cooling of the pre-cooked kokorec to room temperature; (5) Removing of spits from the kokorec; (6) Freezing at –18°C; (7) Cutting into slices (about 1.5 cm thickness); and (8) The final cooking on charcoal grill (6–8 min).
Sample preparation
The cooked samples were ground through a 3 mm plate grinder (Kitchen Aid, Classic Model, USA). The minced kokorec samples were frozen at –18°C until PAH analysis and stored at 4°C for physicochemical analyses.
Experimental design
In this study, two different types of treatments were applied. Beef and lamb small intestines were used for small intestine treatment. Lamb tallow fat, lamb subcutaneous fat, and lamb tail fat (250 g animal fat for each group) were used for the other treatment. A total of six different kinds of kokorec groups were produced (interaction of beef small intestines and lamb tallow fat, interaction of beef small intestines and lamb subcutaneous fat, interaction of beef small intestines and lamb tail fat, interaction of lamb small intestines and lamb tallow fat, interaction of lamb small intestines and lamb subcutaneous fat, and interaction of lamb small intestines and lamb tail fat). The experimental design of the study is shown in .
Extraction and clean-up of PAHs
Extraction and clean-up procedures were carried out based on the method described by Farhadian et al.[Citation25] and Janoszka et al.[Citation26] with minor modifications. Each kokorec sample (5 g) was homogenized for 2 h in 15 mL of cold NaOH solution (1 M). Each sample was mixed with Extrelut refill material (diatomaceous earth, 17 g) at the stationary phase and the mixture was placed in a 20 mL Extrelut column. Then the column was connected to a PRS Solid Phase Extraction (SPE) column and the PAH fraction was eluted with dichloromethane (60 mL). The dichloromethane extract was evaporated by a rotary evaporator to dryness and the residue was redissolved in 1 mL n-hexane, which was then placed on top of the column, which was packed with activated silica gel (10 g) and preconditioned by using n-hexane (25 mL). The column was eluted with 60:40 (v/v) n-hexane and dichloromethane (60 mL) to collect the PAH fraction. The solvent was evaporated and the PAH residue was dissolved in acetonitrile (1 mL).
Limit of detection (LOD) and limit of quantification (LOQ)
The LOD and LOQ were calculated by using signal-to-noise ratios (S/N) of S/N = 3 and S/N = 10, respectively.[Citation27] In the present study, the LOD and LOQ of PAHs were determined by injecting three replicates of the lowest concentration of each standard (PAH8: BaA, Chry, BbF, BkF, BaP, DhA, BgP, IcP) into HPLC.
Recovery studies
Recovery rates for different PAHs (BaA, Chry, BbF, BkF, BaP, DhA, BgP, IcP) in raw kokorec samples were determined by the standard addition method.[Citation28] The amounts of these PAHs were corrected for by spiking four levels (1, 5, 10, and 50 ng/g) in four replicates.
HPLC analysis
PAH analysis was carried out based on the method described by Farhadian et al.[Citation25] with some modifications using an HPLC instrument (ThermoScientific UltiMate® 3000, USA) equipped with a 600 controller pump, a fluorescence detector, ThermoScientific (λex = 207 nm, λem = 500 nm) and a 20 µL loop injector. The mixture and extracts of PAHs were separated on a Hypersil Green PAH (ThermoScientific, USA) column (150 × 2.1 mm, 3 µm particle size). The mobile phase consisted of 50% acetonitrile and 50% purified water at a flow rate of 0.6 mL/min. Separation was performed under isocratic conditions. The quantification of PAHs was performed using an external calibration curve method. The quantification of PAH8, BaA, Chry, BbF, BkF, BaP, DhA, BgP, and IcP was carried out through the external standard method.
pH and total lipid analyses
The pH was measured with a pH meter (WTW 315i set model, Weilheim, Germany) according to AOAC.[Citation29] Samples were analyzed for total lipid according to the Soxhlet method described in 960.39 of AOAC for meat samples.[Citation29]
Statistical analysis
In this study, a completely randomized design was employed (two replicates). The descriptive statistics and two-way analysis of variance (ANOVA) were performed by using the Minitab 16 (Minitab Inc., PA, USA) statistical program.
Results and discussion
The total lipid content and pH values of the kokorec samples are given in . The pH values of the samples were between 6.92 and 7.07. The differences between the pH values of the beef kokorec samples and lamb kokorec samples were not statistically significant (p > 0.05). The total lipid contents of the groups were between 24.04 and 40.39%. This difference between the samples is not statistically significant (p > 0.05). Makarnaci[Citation23] reported that the total lipid contents of raw kokorec samples were between 6.12 and 8.12%. In this study, the total lipid contents of the samples were not similar to the findings of Makarnaci[Citation23] because the cooking process seemed to result in a higher fat content. As a result of cooking kokorec, since the amount of water decreases, the total lipid content increases in general. Therefore, the fat content in cooked kokorec is higher than that in raw kokorec.
Table 1. pH values and total lipid contents of kokorec samples.
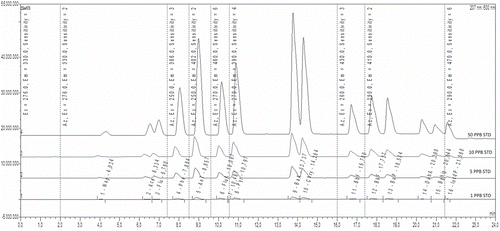
An HPLC chromatogram from mixed stock solutions is shown in . The LOD, LOQ, R2, values and recoveries are presented in . The LOD and LOQ values ranged from 0.027 to 0.125 and 0.090 to 0.869 µg/kg, respectively, for all eight PAH (PAH8) standards. The correlation coefficient (R2) values of PAH8 were between 0.9991 and 0.9999. Recoveries for kokorec samples were between 49.03 and 92.57%.
Table 2. Limits of detection and quantition (LOD and LOQ), R2 and recovery values obtained for PAH8 standards.
In the present study, concentrations of PAH8 analyzed and the four marker PAHs (PAH4) were measured in kokorec produced from beef and lamb small intestines by adding various animal fats. shows the determination of PAH8 in kokorec groups by HPLC-Fluorescence Detection (FD). It was determined that the average BaA, Chry, BbF, BaP and BgP concentrations of the kokorec groups ranged from 0.47 to 4.86, 0.56 to 5.60, 0.50 to 5.37, 0.63 to 7.73, and 0.47 to 6.81 µg/kg, respectively. Beef kokorec and tail fat interaction had significantly (p < 0.05) the highest concentrations for all PAH8s while lamb kokorec and tail fat interaction decreased significantly (p < 0.05) the contents of PAH8, and this interaction had the lowest concentrations of PAH8. Indeno[1,2,3-cd]pyrene was not detected in lamb kokorec and tail fat interaction. BkF was not quantified in beef kokorec and tallow fat interaction, lamb kokorec and subcutaneous fat and lamb kokorec and tail fat interactions, and also DhA was not quantified in lamb kokorec and tail fat interaction.
Table 3. Polycyclic aromatic hydrocarbons in beef and lamb kokorec samples (µg/kg).
The results of some studies concerning concentrations of PAHs in meat and meat products are summarized in . Janoszka[Citation30] found 0.01–2.76 µg/kg concentrations of BaA in pork meat and its gravy fried without additives and in the presence of onion and garlic. In a study by Roseiro et al.,[Citation31] Chry was found in concentrations of 1.54–150.56 µg/kg in Portuguese traditional meat products, and 0.61–139.62 µg/kg in blood sausages. Farhadian et al.[Citation32] investigated the effects of marinating on the formation of PAHs in grilled beef meat, and in their study BbF, was found in concentrations of 1.42–5.91 µg/kg. In addition, in Roseiro et al.,[Citation31] BbF was determined in concentrations of 0.07–6.68 µg/kg in Portuguese traditional meat products. BkF was determined in concentrations of 0.12–4.69 µg/kg in processed meat products.[Citation33] EU Regulations (EUR) determined a maximum level of 2.0 µg/kg for BaP (5.0 µg/kg prior to September 2014) in smoked meat and smoked meat products. It was reported that BaP in grilled meat or fish ranged from 0.2 to 50 µg/kg.[Citation34] BaP was determined in concentrations of 0.01–1.61 µg/kg in pork meat and its gravy fried without additives and in the presence of onion and garlic. BaP was determined at between 0.36 and 4.75 µg/kg in Portuguese traditional meat products.[Citation35] In addition, Farhadian et al.[Citation32] reported that BaP was found in concentrations of 0.11–4.78 µg/kg in grilled beef meat. Concentrations of DhA and BgP ranged from 0.05 to 0.55 and 0.01 to 1.12 µg/kg, respectively.[Citation30] Olatunji et al.[Citation33] reported that concentrations of IcP ranged between 0.21 and 31.11 µg/kg in processed meat products.
Table 4. Concentrations of PAHs in meat and meat products.
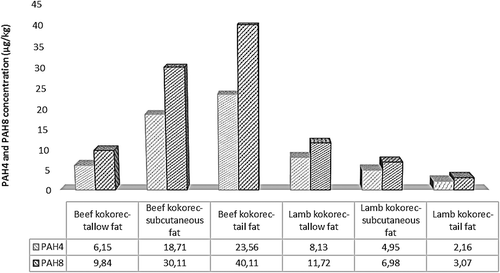
The mean PAH4 and PAH8 concentrations of the kokorec groups are given in . PAH4 and PAH8 values of the samples ranged from 2.16 to 23.56 and 3.07 to 40.11, respectively. The minimum concentration of PAH4 (2.16 µg/kg) was detected in lamb kokorec and tail fat interaction, and the maximum concentration (23.56 µg/kg) was detected in beef kokorec and tail fat interaction.
The results of some studies about concentrations of PAH4 in meat and meat products are summarized in . Duedahl-Olesen et al.[Citation36] determined the sum of PAH4 in pork chops (26 µg/kg), whole marinated chicken (21.4 µg/kg), hamburgers (7.7 µg/kg), marinated on a stick (4.9 µg/kg), and pork chops (4.1 µg/kg). In addition, Rose et al.[Citation37] reported that PAH4 was found in concentrations of between 9.27 and 95.87 µg/kg in beefburgers and 0.30–10.87 µg/kg in beef. Our results were similar when compared with other studies. Also, as a result of our study, the PAH4 levels of the interactions between beef small intestines and subcutaneous fat, and beef small intestines and tail fat, were higher than the PAH4 value that was determined by EUR as a maximum limit of 12 µg/kg (30.0 prior to September 2014). However, it was observed that the regulatory levels of PAHs currently cover only smoked meat and smoked meat products. However, there is no smoking process in the production of traditional kokorec.
Table 5. PAH4 concentrations of meat and meat products.
It was observed that there was no statistical correlation between the PAH concentrations and the fat contents in the kokorec groups. Similarly, it was reported that fat content and smoking regime alone did not influence the PAH concentrations.[Citation38] In another study, Pöhlmann et al.[Citation39] found that the sum contents of the five phenolic compounds did not depend on the fat contents of the sausages. While the fat contents of the kokorec groups did not influence the PAH concentrations, animal fat varieties affected the PAH contents of the kokorec groups. It was determined that lamb kokorec that was made with tail fat had the lowest PAH4 and PAH8 concentrations in the lamb kokorec groups. In the beef kokorec groups, the group that was made with tallow fat had the lowest PAH4 and PAH8 contents.
Conclusion
It was determined that traditional kokorec was among the risky food groups due to the presence of PAHs (BaA, Chry, BbF, BkF, BaP, DhA, BgP, IcP). The PAH concentrations between the six groups of kokorec were determined to be significantly (p < 0.05) different. The highest concentrations of PAHs were determined in beef kokorec and the tail fat group. The interaction of lamb kokorec and the tail fat group had the lowest level of PAHs. Unfortunately, studies on traditional kokorec are very limited, not only in Turkey but across the world. It is well known that the traditional kokorec is very popular in Turkey in today’s world. Some regulations regarding chemical contaminants such as PAH levels and microbial loads are needed in kokorec production. Further studies are needed to minimize the PAH contamination of traditional kokorec such as the effects of heat sources, distance to heat source, charcoal grilling time, and fat content.
Funding
This study was funded by Academic Staff Training Program (OYP) Coordination Unit (2014 OYP–046).
Additional information
Funding
References
- Kilic, B. Current Trends in Traditional Turkish Meat Products and Cuisine. LWT–Food Science and Technology 2009, 42(10), 1581–1589.
- Omurtag, I.; Paulsen, P.; Hilbert, F.; Smulders, F.J.M. Demographic/Socio-Economic Factors and Dietary Habits Determining Consumer Exposure to Foodborne Bacterial Hazards in Turkey Through the Consumption of Meat. Food Security 2013, 5(1), 103–115.
- Dontorou, C.; Papadopoulou, C.; Filioussis, G.; Economou, V.; Apostolou, I.; Zakkas, G.; Salamoura, A.; Kansouzidou, A.; Levidiotou, S. Isolation of Escherichia Coli O157: H7 from Foods in Greece. International Journal of Food Microbiology 2003, 82(3), 273–279.
- Šimko, P. Determination of Polycyclic Aromatic Hydrocarbons in Smoked Meat Products and Smoke Flavouring Food Additives. Journal of Chromatography B 2002, 770(1–2), 3–18.
- Wenzl, T.; Simon, R.; Anklam, E.; Kleiner, J. Analytical Methods for Polycyclic Aromatic Hydrocarbons (Pahs) in Food and the Environment Needed for New Food Legislation in the European Union. TrAC Trends in Analytical Chemistry 2006, 25(7), 716–725.
- IARC. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. In Monographs on the Evaluation of Carcinogenic Risks to Humans, Mitchell, J., Ed.; World Health Organization, Lyon, France, 2010.
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Pork, Beef and Chicken Barbecued at Home by Danish Consumers. Meat Science 2013, 93(1), 85–91.
- Djinovic, J.; Popovic, A.; Jira, W. Polycyclic Aromatic Hydrocarbons (PAHs) in Different Types of Smoked Meat Products from Serbia. Meat Science 2008, 80(2), 449–456.
- Kendirci, P.; Icier, F.; Kor, G.; Onogur, T.A. Influence of Infrared Final Cooking on Polycyclic Aromatic Hydrocarbon Formation in Ohmically Pre-Cooked Beef Meatballs. Meat Science 2014, 97(2), 123–129.
- Stumpe-Vīksna, I.; Bartkevičs, V.; Kukāre, A.; Morozovs, A. Polycyclic Aromatic Hydrocarbons in Meat Smoked with Different Types of Wood. Food Chemistry 2008, 110(3), 794–797.
- EFSA. Polycyclic Aromatic Hydrocarbons in Food, Scientific Opinion of the Panel on Contaminants in the Food Chain. The EFSA Journal 2008, 724, 1–114.
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 As Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Official Journal of the European Union 2011, 215(4), 8.
- Tfouni, S.A.V.; Souza, N.G.; Neto, M.B.; Loredo, I.S.D.; Leme, F.M.; Furlani, R.P.Z. Polycyclic Aromatic Hydrocarbons (PAHs) in Sugarcane Juice. Food Chemistry 2009, 116(1), 391–394.
- Lijinsky, W.; Shubik, P. Benzo[a]pyrene and Other Polynuclear Hydrocarbons in Charcoal-Broiled Meat. Science 1964, 145(3627), 53–55.
- Sundararajan, N.; Ndife, M.; Basel, R.; Green, S. Comparison of Sensory Properties of Hamburgers Cooked by Conventional and Carcinogen Reducing “Safe Grill” Equipment. Meat Science 1999, 51, 289–295.
- Rey-Salgueiro, L.; Garcia-Falcon, M.S.; Martinez-Carballo, E.; Simal-Gandara, J. Effects of Toasting Procedures on the Levels of Polycyclic Aromatic Hydrocarbons in Toasted Bread. Food Chemistry 2008, 108(2), 607–615.
- Rey-Salgueiro, L.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Effects of a Chemical Company Fire on the Occurrence of Polycyclic Aromatic Hydrocarbons in Plant Foods. Food Chemistry 2008, 108(1), 347–353.
- Lijinsky, W.; Ross, E. Production of Carcinogenic Polynuclear Hydrocarbons in the Cooking of Food. Food and Cosmetics Toxicology 1967, 5, 343–347.
- Yılmaz, M.T.; Karakaya, M. Thermal Analysis of Lipids Isolated from Various Tissues of Sheep Fats. Journal of Thermal Analysis and Calorimetry 2009, 101(1), 403–409.
- Chen, B.H.; Lin, Y.S. Formation of Polycyclic Aromatic Hydrocarbons During Processing of Duck Meat. Journal of Agricultural and Food Chemistry 1997, 45, 1394–1403.
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N.; Analysis of 200 Food Items for Benzo(a)pyrene and Estimation of Its Intake in An Epidemiologic Study. Food and Chemical Toxicology 2001, 39, 423–436.
- Kara, R.; Aslan, S.; Yaman, H.; Akkaya, L. Determination of Microbiological Quality of Kokorec Samples Consumed in Afyonkarahisar, Turkey. Kocatepe Veterinary Journal 2013, 6(1), 7–10.
- Makarnaci, N. A Study on the Determination of Microbiological Quality of Kokorecs Trakya University, Graduate School Of Natural Sciences: Tekirdaĝ, 2003.
- Temelli, S.; Saltan, E.S.; Anar, Ş.; Tayar, M. Determination of Microbiological Quality of Kokorecs Consumed in Bursa. İstanbul University Journal of Veterinary Faculty 2002, 28(2), 467–473.
- Farhadian, A.; Jinap, S.; Hanifah, H.N.; Zaidul, I.S. Effects of Meat Preheating and Wrapping on the Levels Of Polycyclic Aromatic Hydrocarbons in Charcoal-Grilled Meat. Food Chemistry 2011, 124(1), 141–146.
- Janoszka, B.; Warzecha, L.; Blaszczyk, U.; Bodzek, D. Organic Compounds Formed in Thermally Treated High-Protein Food, Part 1: Polycyclic Aromatic Hydrocarbons. Acta Chromatographica 2004, 14, 115–128.
- Juan, C.; Zinedine, A.; Moltó, J.C.; Idrissi, L.; Mañes, J. Aflatoxins Levels in Dried Fruits and Nuts from Rabat-Salé Area, Morocco. Food Control 2008, 19(9), 849–853.
- Oz, F. Quantitation of Heterocyclic Aromatic Amines in Ready to Eat Meatballs by Ultra Fast Liquid Chromatography. Food Chemistry 2011, 126(4), 2010–2016.
- AOAC. Official Methods of Analysis, 17th Ed; Association of Official Analytical Chemists, Gaithersburg, MD, USA, 2000..
- Janoszka, B. HPLC-Fluorescence Analysis of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat and Its Gravy Fried Without Additives and in the Presence of Onion and Garlic. Food Chemistry 2011, 126(3), 1344–1353.
- Roseiro, L.C.; Gomes, A.; Santos, C. Influence of Processing in the Prevalence of Polycyclic Aromatic Hydrocarbons in a Portuguese Traditional Meat Product. Food and Chemical Toxicology 2011, 49(6), 1340–1345.
- Farhadian, A.; Jinap, S.; Faridah, A.; Zaidul, I.S.M. Effects of Marinating on the Formation of Polycyclic Aromatic Hydrocarbons (Benzo[a]pyrene, benzo[b]fluoranthene and Fluoranthene) in Grilled Beef Meat. Food Control 2012, 28(2), 420–425.
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Determination of Polycyclic Aromatic Hydrocarbons [PAHs] in Processed Meat Products Using Gas Chromatography–Flame Ionization Detector. Food Chemistry 2014, 156, 296–300.
- IARC. Polynuclear Aromatic Compounds. In Monographs on the Evaluation of Carcinogenic Risks to Humans, Heseltine, E., Ed.; World Health Organization, Lyon, France, 1983.
- Roseiro, L.C.; Gomes, A.; Patarata, L.; Santos, C. Comparative Survey of PAHs Incidence in Portuguese Traditional Meat and Blood Sausages. Food and Chemical Toxicology 2012, 50(6), 1891–1896.
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.H.; Binderup, M.L. Polycyclic Aromatic Hydrocarbons (PAH) in Danish Barbecued Meat. Food Control 2015, 57, 169–176.
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the Formation of PAHs in Foods Prepared in the Home to Determine the Effects of Frying, Grilling, Barbecuing, Toasting and Roasting. Food and Chemical Toxicology 2015, 78, 1–9.
- Gomes, A.; Santos, C.; Almeida, J.; Elias, M.; Roseiro, L.C. Effect of Fat Content, Casing Type and Smoking Procedures on PAHs Contents of Portuguese Traditional Dry Fermented Sausages. Food and Chemical Toxicology 2013, 58, 369–374.
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic Aromatic Hydrocarbons (PAH) and Phenolic Substances in Smoked Frankfurter-Type Sausages Depending on Type of Casing and Fat Content. Food Control 2013, 31(1),136–144.