ABSTRACT
The effect of different extracting solvents (methanol, ethanol and butanol) and cooking treatments such as pressure cooking, microwave cooking and frying was studied in regards to phenolic profile and antioxidant activity of Sponge gourd (L. cylindrica). The antioxidant activity was investigated using different assays, namely, ferric thiocyanate test (FTC), thiobarbituric acid test (TBA), ferric reducing antioxidant power (FRAP) and DPPH free radicals scavenging test. A densitometric HPTLC analysis was performed for the analysis of phenolic acids and flavonoids. The inconsistent effect of cooking treatments on antioxidant potential was observed. In general, frying was most potent cooking treatments to retain the maximum antioxidant capacity. Correlation studies indicated that the phenolic compounds including flavonoids were mainly responsible for ferric reducing power, free radical scavenging activity and percent inhibition activity.
Introduction
Today there is unprecedented interest by consumers, public health organizations, and the medical community to improve health and wellness through dietary means. Clinical trials and epidemiological studies have established an inverse correlation between the intake of fruits and vegetables and the occurrence of diseases including inflammation, cardiovascular diseases, cancer, and aging-related disorders.[Citation1] The naturally occurring compounds such as phenolic acids, flavonoids, tannins, alkaloids, saponins, and terpenoids have attracted great attention from the scientific community for their antioxidant properties and their implication in a variety of biological mechanisms at the base of degenerative processes.[Citation2] The antioxidant activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers. Many mechanisms have been proposed to explain biological protective effects of polyphenols, which, for several years, have been often ascribed mainly to their antioxidant capacity. However, in the past few years, the dimensions of research on polyphenols have expanded beyond antioxidant activity unveiling several nutrition-pharma biological activities of these compounds in light of more complex molecular-level mechanisms.[Citation3] Studies have demonstrated that, besides antioxidant and anti-inflammatory capacities, phenolics may engage with cellular signalling flow, controlling the action of transcription factors and subsequently affecting the expression of those genes involved in cellular metabolism and cellular survival.[Citation2,Citation4]
Luffa cylindrica, commonly known as sponge gourd, has been found to be a unique gourd vegetable belonging to the family of Cucurbitaceae. It is a subtropical vegetable and widely cultivated in India, Brazil, and the USA.[Citation5,Citation6] It is considered as an excellent source of all the essential constituents required for good health of humans.[Citation7] There have been many studies on the phytochemicals and their antioxidant potential in L. cylindrica. The phytochemical constituents including alkaloids, flavonoids, saponins, and steroids possessing antioxidant activity have been reported in L. cylindrica.[Citation8] Lee et al.[Citation9] studied the total phenol content of L. cylindrica in various extracts, and it was reported to be in the range of 0.63–0.75 mg gallic acid. In another study, Azeez et al.[Citation10] reported the total phenol content in various extracts of pulp and peel of sponge gourd in the range of 0.94–14 mg GAE/g. Sponge gourd has been reported to contain 20.74 mg/g of total phenolics, 17.94 mg/g of flavonoids, 0.5 mg/g of total anthocyanins, and 1.2 mg/g of ascorbic acid.[Citation11] The considerable amount of flavonoids in L. cylindrica has been reported in previous studies.[Citation9,Citation11,Citation12] Extensive investigations have led to isolation of several bioactive compounds from L.cylindrica which includes p-coumaric acid, 1 -O-feruloyl-D-glucose, 1 -O-caffeoyl-D-glucose, 1 -O-(4-hydroxybenzoyl) glucose, diosmetin-7-O-D-glucuronide methyl ester, apigenin, and luteolin.[Citation13] The antioxidant activity such as percent inhibition, superoxide anion radical scavenging activities, and DPPH free radical scavenging of various extracts of L. cylindrica has also been reported in previous studies.[Citation9,Citation14]
In Asian countries including India, vegetables are consumed after different cooking treatments such as pressure cooking, microwave cooking, frying, etc. These treatments may affect the efficacy and concentration of the bioactive constituents present in plant foods. Previous studies conducted on various vegetables including gourd vegetables showed that total polyphenol content and antioxidant activity of the cooked vegetables could be higher or lower in comparison to fresh vegetables. Moreover, the extraction mechanism and type and polarity of extracting solvents have also been reported to exert significant effects on the extractability of phytochemicals and hence the in vitro antioxidative acitivity of plant extracts.[Citation15] Boiling has been reported to increase in the free radical scavenging activity of Luffa acutangula.[Citation16] Variable effects of different cooking treatments have been reported by Sengul et al.[Citation17] on various vegetables including beet root, turnip, cabbage, and broccoli. There have been no systematic studies on the effect of different cooking treatments on the phenolic profile and antioxidant profile of L. cylindrica; therefore, the present study was undertaken to investigate and quantify the effects of different cooking methods and extracting solvents on the total phenol content and antioxidant activity of L. cylindrica extracts.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), trichloroacetic acid (TCA), thiobarbituric acid (TBA), β carotene, tween 20, linoleic acid, 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), phenolic acids (p-coumaric, tannic, benzoic, gallic, ellagic, chlorogenic acids), flavonoid standards (quercetin and rutin) were purchased from Himedia (India). Folin–Ciocalteu reagent, HPLC-grade solvents such as methanol, chloroform, hexane, formic acid, acetonitrile and HPLC-grade water were purchased from Merck (Darmstadt, Germany). Phenolic acid standards (vanillic acid and caffeic acid) and flavonoid standards (kaempferol, myricetin, catechin, luteolin, and apigenin) were purchased from Sigma-Aldrich (USA). The ferrous chloride was purchased from Fisher scientific (Fair Lawn. New Jersey). The HPTLC Silica gel 60F254 plates were purchased from Merck (Darmstadt, Germany).
Cooking of vegetables
Cooking conditions were optimized by preliminary experiments carried out for each vegetable. For all cooking treatments, the minimum cooking time to reach a similar tenderness for an adequate palatability and taste, according to the Indian eating habits, was used. Small pieces (3 × 0.5 × 0.5 cm) of vegetable (500 g) were placed in pressure cooker (134 mm diameter, Hawkins) containing 100 ml water. The vegetables were cooked on low flame for 7 min after the pressure existed in the cooker. The cut and pinched pieces were placed in a glass dish and cooked at 110°C for 20 min in a microwave oven (IFB model: 25SC3). Vegetable pieces of approximately 0.25 cm thickness were placed in a frying pan (170°C) containing 80 ml of soya refined oil and stirred until the vegetable became crisp – tender (10 min.). The excess oil was drained off, and the vegetable sample was recovered.
Preparation of vegetable extracts
The macerates of vegetable samples were extracted with absolute methanol, ethanol, and n-butanol separately for 6 days at room temperature with intermediate shaking. The extract after filtration and centrifugation was concentrated using rotary evaporator at 45°C under reduced pressure (97.3 kPa) until the weight became constant and the extract was stored in dark at −20°C for further analysis.
Phytochemical screening and total phenol content
The extracts were screened for phytochemicals to identify the various constituents as described by standard methods as used by Sofowara[Citation18] and Harborne.[Citation19] The total phenolic content of the extracts was determined using Folin–Ciocalteu reagent.[Citation20] Gallic acid was used as the standard, and the results were expressed as gallic acid equivalent (mg of GAE/100 g dwb) using the regression equation of the standard curve for gallic acid (Y = 4.262x + 0.043; R2 = 0.997).
Determination of total flavonoids
The method as reported by Meda et al.[Citation21] was used for determination of flavonoids. Quercetin was used as the standard, and results were calculated from the regression equation of Y = 14.32x + 0.047; R2 = 0.990) and expressed in terms of quercetin equivalent (mg of QE/100 g dwb).
Determination of tannin content (Vanillin–HCl method)
Condensed tannins were determined by slight modification of the vanillin method.[Citation22] Diluted extract (1.00 ml) was mixed with 5 ml of vanillin reagent (equal volume of 1% vanillin in methanol and 8% concentrated HCl in methanol) in a test tube incubated at 30°C in a water bath for about 20 min. To correct the background colour, similar samples were prepared with the addition of 5 ml of 4% HCL in methanol. The regression equation Y = 0.5523x + 0.0273; R2= 0.998 was used to express the results as mg equivalents of catechin/100 g dwb.
Determination of β-carotene
The method as reported by Santra et al.[Citation23] along with some modifications was used for determination of β-carotene. Sample extract (0.1 g) was mixed with 10 ml of water saturated n-butanol and kept in the dark for 16–18 h for extraction of β-carotene. The contents were centrifuged at 1990g for 10 min, and the absorbance of collected supernatant was measured at 440 nm. The amount of β-carotene was calculated using regression equation Y = 0.1398x + 0.0463 (R2 = 0.9809).
Determination of antioxidant activity of extracts
Ferric thiocyanate (FTC) method
The FTC method was adapted as described by Kikuzaki and Nakatani.[Citation24] The reaction mixture [A mixture of 1 mg of vegetable extracts in 1 ml of 99.5% ethanol, 1.025 ml of 2.51% linoleic acid in 99.5% ethanol, 2 ml of 0.05 M phosphate buffer (pH 7.0), and 0.975 ml of distilled water] contained in a screw-cap vial was incubated under dark conditions at 40°C. To 0.05 ml of this reaction mixture was added 4.85 ml of 75% (v/v) aqueous ethanol, 0.1 ml of 30% aqueous ammonium thiocyanate, and 0.1 ml of 0.02 M ferrous chloride in 3.5% hydrochloric acid. Precisely 3 min after the addition of ferrous chloride into the reaction mixture, the absorbance of red colour was measured against the blank at 500 nm. The water instead of sample in the reaction mixture was used as control, and BHT and vitamin E were used as standard. The absorbance was measured at an interval of 24 h until one day after the absorbance of control reached its maximum value, i.e., on 5th day. The percent inhibition was calculated on the final day by the following formula.
Thiobarbituric acid (TBA) method
The samples prepared for FTC method were also used to measure the percent inhibition by TBA method as described by Kikuzaki and Nakatani.[Citation24] To 0.5 ml of the reaction mixture as prepared in FTC method, 1.0 ml of 20% aqueous trichloroacetic acid (TCA) and 1 ml of 0.67% aqueous thiobarbituric acid (TBA) solution were added. The mixture was placed in a boiling water bath for 20 min. After cooling, it was centrifuged at 885×g for 25 min. The absorbance of the supernatant was measured at 532 nm. Antioxidant activity was recorded on the final day of the FTC assay, and the % inhibition was measured by the formula as used in the FTC method.
Ferric reducing antioxidant power (FRAP) method
FRAP assay was adapted as described by Moyer et al.[Citation25] Based on the measured absorbance, the concentration of FeSO4 was measured (mM FeSO4/100 g dw basis) from the regression equation of the standard curve of FeSO4 (Y = 0.5783x 0.0042; R2 = 0.9991).
Evaluation of the free radical scavenging activity by DPPH assay
DPPH free radical scavenging activity assay used by Chan et al.[Citation26] was adapted with slight modification. Ascorbic acid was used as the standard. The mixture was shaken vigorously and allowed to remain in dark for 30 min. The absorbance of the resulting solution was measured at 517 nm. The scavenging activity of each extract on DPPH radical was calculated using the following equation, and the results were expressed as IC50 (The effective concentration of the extract required for 50% scavenging of DPPH).
HPTLC analysis of phenolic acids and flavonoid compounds
A densitometric HPTLC analysis was performed for the analysis of phenolic acids and flavonoids. The standard stock solution (0.1 mg/ml) and sample (100 mg/ml) was prepared in HPTLC-grade methanol. The sample (8 μL) was applied with a 100 μL sample syringe using automatic Linomat-5 system (CAMAG, Switzerland). The stock solutions of the reference compounds were prepared in methanol at different concentration levels (2–8 μL). The plates were developed in a vertical glass chamber (CAMAG) until the respective mobile phase, i.e., solvent system I (6.4 chloroform: 3.9 hexane: 2.0 methanol: 0.5 formic acid) for detection of gallic acid, caffeic acid, quercetin, apigenin, kaempferol, and chlorogenic acid; solvent system II (4 chloroform: 1 hexane: 1 methanol: 1 formic acid) for detection of p-coumaric acid, luteolin, myricetin, catechin and ellagic acid and solvent system III (4.5 acetonitrile: 1.0 methanol: 0.5 water) for detection of ferulic acid, benzoic acid, cinnamic acid, and vanillic acid) respectively rose to 80% of the plate height. The densitometric evaluation was performed with a TLC scanner 3 (CAMAG) at wavelength 254 nm. The plate image was documented by TLC visualizer documentation system (CAMAG). The quantification and documentation were done by winCAT software. The concentration of each compound was determined by using calibration curve prepared by plotting the peak area versus concentration of standard compound.
Statistical analysis
Three independent determinations were made for each parameter (n = 3), and the results were reported as mean ± standard deviation (SD). Analysis of variance was performed by two way ANOVA analysis (SPSS 19.0) followed by Tukey’s HSD post hoc comparison test at p < 0.05. Pearson correlation coefficients among various antioxidant assays and phytochemicals were performed using SPSS 19.0 software.
Results and discussion
Phytochemical screening
The phytochemicals screening of various extracts of L. cylindrica confirmed the presence of considerable amounts of flavonoids, tannins, saponins, terpenoids, and alkaloids in most of the extracts of the raw as well as cooked forms of the vegetable (). The screening results were further confirmed by quantitative testing of phenols, flavonoids, tannins, and carotenoids. Tannins were absent in ME and BE of raw sample, but their presence was revealed in the extracts of cooked samples. The biological mechanism of tannins as antioxidants has been reported through iron sequestration, hydrogen bounding, or specific interactions with vital proteins such as enzymes.[Citation27] Saponins were however tested positive only in the ME and EE of L. cylindrica. Previous studies showed the presence of considerable amount of saponins and terpenoids in this vegetable.[Citation28,Citation29] Irshad et al.[Citation8] also screened the moderate concentrations of alkaloids, flavonoids, tannins, and saponins in L. cylindrica. Saponins constitute a key ingredient in traditional Chinese medicine and are responsible for many of the attributed biological effects, including an inhibitory effect on inflammation.[Citation30] The results of the present study on phytochemicals screening are also indicative of the presence of flavonoids as the most prominent phytochemicals in L. cylindrica. Flavonoids are considered as most popular natural antioxidants and effective secondary metabolic products as they help to provide protection against oxidation at cellular level by interfering in enzyme activity, chelation of redox active metals, and by scavenging free radicals.[Citation31]
Table 1. Phytochemical screening of the extracts of Luffa cylindrica.
Phenolic profile of the cooked vegetables
summarises phenolic profile of raw and cooked vegetable samples of L. cylindrica. The average value of TPC irrespective of extracting solvents showed a significant enhancement (p < 0.05) by 14.9% in pressure cooked and 64.4% in fried sample, whereas no change was observed in microwaved sample when compared to the raw counterpart. Previous studies have also reported the increase in phenolic content during pressure cooking and frying in broccoli[Citation32] and green paprika,[Citation33] respectively. This retention of TPC could be due to the release of bound phenolic acids resulting from the breakdown of cellular constituents and cell walls during processing, disassociation of conjugate phenolic forms, and formation of by-products.[Citation34] Individual phenolics may sometimes increase because heat can break supra molecular structures, releasing the bound phenolic, which reacts better with the Folin-Ciocalteau reagent.[Citation35] However, the loss in phenolic content during microwave cooking may be due to the higher cooking time as compared to other cooking methods. The reduction in TPC could also be ascribed to the breakdown of some heat labile phenolic compounds[Citation36] and temperature induced chemical oxidation that could destroy the antioxidant compounds in vegetables.[Citation37] Previous studies have also reported decrease in TPC after microwave cooking of different vegetables.[Citation38] The significant impact of extraction solvents on the total phenols extraction was observed (216.0 at df = 2, p = 0.000). TPC of various extracts regardless of cooking treatments was in order EE > BE > ME. The peak activity of EE was due to its highest polarity and lowest viscosity as compared to the methanol and butanol.[Citation39] A significant interaction effect (F = 585.1 at df = 6, p = 0.000) of cooking treatments and extraction solvents showed an inconsistent effect of cooking treatments on the TPC of various extracts. Flavonoids are considered as most popular natural antioxidants and effective secondary metabolic products.[Citation31] The flavonoids are the main bioactive compounds found in fruits and vegetable, and in particular, vegetables flavonoids have been reported to be dominated by glycosidic flavonols.[Citation40] The average flavonoids content of the raw sample irrespective of extracting solvents was 72.1 mg QE/100 g (dwb), whereas in cooked samples, this value significantly varied from 43.4 to 262.7 (F = 93918.3 at df = 3, p = 0.000) suggesting the inconsistent effect of cooking methods. Wide variations in flavonoids content (150–630 mg/100 g) in different extracts of L.cylindrica have been previously reported.[Citation9,Citation12] The effect of solvents in extracting out the flavonoids was also significant (F = 20822.3 at df = 2, p = 0.000), and the flavonoids content of various extracts was in the order of BE > EE > ME. Fairly, a similar finding was observed for Punica granatum fruit in which butanol influenced the higher extraction of flavonoids as compared to the ethanol and aqueous extracts.[Citation41] The significant interaction effect of cooking treatments and extraction solvents showed that the total flavonoids content has declined markedly (p < 0.05) after microwave cooking in all the extracts as compared to their raw counterparts and the maximum drop was observed in EE (46.4%) followed by BE (41.0%) and ME (21.5%). However, in case of fried sample, all the extracts were observed to be proficient in extracting the flavonoids as compared to their respective raw counterparts. It has been reported that high heat treatment increased the level of free flavonols.[Citation42]
Table 2. Total phenols, flavonoids, tannins, and carotenoid contents of various extracts of differently cooked L. cylindrica.
The average tannin content of the cooked samples irrespective of the extraction solvents varied from 12.1 to 14.8 mg CE/100 g when compared to the tannin content of raw sample (24.1 mg CE/100 g) suggesting the declining effect of heat processing treatments on tannin content. The results of present investigation are in agreement with observations of Zhang and Hamauzu[Citation43] and Racchi et al.[Citation44] who reported the loss of tannic acid after different cooking processes. During the thermal treatment, most important reactions are hydrolysis, oxidation, polymerisation, and interaction of composition and reactions of thermal decomposition[Citation45] due to which reduction in tannins content is possible. Regardless of cooking treatments, the average value of tannin content of different extracts ranged from 15.2 to 17.4 mg CE/100 g. The significant interaction effect (F = 10.2 at df = 6, p = 0.000) of the cooking treatments and the extraction solvents showed that the tannin content varied among different extracts in relation with the cooking treatments. All types of extracts of differently cooked vegetable samples exhibited decreased tannin content over their respective raw counterparts. The extent of the decrease was maximum in ME of microwaved sample (79.6%) followed by ME of fried (67.3%) and EE of pressure cooked samples (61.2%).
Total carotenoids content
The average value of carotenoids content of cooked vegetable samples regardless of the extraction solvent ranged from 15.0 to 18.2 mg β-carotene/100 g (dwb) when compared to carotenoids content of 9.2 mg β-carotene/100 g (dwb) in the raw vegetable sample (). Pressure cooking was the most effective cooking treatment in retaining the carotenoids (97.8% increase) followed by frying (85.9% increase) and microwave cooking (63.0% increase). Previous studies have also observed the increase in carotenoids content after various cooking treatments because of their better extractability and enhanced bioavailability from heat treatment.[Citation46] Butanol was found to be most efficient solvent for extracting carotenoids (21.5 mg β-carotene/100 g, dwb), probably because of the fact that carotenoids are fat soluble compounds being more soluble in a less polar solvent like butanol. Nonetheless, in terms of interactive effects of cooking treatments and the extraction solvents, there was a significant interaction effect (F = 2206.3 at df = 6, p = 0.000). Different extracts of the pressure cooked sample revealed an increased recovery of carotenoids in comparison to their raw counterparts, and this recovery was maximum in BE (142.6% increased) followed by ME (139.1% increased) and EE (24% increased). However, in microwave cooking, most abrupt retention in the carotenoid content was reported in BE followed by EE and ME as compared to their raw vegetable sample.
Total antioxidant activity
As large number of phytochemicals are embedded in the plant matrix. Therefore, a number of different methods might be necessary to adequately assess the in vitro antioxidant potential.
Percent inhibition as measured by FTC method
Antioxidant activity of ME, EE, and BE of raw and cooked vegetable samples of L. cylindrica in terms of measurement of inhibition of peroxidation is shown in . The extracts of different samples of L. cylindrica inhibited 6.1–35.4% peroxidation of linoleic acid after an incubation period of 96 h as measured by FTC assay. However, the antioxidant activity of all the extracts in terms of measurement of percent inhibition was significantly (p < 0.05) lower than synthetic antioxidant BHT (88.8%) and vitamin E (74.6%). Irrespective of cooking treatments, significant differences (F = 777.1 at df = 2, p = 0.000) were observed in percent inhibition, and it was found maximum in ME (25.6%) followed by EE (20.2%) and BE (14.3%). Regardless of extraction solvents, the percent inhibition was observed to be inconsistent after various cooking treatments, and it was found to be superior in pressure cooked and fried samples in comparison to raw sample. The interactive effect showed that percent inhibition in pressure cooked sample was significantly higher (p < 0.05) in ME and EE by 169.4% and 3.9%, respectively, while it was lower by 66.1% in BE as compared to raw vegetable extracts. The extracts of microwaved samples showed decreased peroxidation inhibition as compared to their raw counterparts. However, in case of fried sample, all the extracts revealed an increased percent inhibition in comparison to their raw counterparts.
Table 3. Antioxidant activity of various extracts of differently cooked vegetable of L. cylindrica as determined by FTC, TBA, and FRAP assays.
Percent inhibition as measured by TBA method
Regardless of cooking treatments, the percent inhibition by various extracts followed the same order as measured by TBA assay (). The main effect of the cooking methods was significant (F = 2844.5 at df = 3, p = 0.000), but the contradictory results were observed after different cooking treatments. The interactive effects of cooking treatments and extracting solvents showed that the EE of raw sample was most effective in inhibiting the oxidation. However, in cooked samples, the maximum increased level of inhibition was revealed by ME of pressure cooked sample followed by ME of fried and EE of fried sample. In general, frying was most effective in increasing the percent inhibition by various extracts of L. cylindrica. The percent inhibition by the various extracts of fried sample varied from 38.6% to 44.6% in comparison to 25.8% to 29.0% of raw sample.
FRAP assay
Significant differences were observed in the FRAP values among all the cooking treatments in their respective extracts (p < 0.05). The average FRAP values as shown by various extracts regardless of cooking treatments varied from 905.5 to 1313.0 μM FeSO4/100 g being highest in EE followed by BE and ME (F = 626.1 at df = 2, p = 0.000). The lower, reducing power of ME might be due to the lower polyphenolic concentration in ME, which had been found to contribute to antioxidant activity by various reaction mechanisms. Regardless of extraction solvents, the significant effect of cooking on ferric reducing power was observed when compared to raw vegetable sample, and it was found in the order of fried > pressure cooked > microwave cooked (F = 2950 at df = 3, p = 0.000). Comparatively, the increased FRAP level in L. cylindrica after frying might be due to the newly formed flavonoids such as quercetin and catechin. Further, flavonoids have more hydroxyl groups and hence high antioxidant activity if compared to phenolic acids.[Citation39] Increased reducing power after assorted cooking treatments has been reported in earlier studies also.[Citation32,Citation47] The interaction effect of cooking treatments and the extraction solvents was also significant (F = 346.5 at df = 6, p = 0.000). The effect of pressure cooking was observed to be inconsistent in regard to their reducing power as ME was more effective to retain the antioxidant activity than the EE and BE as compared to their respective raw vegetable. As compared to the raw samples, all the extracts of fried samples were more effective with respect to their reducing power, and the increased FRAP value was in the order of BE > ME > EE.
Free radical scavenging activity
The percent scavenging activity of 30 μg/ml ascorbic acid (92.0%) was observed to be highest in respect to various raw and cooked samples of L. cylindrica (). The percent scavenging activity of the raw samples irrespective of extracting solvents was 12.1%, which varied from 5.7 to 20.5% in cooked samples, suggesting that the effect of different heat processing treatments on the percent scavenging was highly significant (F = 1693.3 at df = 3, p = 0.000) but inconsistent. In previous studies, the free radical scavenging activity of L. cylindrica was observed in the range of 6.3–55.2% in various concentrations of extracts.[Citation12,Citation13] The average increase in scavenging activity after pressure cooking and microwave cooking may be attributed largely due to the formation of catechin (as determined by HPTLC). The catechin has good hydrogen donating ability because of attachment of many hydroxyl groups. Apart from this, the increase in scavenging activity might be due to thermal inactivation of oxidative enzymes and release of potent radical-scavenging antioxidants from thermally destructed cells.[Citation33] The earlier study showed that antioxidant capacity of different vegetables increased during cooking procedure compared to the fresh vegetables.[Citation48,Citation49] Boiling has been reported to increase in the free radical scavenging activity of Luffa acutangula.[Citation16] In contrast, antioxidant capacity was also reported to decrease after thermal treatment of various vegetables.[Citation39] Regardless of cooking treatments, the percent scavenging at 30 μg/ml of various extracts was in the order of BE > EE > ME. The interaction effect of heat processing treatments and the extraction solvents was also significant (F = 751.8 at df = 6, p = 0.000), and the results showed that percent scavenging activity of pressure cooked and microwave cooked samples was significantly higher (p < 0.05) in their EE and BE in comparison to the respective extracts of raw vegetable. However, the fried sample exhibited decreased scavenging activity in all types of extracts over their raw counterparts. summarises the results of IC50 values of raw and cooked vegetable samples of L. cylindrica. Regardless of the extraction solvent, free radical scavenging activity as assessed by IC50 values decreased by 14.3% and 52.9% after pressure cooking and microwave cooking, respectively, whereas it increased by 111.9% after frying. Regardless of cooking treatments, the average IC50 value was observed to be highest in ME (212.6 μg/ml) followed by BE (200.5 μg/ml) and EE (164.4 μg/ml). Nevertheless, the IC50 values of all extracts were significantly (p < 0.05) higher than ascorbic acid (5.05 μg/ml).
Table 4. Free radical scavenging activity of various extracts of cooked vegetable of L. Cylindrica as measured by DPPH assay.
Identification of phenolic acids and flavonoids by HPTLC
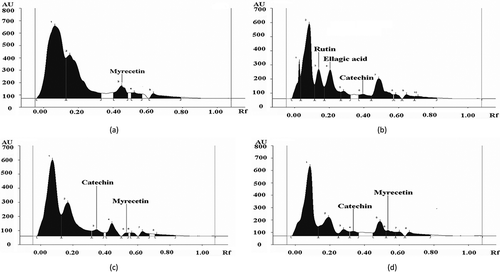
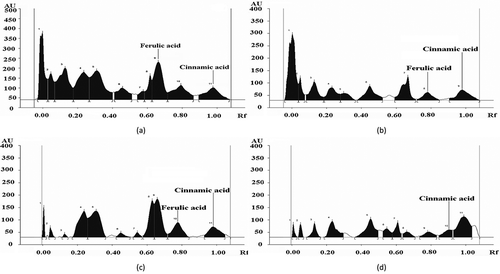
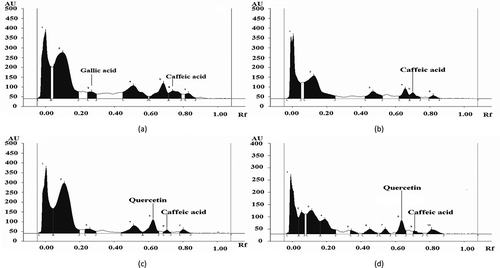
There is a wide degree of variation between different phenolic compounds in their effectiveness as an antioxidant.[Citation50] Considering the fact that methanol is considered as the universal extraction solvent, the ME of different samples was identified and quantified for their phenolic acids and flavonoid compounds. The distribution of phenolic acids and flavonoids in ME of L. cylindrica as studied by HPTLC is presented in and –. Gallic acid (26.8 μg/ml) as a phenolic acid was identified only in the raw sample extract. Caffeic acid (18.4 μg/ml) and cinnamic acid (8.6 μg/ml) were reported in raw vegetable sample of L. cylindrica and its concentration decreased in all the cooked sample extracts. This decrease in concentration may be due to the breakdown of phenolic acid after the heat treatments. The ferulic acid (49.6 μg/ml) was detected in raw sample extract, and its retention was observed after pressure cooking and microwave cooking. Ellagic acid (78.8 μg/ml) and rutin (79.3 μg/ml) were observed as most prominently present phenolic acid in pressure cooked samples. Rutin has been studied for beneficial human health effects, such as antioxidant effect and anti-inflammatory effect.[Citation51] Moreover, the high antioxidant activity as measured by FTC and TBA assay in ME of pressure cooked sample may be due to the presence of high concentration of rutin, a flavonoid, which is more reactive because of the higher number of -OH group attached, as the free radical scavenging ability increases with the increase of number of phenolic -OH groups.[Citation52] Interestingly, the catechin was newly formed compounds observed after pressure cooking treatments although; this was not identified in raw L. cylindrica. Catechin is a kind of flavan-3-ols. The formation of catechin in pressure cooking may be attributed to release of basal structural compound (catechin) from condensed tannins through a depolarisation process upon thermal and pressure conditions. Moreover, the formation of new phenolic compounds after heat treatments was due to the availability of precursors formed by non-enzymatic inters conversion between phenolic molecules.[Citation53] The results presented here showed that heat processing treatments could make the phenolic acids and flavonoids different from that of uncooked form. The most destructive effect of heat processing was on gallic acid and caffeic acid, which were prominently detected in raw sample, but after various cooking treatments, these acids were highly reduced or not detected at all. This degradation of phenolic compounds may also happen due to the epimerisation, dimerisation, hydrolysis, oxidative and polymerisation reaction.[Citation54] Myricetin (35.8 μg/ml) was detected in raw sample extracts, and its retention was observed after microwave cooking (20.9 μg/ml) and frying (30.9 μg/ml). Quercetin was not identified in the raw sample extract, but it was detected in microwave cooked (55.4 μg/ml) and fried (45.2 μg/ml) samples. Interestingly, catechin was newly formed compounds in the cooked samples as it was not identified in raw sample of L. cylindrica. The previous study as demonstrated by DU et al.[Citation13] had also shown the presence of various polyphenolic compounds such as p-coumaric acid, apigenin, and luteolin in L. cylindrica.
Table 5. Identification and quantification of phenolic acids and flavonoids (μg/ml of extract) in ME as determined by HPTLC.
Figure 1. HPTLC profile of ME of L. cylindrica as developed in chloroform: hexane: methanol: formic acid (6.4: 3.9: 2.0: 0.5) (a) raw, (b) pressure cooked, (c) microwave cooked, and (d) fried.
Correlation studies
The results of correlation studies showed moderate positive correlations of FTC assay with total phenols (r = 0.711, p < 0.01) () suggested that phenol may be the main phytochemical responsible for the oxidation of linolic acid. Similarly, the positive correlation was observed between percent inhibition (as measured by TBA assay) and phenol content (r = 0.737, p < 0.01) indicating that the high concentration of phenol inhibits malonaldehyde formation and was responsible for the antioxidant activity of L. cylindrica. These findings are in agreement with the previous studies which have reported the positive correlation between phenol content and total antioxidant activity.[Citation55] The strong correlation (r = 0.989, p < 0.01) between FTC and TBA assay showed that the increase in peroxide level caused the formation of malonaldehyde compounds.[Citation56] Interestingly, although the TPC and flavonoids content of the ME of the vegetable samples was the highest, whereas the antioxidant activity as measured by FTC and TBA assay was lowest. This observation in the present study suggested a wide variation in the nature and kind of flavonoids compounds recovered in the different solvents and hence differences in the antioxidative potency of the extracts. The effectiveness of phenolics and flavonoids as antioxidants is not only because of their composition or relative amount but also influenced by the degree of polymerisation, concentration, and interaction of their diverse chemical structures to the colorimetric assays.[Citation57] A highly significant and positive correlation of the FRAP value with phenols content (r = 0.909, p < 0.01) and moderate positive but significant correlation with flavonoid content (r = 0.702, p < 0.01) concluded that phenols and flavonoids may be the main compounds responsible for the reduction the ferrous ions into ferric ions in their respective extracts. Previous studies have also reported positive correlation of antioxidant activity (as measured by FRAP assay) with phenols[Citation58] and flavonoids.[Citation59] Moreover, a significant and positive correlation of the DPPH value with phenol content (r = 0.880, p < 0.01) and flavonoids content (r = 0.756, p < 0.01) indicated that the change in free radical scavenging activity of heat processed sample may be due to the change in phenolic acids and flavonoids content after the cooking treatments. The number and position of hydroxyl groups in the phenolic compounds may directly contribute to the antioxidant activity and have a critical role in scavenging free radicals.[Citation52] Sun and Ho[Citation60] also reported a significant correlation between total phenols and scavenging effect of DPPH.
Table 6. Pearson correlation coefficients (r) between phytochemicals and antioxidant activity.
Conclusion
From the results of the present study, it could be concluded that cooking methods, as well as extraction solvents, had significant effects on the recovery of polyphenolic compounds available in L. cylindrica. ME was the most effective in respect to the antioxidant activity as measured by FTC and TBA assays. From the results, it could be inferred that cooking had both positive and negative impact on the concentration of phytochemicals and their antioxidant activity. In general, frying emerged as a most effective cooking treatment in retention of phenolics as well as antioxidant activity. However, the microwave cooking showed the most deleterious effect in respect to the polyphenols and antioxidant activity. The correlation study concluded that the phenolic acid and flavonoids are mainly responsible compounds for their antioxidant activity. From the observations of quantitative testing and HPTLC profiles of the extracts, it can also be concluded that there could be even wider range of phytochemicals present in L. cylindrica. Therefore, evaluating the biological activity of isolated bioactive compounds of L. cylindrica singularly and in combination with others in vitro and in vivo models will then further offer new insight into the exact mechanisms of action of L. cylindrica on health benefits. This research could be of practical importance as it may help to provide information to the database related to the polyphenolic compounds of differently cooked sponge gourd, which could help in the establishment of RDA value of polyphenolic compounds for the consumers of sponge gourd.
Acknowledgements
We are grateful for the support from the Professor Munish Garg from Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak (Haryana) India.
Funding
This study was financially supported by University Grants Commission, New Delhi.
Additional information
Funding
References
- Wang, L.; Manson, J.E.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. Fruit and Vegetable Intake and the Risk of Hypertension in Middle-Aged and Older Women. American Journal of Hypertension 2012, 25(2), 180–189.
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. Journal of Agricultural and Food Chemistry 2014, 62(18), 3867–3876.
- Visioli, F.; Lastra, C.A.D.L.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; Edeas, M. Polyphenols and Human Health: A Prospectus. Critical Reviews in Food Science and Nutrition 2011, 51(6), 524–546.
- Forbes-Hernandez, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The Effects of Bioactive Compounds from Plant Foods on Mitochondrial Function: A Focus on Apoptotic Mechanisms. Food and Chemical Toxicology 2014, 68, 154–182.
- Oboh, I.O.; Aluyor, E.O. Luffa Cylindrica an Emerging Cash Crop. African Journal of Agricultural Research 2009, 4(8), 684–688.
- Laidani, Y.; Hanini, S.; Henini, G. Use of Fiber Luffa cylindrica for Waters Treatment Charged in Copper. Study of the Possibility of its Regeneration by Desorption Chemical. Impact of Integrated Lean Energy on the Future of the Mediterranean Environment. Energy Procedia 2011, 6, 381–388.
- Prajapati, R.P.; Kalaria, M.V.; Karkare, V.P.; Parmar, S.K.; Sheth, N.R. Effect of Methanolic Extract of Lagenaria siceraria (Molina) Standley Fruits on Marble-burying Behavior in Mice: Implications for Obsessive–Compulsive Disorder. Pharmacognosy Research 2011, 3(1), 62–66.
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant Capacity and Phenolic Content of the Aqueous Extract of Commonly Consumed Cucurbits. International Journal of Food Proprties 2010, 17(1), 179–186.
- Lee, G.O.; You, Y.H.; Hwang, K.T.; Lee, J.M.; Lee, H.J.; Jun, W.J. Physicochemical Characteristics and Antioxidant Activities of Luffa cylindrica (L.) Roem. Journal of the Korean Society of Food Science and Nutrition 2012, 41(6), 733–738.
- Azeez, M.A.; Bello, O.S.; Adedeji, A.O. Traditional and Medicinal Uses of Luffa cylindrica: a Review. Journal of Medicinal Plants 2013, 1(5), 102–111.
- Reddy, B.P.; Reddy, A.R.; Reddy, B.S.; Mohan, S.V.; Sarma, P.N. Apoptosis Inducing Activity of Luffa acutangula in Leukemia Cells (HL60). International Journal of Pharmaceutical Research and Development 2010, 2(10), 109–122.
- Sharma, D.; Rawat, I.; Goel, H. Antioxidant and Prebiotic Potential of Some Cucurbits. Research Journal of Medicinal Plant 2012, 6(7), 500–510.
- Du, Q.; Xu, Y.; Li, L.; Zhao, Y.; Jerz, G.; Winterhalter, P. Antioxidant Constituents in the Fruits of Luffa cylindrica (L.) Roem. Journal of Agricultural and Food Chemistry 2006, 54(12), 4186–4190.
- Yadav, B.S.; Yadav, R.; Yadav, R.B.; Garg, M. Antioxidant Activity of Various Extracts of Selected Gourd Vegetables. Journal of Food Science and Technology 2016. 53(4), 1823–1833.
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. Journal of Pharmaceutical and Biomedical Analysis 2006, 41(5), 1523–1542.
- Ansari, N.M.; Houlihan, L.; Hussain, B.; Pieroni, A. Antioxidant Activity of Five Vegetables Traditionally Consumed by South-Asian Migrants in Bradford, Yorkshire, UK. Phytotherapy Research 2005, 19(10), 907–911.
- Sengul, M.; Yildiz, H.; Kavaz, A. The Effect of Cooking on Total Polyphenolic Content and Antioxidant Activity of Selected Vegetables. International Journal of Food Properties 2014, 17(3), 481–490.
- Sofowora, A. Research on Medicinal Plants and Traditional Medicine in Africa. The Journal of Alternative and Complementary Medicine 1996, 2(3), 365–372.
- Harborne, J.B. Phytochemical Methods; 2nd ed.; Chapman and Hall Publications: London, 1984.
- Zhou, K.; Yu, L. Total Phenolic Contents and Antioxidant Properties of Commonly Consumed Vegetables Grown in Colorado. LWT – Food Science and Technology 2006, 39(10), 1155–1162.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as their Radical Scavenging Activity. Food Chemistry 2005, 91(3), 571–577.
- Burns, R.E. Method for Estimation of Tannin in Grain Sorghum. Agronomy Journal 1971, 108(2), 649–656.
- Santra, M.; Santra, D.; Rao, V.; Taware, S.; Tamhankar, S. Inheritance of I2-Carotene Concentration in Durum Wheat (Triticum turgidum L. ssp. durum). Euphytica 2005, 144(1–2), 215–221.
- Kikuzaki, H.; Nakatani, N. Antioxidant Effects of Some Ginger Constituents. Journal of Food Science 1993, 58(6), 1407–1410.
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, and Ribes. Journal of Agricultural and Food Chemistry 2002, 50(3). 519–525.
- Chan, E.; Lim, Y.; Omar, M. Antioxidant and Antibacterial Activity of Leaves of Etlingera Species (Zingiberaceae) in Peninsular Malaysia. Food Chemistry 2007, 104(4), 1586–1593.
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30(12), 3875–3883.
- Liang, L.; Lu, L.; Cai, Y.; Liu, C. Studies on the Chemical Components from Leaves of Luffa cylindrica. Journal of Chinese Medicinal Materials 1994, 18(8), 401–402.
- Shuling, X.; Zhapu, F.; Xianyi, Z. Studies on the Chemical Constituents of Luffa cylindrica (L.) Roem. Journal of Chinese Medicinal Materials 1994, 4, 18–19.
- Liu, J.; Henkel, T. Traditional Chinese Medicine (TCM): Are Polyphenols and Saponins the Key Ingredients Triggering Biological Activities? Current Medicinal Chemistry 2002, 9(15), 1483–1485.
- Gyamfi, M.A.; Aniya, Y. Antooxidant Properties of Thonningianin A. Isolated from the African Medicinal Herb, Thonningia Sanguinea. Biochemical Pharmacology 2002, 63(9), 1725–1737.
- Ng, Z.X.; Chai, J.W.; Kuppusamy, U.R. Customized Cooking Method Improves Total Antioxidant Activity in Selected Vegetables. International Journal of Food Sciences and Nutrition 2011, 62(2), 158–163.
- Chuah, A.M.; Lee, Y.C.; Yamaguchi, T.; Takamura, H.; Yin, L.J.; Matoba, T. Effect of Cooking on the Antioxidant Properties of Coloured Peppers. Food Chemistry 2008, 111(1), 20–28.
- Haard, N.F.; Chism, G.W. Characteristics of Edible Plant Tissues. In Food Chemistry; Fennema, O.R.; Ed.; Marcel Dekker Inc.: New York, 1996; 943–1011.
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhe, R.; Van Camp J. Total and Individual Carotenoids and Phenolic Acids Content in Fresh, Refrigerated and Processed Spinach (Spinacia oleracea L.). Food Chemistry 2008, 108(2), 649–656.
- Hunter, K.J.; Fletcher, J.M. The Antioxidant Activity and Composition of Fresh, Frozen, Jarred and Canned Vegetables. Innovative Food Science & Emerging Technology 2002, 3(4), 399–406.
- Rawson, A.; Hossain, M.B.; Patras, A.; Tuohy, M.; Brunton, N. Effect of Boiling and Roasting on the Polyacetylene and Polyphenol Content of Fennel (Foeniculum vulgare) Bulp. Food Research International 2013, 50(2), 513–518.
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of Cooking Methods on Polyphenols, Pigments and Antioxidant Activity in Potato Tubers. LWT – Food Science and Technology 2012, 45(2), 161–171.
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total Antioxidant Activity and Phenolic Content in Selected Vegetables. Food Chemistry 2004, 87(4), 581–586.
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. International Journal of Molcular Science 2007, 8(9), 950–988.
- Amjad, L.; Shafighi, M. Evaluation of Antioxidant Activity, Phenolic and Flavonoid Content in Punica granatum var. Isfahan Malas Flowers. International Journal of Agriculture and Crop Sciences 2013, 5(10),1133.
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Michae, E.J.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato Based Products. Journal of Agricultural and Food Chemistry 2000, 48(7), 2663–2669.
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and their Changes during Conventional and Microwave Cooking. Food Chemistry 2004, 88(4), 503–509.
- Racchi, M.; Daglia, M.; Lanni, C.; Papetti, A.; Govoni, S.; Gazzani, G. Antiradical Activity of Water Soluble Components in Common Diet Vegetables. Journal of Agricultural and Food Chemistry 2002, 50(5), 1272–1277.
- Rakic, S.; Maletic, R.O.; Perunovic, M.N.; Svrzic, G. Influence of Thermal Treatment on Tannin Content and Antioxidation Effect of Oak Acorn Quercus Cerris Extract. Journal of Agricultural Sciences, Belgrade 2004, 49(1), 97–107.
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of Two Cooking Procedures on Phytochemical Compounds, Total Antioxidant Capacity and Colour of Selected Frozen Vegetables. Food Chemistry 2011, 128(3), 627–633.
- Lin, Ch.-H.; Chang, Ch.-Y. Textural Change and Antioxidant Properties of Broccoli under Different Cooking Treatments. Food Chemistry 2005, 90(1), 9–15.
- Faller, A.L.K.; Fialho, E. The Antioxidant Capacity and Polyphenol Content of Organic and Conventional Retail Vegetables after Domestic Cooking. Food Research International 2009, 42(1), 210–215.
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chemistry 2005, 93(4), 713–718.
- Horax, R. Hettiarachchy, N. Islam, S. Total Phenolic Contents and Phenolic Acid Constituents in 4 Varieties of Bitter Melons (Momordica charantia) and Antioxidant Activities of their Extracts. Journal of Food Science 2005, 70(4), C275–C280.
- Koda, T.; Kuroda, Y.; Imai, H. Protective Effect of Rutin Against Spatial Memory Impairment Induced by Trimethyltin in Rats. Nutrition Research 2008, 28(9), 629–634.
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of Antioxidant Activity and Antioxidant Compounds in Wild Edible Mushrooms. Journal of Food Composition and Analysis 2007, 20(3), 337–345.
- Vega-Galvez, A.; Uribe, E.; Perez, M.; Tabilo-Munizaga, G.; Vergara, J.; Garcia-Segovia, P.; Lara, E.; Scala, K.D. Effect of High Hydrostatic Pressure Pretreatment Ondrying Kinetics, Antioxidant Activity, Firmness and Microstructure of Aloe Vera (Aloe Barbadensis miller) Gel. LWT – Food Science and Technology 2011, 44(2), 384–391.
- Okumura, H.; Ichitani, M.; Takihara, T.; Kunimoto, K.K. Effect of Cyclodextrins on the Thermal Epimerization of Tea Catechins. Food Science and Technology Research 2008, 14(1), 83–88.
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica charantia L.) Leaf, Stem and Fruit Fraction Extracts in Vitro. Food Chemistry 2008, 110(4), 881–890.
- Zin, Z.M.; Abdul-Hamid, A.; Osman, A. Antioxidative Activity of Extracts from Mengkudu (Morinda citrifolia L.) Root, Fruit and Leaf. Food Chemistry 2002, 78(2), 227–231.
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural Antioxidants From Residual Sources. Food Chemistry 2001, 72(2), 145–171.
- Sultana, B.; Anwar, F.; Iqbal, S. Effect of Different Cooking Methods on the Antioxidant Activity of Some Vegetables from Pakistan. International Journal of Food Science and Technology 2008, 43(3), 560–567.
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical Scavenging, Antioxidant and Metal Chelating Activities of Annona Cherimola Mill. (cherimoya) Peel and Pulp in Relation to Their Total Phenolic and Total Flavonoid Contents. Journal of Food Composition and Analysis 2012, 25(2), 179–184.
- Sun, T.; Ho, C.T. Antioxidant Activities of Buckwheat Extracts. Food Chemistry 2005, 90(4), 743–749.