ABSTRACT
The texture properties (hardness, adhesiveness, cohesion, elasticity) of composite gels with different ratios (0:100, 6:94, 8:92, 10:90, 12:88, and 14:86, respectively) of soy 7S globulin to corn starch (amylose, amylopectin) were studied. Furthermore, the morphology and crystal structure of the mixed gels were investigated. The results showed: the addition of soy 7S globulin could weaken the hardness of corn starch (amylose, amylopectin) and increased the adhesiveness of corn amylose significantly (p < 0.05). Generally, when the additive amount of soy 7S globulin was 10%, the composite gels of soy 7S globulin and corn starch (amylose, amylopectin) appeared to have the best consistency, cohesion, and elasticity.
Introduction
Soybean, also called “clycine max” is the transliteration of its ancient Chinese name shū (meaning “bean”), which originates in China.[Citation1] Soybeans contain about 40% protein, one of the main usable components in processing.[Citation2,Citation3] Soy protein components can be divided into four categories based on the sedimentation coefficient: 2S, 7S, 11S, and 15S4. Soy 7S globulin accounts for about 37% of the total soy protein content.[Citation4]
Starch is a polymer carbohydrate comprising many glucose molecules and is formed after dehydration–polymerization.[Citation5,Citation6] Starch can be divided into amylose and amylopectin; amylose has strong retrogradation properties, and amylopectin has quite higher molecular weight among natural polymeric compounds. Significant differences in some properties exist between them. Amylopectin is water soluble and produces the stable solution with very high viscosity. The viscosity of starch paste primarily originates from amylopectin.
Many interactions exist among complex biomacromolecules, and the dominant interaction depends on molecular characteristics. Both starch and protein are biomacromolecules found in food and significantly affect food texture and rheology. Protein–starch interactions exist in the food emulsion can be divided into strong (e.g., covalent interaction and electrostatic interaction) and weak interactions.[Citation7] In addition to being nutrients, they can both be used as gelatinizer, thickener, or stabilizer, thereby crucially affecting the texture, rheological, physical, and chemical properties of food. Usually, gelatinization, emulsification, and foaming properties of protein are greatly improved by adding starch. Consequently, the texture and processing characteristics of colloidal food are ultimately affected.
The interest in research on the interactions between protein and starch is increasing. Food quality can be significantly improved by exploiting the interactions between these two macromolecules. This article mainly explored texture properties and micromorphology of the composite gels with soy 7S globulin and corn starch (amylose, amylopectin). The properties of composite gel with soy 7S globulin and corn starch and the mechanism of interaction between them can serve as a guide for researches on food hydrocolloids processing.
Materials and methods
Fractional extraction of soybean 7S globulin
The fractional extraction of soybean 7S globulin procedure was followed according to the method reported by Saio and Watanabe.[Citation8] To 25 L of 10 mmol/L calcium chloride solution, 2.5 kg of defatted soy meal (Shandong Yu-Wang Industrial Co. Ltd., Jinan, Shandong, China) were added with stirring. The mixture was stirred for 2 h and centrifuged (3000 × g) with a centrifuge. The extract was adjusted to pH 4.5 and centrifuged (8000 × g) to whey. The precipitated protein was suspended in water, adjusted to pH 7.0 and placed in molecular porous membrane tubing (MWCO: 12~14 kDa; Spectrum Medical Industries Inc., Houston, Texas, America) and dialyzed against 2 L of reverse osmosis purified water at 4°C. The water was changed every 2 h for the initial 6 h and then after 12 h, and subsequently freeze dried, denoted as “7S.”
Experimental methods
Extraction and purification of amylose and amylopectin present in corn
For the crude separation of amylose and amylopectin, 20 g corn starch was initially weighed, and then a small amount of absolute ethyl alcohol was added. After subsequently adding 400 mL of NaOH solution (0.5 mol/L), the mixture was agitated for 10 min at 100°C, centrifuged for 10 min after cooling at 10°C and 10,000 r/min, and neutralized in diluted hydrochloric acid solution until pH 7.0 was reached. Agitation was performed for 15 min in a boiling water bath at a certain constant temperature after adding 200 mL of butanol-isoamylol (3:1) mixture. The mixture was stored in a refrigerator at 4°C overnight after cooling. After centrifugation for 10 min at 10°C and 10,000 r/min, a precipitate of crude amylose and a centrifugate of crude amylopectin were obtained.
For the purification of amylose, crude amylose was initially dissolved in distilled water saturated with 400 mL of butanol, heated, and dissolved until the solution became transparent. The mixture was then stored in a refrigerator at 4°C for 24 h after cooling to room temperature, and centrifuged for 25 min at 10°C and 10,000 r/min. The resulting precipitate was filtered with a sand core funnel, rinsed with absolute ethyl alcohol, allowed to settle, and dried for 6 h in a vacuum oven (˂40°C), forming pure amylose powder.
For the purification of amylopectin, the centrifugate was initially placed in a separating funnel until the solution divided into three layers. The below emulsoid solution was then taken out, mixed with 100 mL of butanol-isoamylol (1:1), agitated for 10 min at 100°C, and stored in a refrigerator at 4°C for 48 h after cooling (room temperature). After centrifugation for 10 min at 10°C and 10,000 r/min, absolute ethyl alcohol was added to the centrifugate in the 2:1 ratio, and the mixture was stored in a refrigerator at 4°C for 24 h. The precipitate was dissolved in hot NaOH solution (0.5 mol/L, 40°C), purified several times by the previous method, rinsed with absolute ethyl alcohol, and sampled for 6 h after drying in a vacuum oven (40°C). The product was pure amylopectin powder.
Gel preparation for the protein–starch coexistence system
With the protein–starch coexistence system giving priority to starch, protein (protein/starch, w/w) was added to starch at different ratios (0:100, 6:94, 8:92, 10:90, 12:88, and 14:86, respectively). After mixing evenly, the 10% (w/v) solutions made up with distilled water were prepared. Afterward, those solutions were placed in a water bath at 95°C for 20 min and then in another water bath at 4°C for 20 h. The composite gel with soy 7S globulin and corn amylose was denoted as “7S-amylose,” whereas that of soy 7S globulin and corn amylopectin was denoted as “7S-amylopectin.”
Determination of gel texture properties
The protein gel was stabilized for 30 min at room temperature, and gel texture were determined using Model TA-XT2 texture analyzer (Stable Micro Systems Co. Ltd., Godalming, Surrey, England). A puncture test was conducted using a P/0.5 probe with a head speed at 1.0 mm/s, 0.5 mm/s during puncture, 0.5 mm/s during retraction, puncture distance of 10 mm, and time interval of 3 s. Every sample was measured five times and the test data were recorded.
Scanning electron microscopy (SEM)
The sample gels were cut into small pieces (<2 mm cube) with a razor blade, and then immersed in 2.5% glutaraldehyde and rinsed five times with distilled water followed by immersing in 2% OsO4 for 2 h at 4°C. The samples were rinsed for 1 h in distilled water before being dehydrated by 30, 50, 70, 85, 95, and 100% of ethanol. After dehydration, critical-point drying was done using liquid carbon dioxide. Dried samples were mounted on stubs and sputter coated with gold. The microstructure of soy protein gels were observed under a scanning electron microscope (1910FE, AMRAY) at 5 kV.
Statistical analysis
Values given in the figures are the means of quintic, and error bars indicated the standard deviation. The statistical significance of differences among means was evaluated by Duncan’s test at p < 0.05. Statistical analysis was performed using SPSS software version 22.0.
Results and discussion
Texture properties of 7S-amylose and 7S-amylopectin composite gels
The texture properties of the composite gels of soy 7S globulin with starch (corn amylose, corn amylopectin) were measured using texture analyzer. The effects of different contents of soy 7S globulin on the hardness, adhesiveness, cohesion, and elasticity of the composite gels were analyzed. Starch polysaccharides are flexible to an extent because of the revolution of glucosidic bonds. If residues of glycans contained reducing groups (i.e., free hydroxyls and carboxyl), reducing ends of molecules would be formed by them, and many natural polysaccharides would be bonded by combining the reducing ends of glycans and protein. The compound of protein and polysaccharide is generally recognized to be formed under interactions of protein and polysaccharide, such as covalent bond, electrostatic force, hydrogen bond, van der Waals forces, hydrophobic interaction, electrovalent bond, volume exclusion, and molecular entanglement.[Citation9] These functions lie between different fragments and side chains of the two types of macromolecules, thereby maintaining the microstructure of composite gels. Furthermore, which chemical interactions that play dominant roles in composite gels depend on the compositions and structure characteristic of biomacromolecules.[Citation10–Citation13]
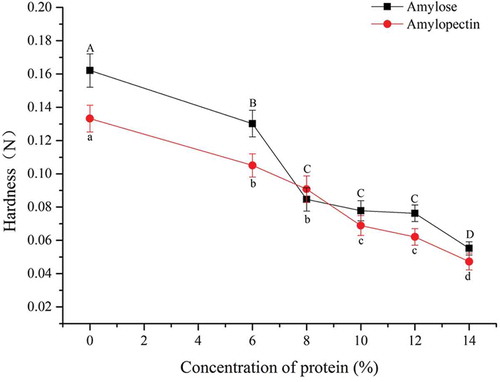
Hardness
showed that the gel hardness of corn amylose was 21% higher than that of pure corn amylopectin. Kshirod et al.[Citation14] found that the α-amylase and β-amylase contents of rice decreased with prolonged storage, thereby leading to increased amylose content, decreased lower amylopectin content, increased hardness, and decreased adhesiveness of steamed rice. This result indicated that amylose presented higher hardness but lower adhesiveness than amylopectin. The gel hardness of 7S-amylose coexistence system after adding soy 7S globulin was prominently higher than that of 7S-amylopectin when the soy 7S globulin additive amount was below 8%. Whereafter, with the soy 7S globulin additive amount continue to increase, the 7S-amylose and 7S-amylopectin presented approximate hardness. In addition, the hardness of all composite gels decreased with increased content of soy 7S globulin. This finding indicated that adding soy 7S globulin would weaken the composite gel hardness of both 7S-amylose and 7S-amylopectin. Michiko Watanabe et al.[Citation15] disposed rice with protease under high pressure, thereby lowering the albumin and globulin contents of rice, more quickly releasing starch from rice, and increasing the contribution of starch to the grain texture of rice. The results showed that the hardness and luster of rice increased. Furthermore, there was a remarkable direct proportion between starch content and rice hardness (p < 0.05), whereas there was a remarkable inverse proportion between globulin content and rice hardness (p < 0.05). These results are consistent with our present experimental results to a certain extent.
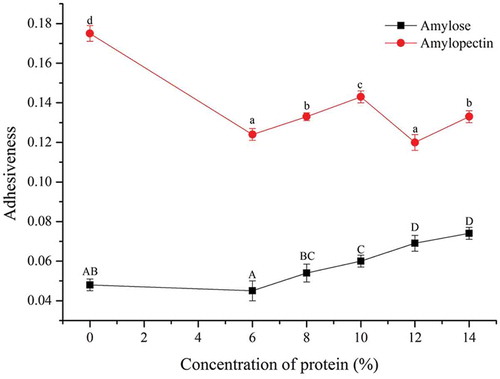
Adhesiveness
showed that the gel adhesiveness of pure corn amylopectin was 364% higher than that of pure corn amylose. With different proportions of soy 7S globulin added to amylopectin and amylose, the composite gels adhesiveness showed different change trends. With soy 7S globulin added to amylopectin, the composite gel adhesiveness of 7S-amylopectin changed in a wave-like manner, and when the additive amount accounted for 10%, the composite gel adhesiveness of 7S-amylopectin reached the peak (p < 0.05). The composite gel adhesiveness increased with different amounts of soy 7S globulin added to amylose. Chedid et al.[Citation16] put forward that adding protein would increase the adhesiveness of starch. This finding was due to the fact that adding protein could affect starch pasting properties and thus, the composite gels adhesiveness. Perez et al.[Citation17] found that the content of amylose was negatively related to its adhesiveness (p < 0.05) and positively correlated with its hardness (p < 0.05). Therefore, in the present experiment, when the additive amount of soy 7S globulin gradually increased and the proportion of amylose decreased, the composite gel adhesiveness of 7S-amylose increased.
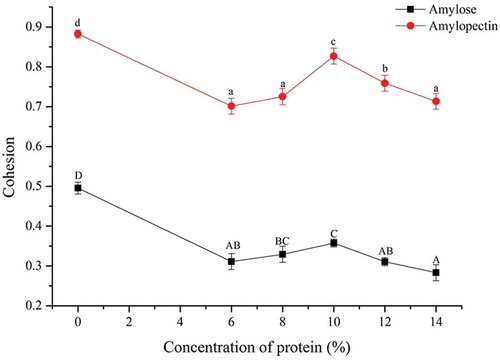
Cohesion
Cohesion is the maintenance of the intact nature of food while the tight junction microstructure of food is being damaged by chewing. The composite gel has good cohesion and is strongly coupled with macromolecule compatibility. This compatibility refers to the abilities of biomacromolecules to disperse evenly in the composite system and the stability of such biomacromolecules. Biomacromolecules are usually compatible with one another merely at a specific proportion and temperature. showed that the gel cohesion of pure corn amylopectin was 78% higher than that of pure corn amylose, which indicated that under the same experimental conditions, pure corn amylopectin had better gelling effects and higher colloidal integrity. With increased additive amount of soy 7S globulin, the two types of composite gels appeared fluctuating cohesion trends. Cohesion of mixed gels were optimum after adding 10% soy 7S globulin (p < 0.05), which showed that regardless of corn amylopectin or amylose content, compatibility was optimum when the additive amount of soy 7S globulin was 10%. The same was true for colloidal cohesion.
Figure 3. Effects of different ratios of soybean 7S globulin on the cohesion of mixed gels. Each data is the means and standard deviations of five measurements. Different letters indicate significant differences (p < 0.05).
However, even though good texture features of one food benefited endowing nice taste while eating, it did not implicate that this food can be digested easily and quickly in the gastrointestinal tract. The difficulty degree of digestibility of starch affects postprandial glucose response, which will lead to the occurrence of chronic diseases related with diet (e.g., diabetes, angiocardiopathy, and glycogen storage disease). Many researchers[Citation18–Citation20] proposed that the network structure on account of interaction between protein and starch could hinder amylase from touching with starch, which would result in the digestibility of starch declining dramatically (p < 0.05); whereas the digestibility of starch did not decline significantly (p < 0.05) when protein and starch were merely physically blended.
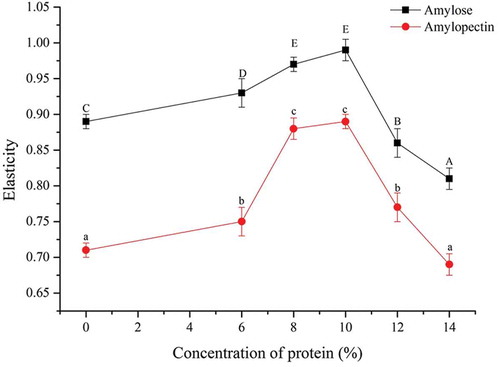
Elasticity
showed that the gel elasticity of pure corn amylose was 25% higher than that of pure corn amylopectin. Bhattacharya et al.[Citation21] proposed that the elasticity of starch grain formed by amylopectin under the intertwining roles was higher than that of amylose. With increased additive amount of soy 7S globulin, the composite gel elasticity of 7S-amylopectin increased and then decreased, and the peak value was reached when the additive amount of soy 7S globulin was 8~10% (p < 0.05). The composite gel elasticity of 7S-amylose considerably decreased when the additive amount of soy 7S globulin exceeded 10% (p < 0.05). In general, when the additive amount of soy 7S globulin was 8~10%, the composite gel elasticity of 7S-amylose and that of 7S-amylopectin were optimum (p < 0.05). Many studies have also shown that gel elasticity was also high, with relatively neat and uniform voids, as well as improved cohesion.[Citation1]
Composite gel systems of soy 7S globulin with corn amylose and amylopectin by SEM
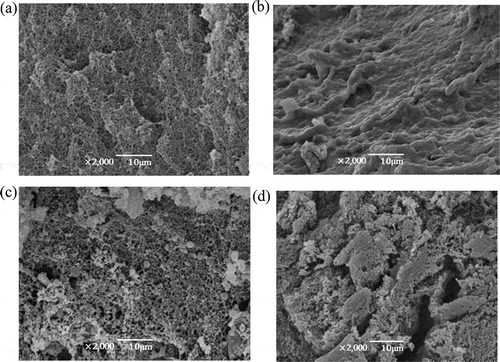
The particle morphology of the composite gel systems of soy 7S globulin with corn amylose and amylopectin was observed by SEM. To determine morphological and crystallization variations in the composite gel systems, the added amounts of soy 7S globulin were varied: 0% (single amylose or amylopectin), 6% (the point at which textural properties mutationally changed), 10% (the point at which cohesion was best), and 14% (the maximum additive amount) while fixing the total mass.
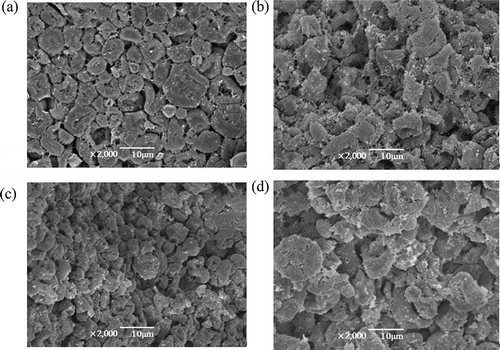
SEM characterization of the composite gel of soy 7S globulin and corn amylose
showed the SEM image of the 7S amylose composite gel, in which total mass was fixed and the added amount of soy 7S globulin was varied. As shown in the micrograph of pure corn amylose (), grains were round, oval, and polyhedral with firm arrangement. As shown in , when the additive amount of soy 7S globulin was 6%, particles were of different sizes and shaped as irregular polygons with chaotic and random arrangement. As shown in , when the additive amount of soy 7S globulin was 10%, the composite gel appeared compact and relatively orderly surface microtopography with granular particles. As shown in , when the additive amount of soy 7S globulin was 14%, the composite gel presented similar surface microtopography with that of the composite gel added 6% soy globulin.
SEM characterization of the composite gel of soy 7S globulin and corn amylopectin
showed the SEM image of the 7S-amylopectin composite gel, in which total mass was fixed and the added amount of soy 7S globulin was varied. showed the micrograph of pure corn amylose, in which pure corn amylopectin gel was characterized by a compact microstructure, smooth surface microtopography and uniform voids. showed a micrograph of the composite gel, in which the added amount of soy 7S globulin was 6%. The surface microtopography was found to be unevenly wrinkled. The micrograph of the composite gel when added amount of soy 7S globulin was 10%, shown in . The net-like structure with a relatively even and meticulous mesh distribution was observed. showed a micrograph of the composite gel, in which the added amount of soy 7S globulin was 14%. The surface microtopography of composite gel was uneven and coarse, with obvious trench cracks and deep collapse, indicating obvious gel deterioration.
Conclusion
The addition of soy 7S globulin could weaken hardness of the composite gel with soy 7S globulin and starch. When the added amount of soy 7S globulin was 10%, the composite gel of 7S-amylopectin was the optimum in terms of adhesiveness, cohesion, and elasticity; when the added amount of soy 7S globulin was 10%, the composite gel of 7S-amylose was the optimum in terms of cohesion and elasticity. Overall, when the added amount of soy 7S globulin was 10%, the compatibility between soy 7S globulin and corn starch (amylose, amylopectin) were the optimum. In addition, when the added amount of soy 7S globulin was 10%, the microsurface of the 7S-amylose composite gel appeared evenly distributed with granular particles; whereas that of 7S-amylopectin composite gel appeared compact and uniform lattice surface microtopography. Further research would be desirable to explore the effects of the varying amounts of β-conglycinin on the difficult level of digestibility of corn starch.
Funding
This research work was supported by the National Natural Science Foundation of China (No. 31301427).
Additional information
Funding
References
- Huang, X.; Li, C.; Yang, F.; Xie, L.; Xu, X.; Zhou, Y.; Pan, S. Interactions and Gel Strength of Mixed Myofibrillar with Soy Protein, 7S Globulin and Enzyme-Hydrolyzed Soy Proteins. European Food Research and Technology 2010, 231, 751–762.
- Foegeding, E.A.; Luck, P.J.; Davis, J.P. Factors Determining the Physical Properties of Protein Foams. Food Hydrocolloids 2006, 20, 284–292.
- Palazolo, G.G.; Sobral, P.A.; Wagner, J.R. Impact of Sample Aging on Freeze-Thaw Stability of Oil-in-Water Emulsions Prepared with Soy Protein Isolates. International Journal of Food Properties 2016, 19, 2322–2337.
- Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Environment Friendly Green Composites Based on Soy Protein Isolate—A Review. Food Hydrocolloids 2015, 50, 174–192.
- Tian, B.Q.; Wang, C.; Wang, L.; Xie, B.J. Granule Size and Distribution of Raw and Germinated Oat Starch in Solid State and Ethanol Solution. International Journal of Food Properties 2016, 19, 709–719.
- Yu, K.; Wang, Y.J.; Wang, Y.; Guo, L.; Du, X.F. Effects of Annealing and Additives on the Gelatinization, Structure, and Textural Characteristics of Corn Starch. International Journal of Food Properties 2016, 19, 1272–1281.
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative Studies on the Physicochemical Properties of Soy Protein Isolate-Maltodextrin and Soy Protein Isolate-Gum Acacia Conjugate Prepared Through Maillard Reaction. Food Research International 2013, 51, 490–495.
- Saio, K.; Watanabe, T. Differences in Functional Properties of 7S and 11S Soybean Protein. Journal of Texture Studies 1978, 9, 135–157.
- Sittikijyothin, W.; Sampaio, P.; Gonçalves, M. Microstructure and Rheology of β-Lactoglobulin–Galactomannan Aqueous Mixtures. Food Hydrocolloids 2010, 24, 726–734.
- Hoskins, R.; Robb, I.D.; Williams, P.A.; Warren, P. Phase Separation in Mixtures of Polysaccharides and Proteins. Journal of the Chemical Society, Faraday Transactions 1996, 92, 4515–4520.
- Mutch, K.J.; van Duijneveldt, J.S.; Eastoe, J. Colloid–Polymer Mixtures in the Protein Limit. Soft Matter 2007, 3, 155–167.
- Schaink, H.; Smit, J. Protein–Polysaccharide Interactions: The Determination of the Osmotic Second Virial Coefficients in Aqueous Solutions of β-Lactoglobulin and Dextran. Food Hydrocolloids 2007, 21, 1389–1396.
- Branca, C.; Wanderlingh, U.; D’Angelo, G.; Crupi, C.; Rifici, S. Study of the Dynamical Behavior of Sodium Alginate/Myoglobin Aqueous Solutions: A Dynamic Light Scattering Study. Journal of Molecular Liquids 2015, 209, 294–300.
- Bhattacharya, K.R.; Sowbhagya, C.M.; Swamy, I.; Yelandur, M. Importance of Insoluble Amylose As a Determinant of Rice Quality. Journal of the Science of Food and Agriculture 1978, 29, 359–364.
- Watanabe, M.; Miyakawa, J. Production of Hypoallergenic Rice by Enzymatic Decomposition of Constituent Proteins. Journal of Food Science 1990, 55, 781–783.
- Chedid, L.; Kokini, J. Influence of Protein Addition on Rheological Properties of Amylose- and Amylopectin-Based Starches in Excess Water. Cereal Chemistry 1992, 69, 551–555.
- Perez, C.M.; Juliano, B.O. Indicators of Eating Quality for Non-Waxy Rices. Food Chemistry 1979, 4, 185–195.
- Jenkins, D.J.; Thorne, M.J.; Wolever, T.M.; Jenkins, A.L.; Rao, A.V.; Thompson, L.U. The Effect of Starch-Protein Interaction in Wheat on the Glycemic Response and Rate of in Vitro Digestion. American Journal of Clinical Nutrition 1987, 45, 946–951.
- Singh, J.; Dartois, A.; Kaur, L. Starch Digestibility in Food Matrix: A Review. Trends in Food Science & Technology 2010, 21, 168–180.
- Singh, J.; Kaur, L.; Singh, H. Food Microstructure and Starch Digestion. Advances in Food & Nutrition Research 2013, 70, 137–179.
- Reddy, K.R.; Ali, S.Z.; Bhattacharya, K. The Fine Structure of Rice-Starch Amylopectin and Its Relation to the Texture of Cooked Rice. Carbohydrate Polymers 1993, 22, 267–275.
- Perrechil, F.A.; Ramos, V.A.; Cunha, R.L. Synergistic Functionality of Soybean 7S and 11S Fractions in Oil-in-Water Emulsions: Effect of Protein Heat Treatment. International Journal of Food Properties 2015, 18, 2593–2602.