ABSTRACT
This study reports the phenolic composition, antioxidant, antibacterial, larvacidal, and cytotoxic activity of methanol and acetone extracts of Hyacinthella lineata leaves and bulbs. The phenolic composition of H. lineata was determined by HPLC. The most abundant component was gallic acid (421.9µg/g). The β-carotene/DPPH/ABTS/FRAP decoloration method was used to estimate the total antioxidant activity. The total antioxidant activity was the highest for bulb-methanol fraction (65.41 ± 0.05%). The total phenolic content for leaves-methanol extract of the plant was determined as 6.56 ± 4.027mg/mL gallic acid equivalents. Analysis of the antibacterial activity showed that the methanolic-bulb extract are efficient against gram positive and gram negative bacteria. The cytotoxic effect on Artemia salina was assessed by Brine shrimp assay. Brine shrimp lethality assay showed that LC50 values of HBM were obtained as 4.105 ± 2.42μg/ml. The bulb extract of H. lineata showed the highest larvicidal activity against Cx pipiens with value LC50 (64.3275μg/ml). This study suggested that H. lineata may be used as a potential source of antioxidant, and for their biological activity, cytotoxic and antimicrobial properties.
Introductıon
Turkey is a country located in the temperate zone with remarkable plant diversity. This plant diversity is due to varied geographical features and climate diversity, the country’s position as a natural bridge between two continents and also as a midpoint of three different geographical plant zones. Each of the plants which have been classified based on their color, texture, shape, and the chemicals they contain, could be the subject of extensive research. From medical to nutrition uses or from art to decoration, the mark of plants and flowers can be found in all elements.[Citation1]
Hyacinthella Schur (Liliaceae) is a genus of 17 species distributed in mainly Mediterranean regions.[Citation2] Hyacinthella genus is constantly changing place between families (Liliaceae, Hyacinthaceae, recently Asparagaceae). Genus represented 12 species, in which 10 of them are endemic, in Turkey.[Citation3] H. lineata Steudel is an endemic species distributed in province within B1 and B3 square in Turkey. Therefore, determination of morphological and anatomical characteristics of all species in detail will contribute to the state of systematic place of the genus.[Citation4]
Recently, several scientific studies on the floristic, morphology, and anatomy of this genus have been performed. Hyacinthella micrantha (Boiss.) Chouard,[Citation5] H. lineata Chouard,[Citation6–Citation10] H. lazulina K. Persson et J. Persson, H. heldreichii (Boiss.) Chouard and H. campanulata K.Press. & Wendelbo[Citation10] and H. glabrescens[Citation11] endemic species for Turkey have been investigated morphologically and anatomically. H. acutiloba is closely related to H. lineata by morphological characters.[Citation12]
The aim of this study was to investigate the phenolic compositions of the endemic Hyacinthella lineata collected from the center of the country and to determine the antioxidant, antibacterial, larvacidal, and cytotoxic activities of these methanolic and acetone extracts. It gives additional information linked to their phenolic constituents analyzed for the first time by HPLC as well as to their biological activities in order to increase the source of natural active compounds which recently have attracted considerable interest in food, cosmetic, and pharmaceutical industries instead of using more toxic synthetic compounds.
Materıals and methods
Plant materials
Hyacinthella lineata species were collected in the spring 2015 from Honaz locality, near Denizli province, in Turkey. The fresh bulbs and leaves of the plant samples were cleaned and dried in the shadow for extraction. The voucher specimen was deposited at the herbarium of Pamukkale Univercity the Laboratory of botanic under the “PAU2015-2023” number.
Extraction preparation
Dried plant parts (bulbs and leaves) were pulverized. Each ground sample was transferred into a beaker. Methanol and acetone (100 mL respectively) were added and they were put in water bath at 55ºC for 6 h.[Citation13] The extraction mixture was separated from the residue by filtration through Whatman No: 1 filter paper. The plant residue was re-extracted triplicates with methanol and acetone. After the filtration two extracts were combined. The residual solvent of methanol and acetone extracts of samples were removed under reduced pressure at 48–49°C using a rotary evaporator (rotavapor IKA VB 10, Germany). The water in the extract was lyophilized using a freeze dryer (Thermosavant Modulyo D, USA). The yields of these fractions were 3.6 and 2.7 g respectively. Extracts were produced in duplicates and used to assay the biological activity: Hyacinthella (H), Bulb-Methanol (HBM), Bulb-Acetone (HBA), Leaf-Methanol (HLM), Leaf-Acetone (HLA).
Analysis of phenolic contents by HPLC
HPLC procedure described by Parvu et al.[Citation14] Phenolic compounds were evaluated by reversed-phase High Performance Liquid Chromatography (RP-HPLC, Shimadzu Scientific Instruments). The conditions utilized were as follows: C-18 column CTO-10ASVp, 4.6 mm × 250 mm, 5 μm; mobile phase was composed of solvent A (formic acid with 3% methanol) and solvent B (100% acetonitrile); injection volume 20 μg, gradient elution from 15–100% B; run time 45 min and flow rate was 1 mg/min. For analysis, the samples were dissolved in methanol, and 20 μg of this solution was injected into the column. The chromatograms were examined at 280 nm with a LC gradient detector. The phenolic compounds were recognized by comparing retention times and UV absorption spectra with those of pure standards. Gallic acid, 3,4-dihidroksi, 4-dihidroksi, chlorogenic acid, Vanilic acid, Caffeic acid, p-Coumaric acid, Ferulic acid and Cinnamic acid (Germany) were used as Standard. Peaks identified by comparing retention times and UV spectra with authentic standards. The amount of each phenolic compound was expressed as µg/g per gram of the extract.
Antioxidant activity (AA) assays
β-carotene bleaching (BCB) assay
The antioxidant activity of the plant extract (methanol and acetone) was determined according to the β-carotene bleaching (BCB) assay described by Amin and Tan with some modifications.[Citation15] In the BCB assay, antioxidant capacity is determined by measuring the inhibition of the organic compounds and the conjugated hydroperoxides arising from linoleic acid oxidation. Briefly, 4 mL of β-carotene solution (0.1 mg in 1 mL chloroform), 40 mg of linoleic acid and 400 mg of Tween 40 were transferred to a roundbottom flask. The mixture was then evaporated at 50°C by means of a rotary evaporator to remove chloroform. Then, 100 mL of oxygenated distilled water was slowly added to the residue and vigorously agitated to give a stable emulsion. 4.8 mL of this emulsion was placed into test tubes which had 0.2 mg of the sample and 0.2 of the extract in them. For control, 0.2 mL of solvent (methanol and acetone, respectively) was placed in test tubes instead of the extract. As soon as the emulsion was added into the test tubes, initial absorbance was measured with a spectrophotometer (Shimadzu UV-1601, Japanese) to be at 470 nm. The measurement was carried out at 0.5 h intervals for 2 h. All samples were assayed in triplicate. BHT (Butylated hydroxy toluen) was used as standards. The antioxidant activity was measured in terms of successful bleaching of β-carotene by using the following equation. The measurements were made using the equation below:
where AA is the total antioxidant activity, A0 is the initial absorbance of the sample, At is the initial absorbance of the control, A0o is the sample’s absorbance after 120 min, and Ato is the control’s absorbance after 120 min. All samples were assayed in triplicate.
DPPH free radical scavenging assay
Free radical scavenging activity of the extracts (methanol and acetone) was determined using the free radical DPPH.[Citation16] 4 ml of the DPPH’s 0.004% metanolic solution was mixed with 1 ml (200–1000 µg/mL) of the extracts, and their absorbances were measured to be at 517 nm after incubation for 30 min at room temperature the absorbance value of the samples were evaluated against empty control group (where all determinants except the test compound were present). Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Every test was treated three times and the averages as determined. Free radical scavenging activity was measured using the equation below:
where A0 is the absorbance of the control (blank, without extract) and A1 is the absorbance in the presence of the extract. The results were expressed as IC50 (the concentration required to inhibit 50% of the DPPH).
Ferric-reducing antioxidant power (FRAP) assay
The reducing power of the extracts (methanol and acetone) was determined according to the method described by Oyaizu.[Citation17] The different concentrations (40–150 μg/mL) of extracts were mixed with 2.5 mL of phosphate buffer (0.2 M, pH = 6.6) and 2.5 mL of potassium ferricyanide [K3Fe(CN)6] (1%). The mixture was incubated at 50◦C for 20 min. A portion (2.5 mL) of TCA (10%) was added to the mixture, which was then centrifuged for 10 min at 3000 rpm (MSE Mistral 2000, UK). The supernatant of solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%). The FeCitation3+/FeCitation2+ transformation was investigated in the presence of extracts or standards and the absorbance values were measured at 700 nm in a spectrophotometer. Phosphate buffer (pH 6.6) was used as blank solution. BHT were used as standards. The FRAP was expressed as trolox equivalents in mg/g of samples used.
ABTS free radical scavenging assay
Experiments were performed according to Re et al. 1999[Citation18] with small modifications. ABTS and potassium persulfate were dissolved in distilled water to a final concentration of 7 mM and 2.45 mM respectively. These two solutions were mixed and the mixture allowed to stand in the dark at room temperature for 16 h before use in order to produce ABTS radical (ABTS•+). For the study of phenolic compounds the ABTS radical solution was diluted with distilled water to an absorbance of 1.00 at 734 nm. After the addition of 10 μL of sample to 4 mL of diluted ABTS solution, the absorbance was measured at 30 min. All samples were analyzed in triplicate. The ABTS radical-scavenging activity of the samples was expressed as
where Acontrol is the absorbance of the blank control (ABTS solution without test sample) and Asample is the absorbance of the test sample. The results were expressed as IC50 (the concentration required to inhibit 50% of the ABTS).
Determination of the total phenolic content
The total phenolic content of extracts were determined with Folin- Ciocalteau reagent, according to the method of Slinkard and Singleton.[Citation19] Briefly, 0.75 mL of Folin–Ciocalteu reagent (1:9; Folin–Ciocalteu reagent: distilled water) and 100 mL of sample (5 mg/mL) were put into a test tube. The mixture was allowed to stand at room temperature for 5 min. 0.75 mL of 6% (w/v) Na2CO3 was added to the mixture and then mixed gently. The mixture was homogenized and allowed to stand at room temperature for 90 min. The total polyphenol content was determined using a spectrophotometer at 760 nm. The Standard calibration (0.01–0.05 mg/mL) curve was plotted using gallic acid. The total phenolic content was expressed as Gallic Acid Equivalents (GAE) in mg/mL plant extract.
Determination of the total flavonoid content
The total flavonoid content was determined using the Dowd method as adapted by Arvouet-Grand et al.[Citation20] For each extract, 1 mL of methanolic solution (100 μg mL−Citation1) was mixed with 1 mL of aluminum trichloride (AlCl3) in methanol (2%). The absorbance was read at 415 nm after 10 min against a blank sample consisting of a 1 mL of methanol and 1 mL of plant extract without AlCl3. The total flavonoid content was determined on a standard curve using quercetin as a standard. The mean of three readings was used and expressed as mg of quercetin equivalents (QE) per 100 mg of extract or fraction (mgQE/g).
Antibacterial activity
Microwell dilution assay
The antibacterial activity of the plant extract was tested in vitro against the following bacteria: Gr(+); Staphylococcus aureus (ATCC 25923), Staphylococcus haemolyticus (ATCC29970) and Gr(-); Escherichia coli (ATCC 25922) were employed in the study. Inoculates of the strains were prepared from 12 h broth cultures, and suspensions were adjusted to 0.5 McFarland standard turbidity. The ethanol extract of H.lineata were dissolved in DMSO, diluted to the highest concentration tested (100 μg/mL) with distilled, sterile H2O. Then serial two-fold dilutions were made to obtain a concentration range from 10 to 100 μg/mL in 10-mL sterile test tubes containing Nutrient Sabouraud Dextrose Broth. Determination of the minimum inhibitory concentration (MIC) of the extract and cirsimarin against the test bacteria were determined by microdilution method in 96 multi-well microtiter plates.[Citation21]
Cytotoxic activity
Brine shrimp lethality test (BSLT) was applied to analyze the possible cytotoxic activity of the extracts. A. salina eggs (10mg) were incubated in 500mL of seawater under artificial light at 28ºC, pH 7–8. After incubation for 24h, nauplii were collected with a pasteur pipette and kept for an additional 24h under the same conditions to reach the metanauplii (mature larvae) stage. Ten nauplii were drawn through a glass capillary and placed in each vial containing 4.5mL of brine solution. In each experiment, 0.5mL of the plant extract was added to 4.5mL of brine solution and maintained at room temperature for 24h under the light and then dead nauplii were counted.[Citation22] Experiments were conducted along with control and five different concentrations (10–1000µg/mL) of the extract in a set of three tubes ± data was performed by EPA Probit Analysis Program (version 1.5) to determine the LC50.
İnsectisidal activity of extracts against the larvae of culex pipiens L. (Diptera: Culicidae)
Cx. pipiens used in the assays originated from Arapsuyu, Antalya, and were collected from a pool in August 2015. The larvae were reared at 12:12 light/dark photoperiod, (60 ± 10)% RH, and (26 ± 2) in an insectary in the Biology Department, Akdeniz University. The third-fourth instar larvae were used for bioassays.
Larvicidal activity of the extracts against Cx. pipiens was assessed by using the method described by Cetin and Yanikoglu.[Citation23] For experimental treatment, 0.5 g of each extract was dissolved in 500 mL distilled water. A series of concentrations ranging from 100 to 1000 µg/mL of dissolved extract were prepared. The extract-water solution was stirred for 30 s with a glass. rod. After approximately 5 min, 20 larvae taken on a strainer with fine mesh were transferred gently to the test medium by tapping. Three replicates of each concentration were run at a time. Mortality was recorded after, 24-, 48- and 72- of exposure, during which fish food was given to the larvae. All experiments were conducted at (26 ± 2)̊C and (60 ± 10)% relative humidity with 12:12 D:L photoperiod. Dead larvae were identified when they failed to move after probing with a needle in the siphon or cervical region. Moribund larvae were those incapable of rising to the surface (within a reasonable period of time) or showing the characteristic diving reaction when the water was disturbed. Larvae were also observed for discoloration, unnatural positions, uncoordination, or rigor.
Statistical analysis
All analyses and tests were run in triplicate and mean values recorded. All the experimental data are presented as mean ± SEM of three individual samples. Data are presented as percentage of inhibition or radical scavenging on different concentration of H. lineata. IC50 and LC50(the concentration required to scavenge 50% of free radicals) value was calculated from the dose-response curves. All of the statistical analyses were performed by means of Microsoft Office Excel 2010 software and SPSS. The results were evaluated using an unpaired t-test and one way analysis of variance ANOVA. The differences were regarded as statistically significant at p < 0.05.
Results and dıscussıon
Identification of phenolic compounds by HPLC
The phenolic compounds contained in the H. lineata methanolic extracts were characterized using HPLC methods. Of the nine standard phenolics analyzed, nine were identified in the extracts. As shown in , nine peaks were observed from the HPLC results, which represented nine types of phenolic acids compounds in H.lineata. The retention time and relative content of these phenolic acids compounds are shown in . The potential effects of these compounds antioxidant activity had been also reported.[Citation24]
Figure 1. HPLC chromatograms of phenolic acids from the H.lineata methanolic extracts.(1) Gallic acid; (2) 3,4-dihydroxy benzoic acid; (3) 4-dihydroxy benzoic acid; (4) chlorogenic acid; (5) vanillic acid; (6) caffeic acid; (7) p-coumaric acid; (8) ferulic acid; (9) cinnamic acid
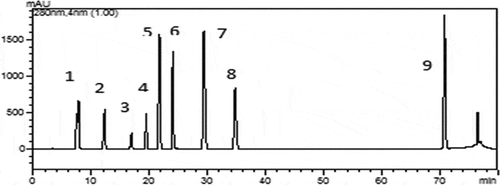
Table 1. HPLC analysis of methanolic extracts for phenolic acids contents.
Antioxidant activity
In general phenolic contents were correlated with those of flavonoid and biological activities.[Citation25,Citation26] The antioxidant activities of the methanolic and acetonic extract and the of H. lineata, determined in our study through four complementary methods, were shown in . The methanolic bulb extract exhibited considerable antioxidant activity. The vital role of the antioxidants is their interaction with oxidative free radicals. The DPPH method with the stable organic radical DPPH is used for the determination of free radical scavenging activity, usually expressed as IC50. The results are expressed as IC50, the amount of antioxidant necessary to decrease the initial concentration of DPPH by 50%. The lower IC50 values indicate a higher antioxidant activity IC50 values 224.62 ± 0.03 (HBM) were and 335.42 ± 0.05 (HBM) for DPPH and ABTS assays, respectively. The ferric-reducing power was 10.75 ± 0.08 mg/gtrolox. Several phenolic acids, mainly Gallic acid, present in the extract have been identified as dominant antioxidant compounds. The presence of these compounds in our extract should be the main cause of its high antioxidant power. Therefore, antioxidant activity may vary, based on the variations in the chemical composition.[Citation27] The antioxidant activity of cilantro methanolic and acetone extract was also evaluated by the BCB method. In the BCB assay, linoleic acid produces hydroperoxides during incubation at 50°C. The presence of hydroperoxides causes rapid discoloration of β-carotene. shows the mean total antioxidant activity of extract of H. lineata. The means of the total antioxidant activity for H. lineata methanol-bulb extract was 65.41 ± 0.05%. BHT, used as the standard, had a higher antioxidant activity (96.04 ± 0.05%) than H. lineata extract.
Table 2. Antioxidant activities of methanol and acetone extracts of H. lineata.
Total phenolic content and total flavonoid content
The total phenolic content of the plant extracts is shown in . Phenolic compounds belong to a group of natural substances found in dietary products, and these compounds have gained considerable attention due to their potent antioxidant activity. In the crude methanol and acetone extracts prepared from the whole plants of H. lineata, total phenolic and flavonoid amounts were determined expressing as gallic acid and quercetin equivalents, respectively (y = 0.0258GAE(mg/mL) – 0.005, R2 = 0.9979 and y = 0.0322 (mgQE/g)- 0.005 R2: 0.997). The highest total phenolic and flavonoid contents of the HLM extracts were determined as 6.56 ± 4.027 mg GAE/mL extract and 96.93 ± 1.072 mgQE/g, respectively. The total flavonoid contents were detected to be higher than the total phenolic contents (). To our knowledge, there is no study about H. lineata regarding these antioxidant activities and total phenolic-flavonoid contents in literature.
Table 3. Total phenolic and flavonoid contents of the extract H.lineata.
In general phenolic contents were correlated with those of flavonoid and biological activities. The biological efficiency of extracts depends on the experimental method, the nature, and the concentration of extracts.[Citation25] In the present study, the antioxidant activity, determined by four different methods, namely DPPH, β-carotene-linoleic acid, FRAP and ABTS is presented in . The vital role of the antioxidants is their interaction with oxidative free radicals. The DPPH method with the stable organic radical DPPH is used for the determination of free radical scavenging activity, usually expressed as IC50. The results are expressed as IC50, the amount of antioxidant necessary to decrease the initial concentration of DPPH by 50%. The lower IC50 values indicate a higher antioxidant activity.[Citation1]
Antibacterial activity
The effects of H. lineata extract the tested bacteria strains were presented in . The antibacterial activities of the methanol extract of H.lineata against bacteria were determined by evaluating the presence of MIC values. The obtained results showed that the tested extracts possessed different antibacterial activity within the concentration range from 100 to >100 μg/mL. Increased concentrations of extracts caused decrease in survival of bacterial cells. Lower antibacterial effect was demonstrated against bacteria strains (MIC was 100 and > 100 μg/mL) in H. lineata extracts.
Table 4. Antibacterial activity of the H. lineata methanol extracts.
Cytotoxic activity
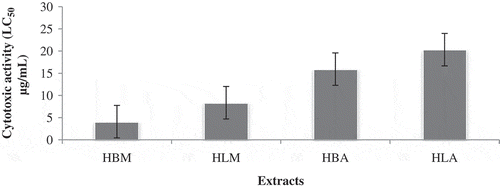
The brine shrimp lethality assay represents a rapid, inexpensive and simple bioassay for testing plant extracts bioactivity which in most cases correlates reasonably well with cytotoxic and anti-tümor properties. Based on the results, the methanol extract (HBM) of H.lineata has showed good toxic to brine shrimp nauplii, with LC50 of 4.105 μg/ml. In addition, the degree of lethality was found to be directly proportional to the concentration of the extract ().
İnsectisidal activity against Cx. pipiens
Toxicities of methanol extracts from H.lineata to young (second and third) instar Cx. pipiens larvae were noted and the LC50 confidence limits for 24, 48, and 72h were calculated (). All the extracts tested demonstrated significant larvicidal activity on C. pipiens, with LC50 values ranging from 64.3275 to 345.2747 µg/mL. A concentration of 1000 µg/mL of the extract was found to be 100% larvicidal. The bulb extract of H.lineata showed highest larvicidal activity against Cx pipiens with value LC50 (64.3275 µg/mL). The insecticidal activity of plant based products (extracts) against different mosquito species has been evaluated by many authors.[Citation28] This study is the first to report on the larvicidal activity of the extracts of H.lineata species against Cx. pipiens ().
Table 5. Larvicidal activity the methanol extracts of H.lineata against Cx. pipiens (% Mortality ± SE).
Table 6. LC50 (24,48 and 72 h) and LC90values of the extracts against Cx. pipiens.
Conclusıon
In conclusion, this ptesents detailed report on the biological activities and chemical compositions of the endemic species H.lineata. It was concluded that, among all the extracts, the methanol and acetone extract of H.lineata exhibited the maximum amount of polyphenolic (total phenolic and total flavonoids) compounds along with the excellent antioxidant activities as revealed by FRAP, ABTS, β-carotene, and DPPH assays. The methanolic extracts, based on HPLC analysis, were mostly represented by phenolic acids. It is apparent that H.lineata contains compounds that might improve the action of natural dietary antioxidant. The cytotoxicity exhibited by the present plants clearly indicates the presence of potent bioactive compounds and they might have insecticidal or pesticidal activity. Therefore, it is suggested that further work be performed on the isolation and identification of the bioactive compounds, which may be useful for therapeutic purpose.
References
- Yıldız, H. Chemical Composition, Antimicrobial, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. International Journal of Food Properties 2016, 19,1593–1603.
- The Plant List. http://www.theplantlist.org/browse/A/Asparagaceae (2010).
- Güner, A.; Özhatay, N.; Ekim, T.; Başer, K.H.C. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, 2000, Vol. 11, ( Supp.2).
- Tekin, M.; Meriç, Ç. Morphological and anatomical investigations on endemic Hyacinthella acutiloba in Turkey. Biological Diversity and Conservation 2013, 8, 161–168.
- Kandemir, N.; Akçin, Ö.E.; Cansaran, A. A Morphological and Anatomical Investigation on Some Geophytes Distributed in the Vicinity of Amasya. Journal of Systematic Botany Weed 2000, 7, 127–147.
- Selvi, S.; Erdoğan, E.; Daşkın, R. The Morphological, Anatomical and Ecological Research on the Hyacinthella lineata (Liliaceae). Ecology 2008, 17(68), 24–32.
- Çelik, A.; Çiçek, M.; Semiz, G.; Karıncalı, M. Taxonomical and Ecological Investigations on Some Geophytes Growing Around Denizli Province (Turkey). Turk Journal Botanic 2004, 28, 205–211.
- Akpulat, H.A.; Celik, N. Flora of Gypsum Areas in Sivas in the Eastern Part of Cappadocia in Central Anatolia, Turkey. Journal of Arid Environments 2005, 61, 27–46.
- Atayeter, E. The morphological and anatomical characteristics of some endemic Hyacinthella Schur (Liliaceae) taxa. 2007. M.Sc. Thesis, Selçuk University, Konya.
- Arslan, E. Polymorphism and phylogenetic relations among Turkish species in Genus Hyacinthella (Liliaceae) as determined by RAPD-PCR. Journal of the Science Faculty of Arts and Sciences 2004, 23, 27–32.
- Yetişen, Y.; Özdemir, K.; Küçüködük, C., Akyol, M. A Morphological and Anatomical Study of Hyacinthella Glabrescens (Liliaceae). Phytologia Balcanica 2012, 18, 319–322.
- Persson, K.; Wendelbo, P. Hyacinthella Schur. In Flora of Turkey and the East Aegean Islands; Davis, P.H. Ed.; Edinburgh Univ. Press: Edinburgh, 1988; Vol. 8., 274–279.
- Mammadov, R.;Ili, P.;Ertem Vaizogullar, H. Antioxidant Activity And Total Phenolic Content Of Gagea Fibrosa and Romulea Ramiflora. Iranıan Journal Chemistry and Chemical Engineering. (IJCCE) 2011, 30(3),23–29.
- Parvu, M.; Toiu, A.; Vlase L.; Parvu E. Determination of Some Polyphenolic Compounds from Allium Species by HPLC-UV-MS. Natural Product Research 2010, 24(10), 1318–1324.
- Amin, I.; Tan, S. Antioxidant Activity of Selected Commercial Seaweeds. Malaysian Journal of Nutrition 2002, 8(2), 167–177.
- Wu, C.; Chen, F.; Wang, X.; Kim, H.; He, G.; Haley-Zitlin, V., Huang, G. Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extract and their Chromatographic Quantification. Food Chemistry 2006, 96, 220–227.
- Oyaizu, M. Studies on Products of Browning Reactions: Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Japanese Journal of Nutrıtıon. 1986, 103, 413–419.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. Antioxidants Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Slinkard, K.; Singleton, V.L. Total Phenol Analyses: Automation and Comparison with Manual Methods. American Journal Enology Viticulture 1977, 28, 49–55.
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A., Legret, P. Standardization of Propolis Extract and İdentification of Principal Constituents. Journal de Pharmacie de Belgique 1994, 49(6), 462–468.
- Satyajit, D.; Sarker, L.N.; Kumarasamy, Y. Microtitre Plate Based Antibacterial Assay İncorporating Resazurin As İndicator Of Cell Growth, And İts Application İn The İn Vitro Antibacterial Screening Of Phytochemicals. Methods 2007, 42, 321–324.
- Maclaughlın, J.L.;Rogers, L.; Anderson, J.E. The Use of Biological Assays to Evaluate Botanicals. Drug Information Journal 1994, 17,–1–13.
- Cetin, H.; Yanikoglu, A. A Study of the Larvicidal Activity of Origanum (Labiatae) Species from Southwest Turkey. Journal Vector Ecology 2006, 31, 118–122.
- Faten Y.; Rym T.; Abennacer B.; Olfa E.; Samir D; Mohamed B.; Chokri M. Essential Oil and Phenolic Compounds of Artemisia herba-alba (Asso.): Composition, Antioxidant, Antiacetylcholinesterase, and Antibacterial Activities. International Journal of Food Properties 2016, 19, 1425–1438.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Evaluation of Antioxidant Activities in Relation to Total Phenolics and Flavonoids Content of Selected Malaysian Wild Edible Plants by Multivariate Analysis. International Journal of Food Properties 2014, 17(8), 1763–1778.
- Ruberto, G.; Baratta, M.T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chemistry 2000, 69, 167–174.
- Elango, G.; Rahuman. A.A.; Bagavan, A., Kamaraj, C., Zahir, A.A.; Venkatesan, C. Laboratory Study on Larvicidal Activity of Indigenous Plant Extracts Against Anopheles Subpictus and Culex Tritaeniorhynchus. Parasitol Research 2009, 104, 1381–1388.