ABSTRACT
The nutritional value of the rhizomes of Kaempferia angustifolia was measured through the antioxidant properties of various extracts and the determination of the bioactive compounds. The chloroform and methanol extracts of the rhizomes of Kaempferia angustifolia showed strong free radical scavenging activities against 1,1-diphenyl-2-picrylhydrazyl (DPPH) with values of 615.92 mg Trolox equivalent (TE)/g each. The methanol extract also exhibited the strongest antioxidant properties in the azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) assay with a value of 38.87 mg TE/g. The hexane extract gave the strongest reducing ability in cupric reducing antioxidant capacity (CUPRAC) assay with a value of 901.76 mg TE/g, whilst the ethyl acetate extract exhibited the strongest reducing ability against ferric reducing antioxidant power (FRAP) with a value of 342.23 mg TE/g. Column chromatographic separation on the rhizomes of Kaempferia angustifolia afforded boesenboxide (1), crotepoxide (2), 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3), kaempfolienol (4), and zeylenol (5), which were elucidated by spectroscopic methods. Kaempfolienol (4) was the strongest free radical agent against DPPH with a value of 443.92 mg TE/g, whilst 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) exhibited the strongest antioxidant properties with values of 42.23, 1497.22, and 781.53 mg TE/g against ABTS, CUPRAC, and FRAP assays, respectively.
Introduction
Metabolism of cell tissues is an essential process for human beings, and it produces oxidant by-products such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). However, excessive production of ROS and RNS can induce oxidative stress, leading to cellular macromolecule damages including DNA lesion and subsequent cellular apoptosis. Free radical-induced oxidative stress has been correlated to various pathogenesis and pathophysiology of chronic diseases including cataract, aging, atherosclerosis, Alzheimer’s disease, Parkinson’s disease, osteoarthritis, diabetes, and cancer as reported earlier.[Citation1–Citation4] Previous studies have shown that dietary antioxidant is a good cancer preventive agent, and antioxidants have been a well-practiced prescription medicine in cancer treatments to reduce side effects and increase the response rate of the therapies.[Citation5]
In the past few decades, phytochemicals from botanical sources have been widely used as anticancer, anti-inflammatory, antitumor, antioxidant, and antimicrobial agents due to their therapeutic efficacies and minimum side effects.[Citation6] Catechin, kaempferol, quercetin, gallic acid, caffeic acid, and α-tocopherol are among the examples of botanical constituents that have been reported to exert good antioxidant and anticancer properties.[Citation7] Zingiberaceae is the largest ginger family of Angiosperms comprising more than 1300 species which have been commonly used as spices and medicinal herbs.[Citation8–Citation10] Kaempferia is one of the genus under the ginger family which is composed of approximately 60 species of small rhizomatous herbs, with some being medicinal rhizomes. One of the medicinal rhizomes of the genus is Kaempferia angustifolia Rosc. in Java.[Citation11] K. angustifolia is less well-known in Malaysia as compared to other Kaempferia species such as Kaempferia rotunda Linn and Kaempferia galanga Linn. It is stemless and tuberous and is distributed widely in forests of Western and Central Java of Indonesia as well as some parts of Thailand. It is locally known as Kunci pepet, Kunci menir, Kunci kunot, and Thao Nhang Haeng.[Citation12] K. angustifolia has a pleasant smell and is often used as masticatory by locals for medicinal purposes such as remedy for cold, cough, stomach ache, diarrhoea, fever, and dysentery. It was reported that phytochemicals including chalcone, triterpene, and cyclohexane oxide derivatives as well as the hexane extract of K. angustifolia exhibited prominent cytotoxicity towards human promyelocytic leukaemia (HL-60) and human breast adenocarcinoma (MCF-7) cell lines.[Citation13,Citation14] Previous studies on the species were mostly carried out on the insecticidal, anti-inflammatory, and anticancer properties of the plant and phytochemicals, and these have shown promising results indicating the potential of phytochemicals and extracts of the species to be developed as pharmaceutical and health-promoting product.[Citation14,Citation15] However, limited studies have been carried out especially on the antioxidant aspects of the plant and its phytochemicals, which thus sparked our interest to study the antioxidant properties of the plant. In this study, the phytochemistry and antioxidant properties in terms of free radical scavenging activities and reducing capacities of the K. angustifolia will be reported.
Methods
Plant material
The rhizomes of K. angustifolia were collected from Java, Indonesia, in 2001 and were identified by Dr. Sugeng Riyanto from Faculty of Pharmacy, Gadjah Mada University. The voucher specimen (No. 16/SR/0601–01) was deposited at the Herbarium of the institution. The plant sample was air dried and cut into small pieces prior to extraction.
Preparation and extraction of plant material
The plant sample (500 g) was extracted successively with hexane, chloroform, ethyl acetate, and methanol. Each solvent extraction which lasted for 3 days (72 h) was repeated twice with the same solvent system. The filtrate was concentrated under reduced pressure using rotary evaporator to give a pale yellow semi-solid residue of hexane extract (2.0 g), a dark green gummy residue of chloroform extract (9.0 g), a yellow semi-solid residue of ethyl acetate extract (2.0 g), and a dark brown gummy residue of methanol extract (2.0 g).
Fractionation and isolation of chemical constituents
The extracts were gravity chromatographed under silica gel Merck Kieselgel 60 PF254 No. 7734 (70–230) and No. 9385 (230–400) mesh using solvent mixtures of hexane, ethyl acetate, and methanol in the order of increasing polarity as eluents. Fractions were collected and pooled on the basis of their similarities in thin-layer chromatography (TLC) profiles. All TLC analyses were conducted on Merck DC-Alufolien 60 F254 and Merck DC-Plastikfolien 60 F254. The potential fractions were further purified by column chromatography and recrystallization to give pure compounds.
The isolation and purification of the chloroform extract resulted in colourless needles of boesenboxide (1, 5.1 mg) and crotepoxide (2, 22.8 mg) along with an amorphous white solid named zeylenol (5, 100. 0 mg) and white needles of kaempfolienol (4, 4.0 mg). The ethyl acetate extract was gravity chromatographed to give yellow crystals of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3, 19.9 mg), kaempfolienol (4, 6.0 mg), and crotepoxide (2, 100.0 mg). The fractionation and separation of the methanol extract gave 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3, 23.4 mg), zeylenol (5, 230.1 mg), and boesenboxide (1, 5.0 mg).
Characterization of chemical constituents
The structures of the chemical constituents were elucidated using spectroscopic techniques including fourier transform infrared spectroscopy (FTIR), liquid chromatography mass spectrometry (LCMS), electron impact mass spectrometry (EIMS), ultraviolet-visible (UV-Vis) and nuclear magnetic resonance (NMR) as well as by comparison with literature.[Citation13,Citation16,Citation17]
In vitro antioxidant assays
The nonenzymatic antioxidant properties of the extracts and isolated chemical constituents of the plant were evaluated through hydrogen atom transfer (HAT)- and single-electron transfer (SET)-based methods. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) were mixed HAT–SET-based assays, while CUPRAC and FRAP assays were SET-based assays.[Citation18] The assays investigated the free radical scavenging activities or reducing capacities of the extracts and chemical constituents by employing chromogenic redox reagents. All samples were prepared in concentrations of 1000 ppm. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was used as the standard in the assays. The antioxidant properties of the samples in the assays were expressed in mg Trolox equivalent (TE)/g of sample, and the calibration curve of Trolox was plotted to evaluate the antioxidant properties of the sample. The absorbance of the sample mixtures in the assays was read via spectrophotometer (BMG Labtech, Germany). All the assays were done in duplicate and were repeated thrice independently. The results were expressed as the mean ± standard deviation (SD).
DPPH assay
The free radical scavenging activity of the sample was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) based on the method described previously.[Citation19] In short, the methanolic DPPH stock solution was prepared by dissolving 40 mg of DPPH in 100 mL methanol. The stock solution was diluted to obtain the absorbance of 1.0 ± 0.01 unit at a wavelength of 517 nm. A sample of 50 µL was mixed with 1.0 mL of methanolic DPPH solution and was allowed to stand in the dark for 30 min at room temperature. The free radical scavenging activity of the sample was measured by spectrophotometer (BMG Labtech) at a wavelength of 517 nm.
ABTS assay
ABTS was used as the chromogenic reagent to determine the free radical scavenging activity of the sample according to the method reported previously[Citation20] with minor modification. Briefly, the working solution was prepared by mixing 7.4 mM of ABTS solution and 2.6 mM potassium persulfate (K2S2O8) solution and was allowed to stand in the dark for 12 h at room temperature to generate the ABTS radical cation. The working solution was diluted to obtain an absorbance of 1.1 ± 0.02 unit at a wavelength 734 nm. About 1.0 mL of diluted working solution was then mixed with 50 µL of sample or Trolox standard and was allowed to stand in the dark for 30 min at room temperature. The free radical scavenging activity of the sample was measured by spectrophotometer (BMG Labtech) at a wavelength 734 nm.
CUPRAC assay
The reducing power of the sample was evaluated by using the adapted method described previously[Citation21] with slight modifications. In brief, the CUPRAC reagent was prepared by mixing 10 mM copper(II) chloride (CuCl2), 7.5 mM neocuproine (Nc), and 1 M ammonium acetate buffer of pH 7.0. About 1.0 mL of CUPRAC reagent was mixed with 50 µL of sample or Trolox standard and was allowed to stand in the dark for 30 min at room temperature. The reducing power of the sample was measured by spectrophotometer (BMG Labtech) at a wavelength of 450 nm.
FRAP assay
The reducing capacity of the sample was investigated by applying the method reported previously.[Citation19] Briefly, the FRAP reagent was freshly prepared by mixing 300 mM acetate buffer of pH 3.6, 10 mM of 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM hydrochloric acid (HCl), and 20 mM iron(III) chloride hexahydrate (FeCl3-6H2O). About 1.0 mL of FRAP reagent was mixed with 50 µL of sample or Trolox standard and was allowed to stand in the dark for 30 min at room temperature. The reducing capacity of the sample was measured by spectrophotometer (BMG Labtech) at a wavelength of 595 nm.
Results
Identification of compounds 1–6
Phytochemical study of the rhizomes of the K. angustifolia afforded several classes of secondary metabolites including cyclohexane oxide derivatives, terpene, and flavonoid. Cyclohexane oxide derivatives were the major compounds present in the plant.
Boesenboxide (1)
Colourless needle; MS (LCMS) m/z (rel. intensity): 871.2221 [2M+Na]+ (12), 463.0797 (16), 447.1055 (100), 425.1235 (68); Rf: 0.60 (hexane:acetone 3:2); IR (UATR) vmax cm−Citation1: 3100, 2950, 1746 (C = O), 1726, 1600, 1450, 1372, 1266 (C-O), 712; UV (EtOH) λmax, nm (log ԑ): 301 (3.02), 276 (3.35), 226 (3.96), 203 (3.90); 1D and 2D NMR spectrum data were in good agreement with the published data.[Citation13]
Crotepoxide (2)
Colourless needle; MS (LCMS) m/z (rel. intensity): 747.1936 [2M+Na]+ (7), 401.0654 (13), 385.0912 (86), 363.1093 (100); Rf: 0.47 (Hexane:Acetone-3.5:1.5); IR (UATR) vmax cm−Citation1: 3024, 1740 (C = O), 1442, 1373, 1237 (C-O), 1048, 899, 714; UV (EtOH) λmax, nm (log ԑ): 273 (3.33), 225 (3.95), 203 (3.90); 1D and 2D NMR spectrum data were in good agreement with previously reported data.[Citation13]
2ʹ-hydroxy-4,4ʹ,6ʹ-trimethoxychalcone (3)
Yellow crystal; EIMS m/z (rel. intensity): 314 [M]+ (100), 297 (13), 207 (44), 180 (37), 143 (23), 134 (46), 121 (61); Rf: 0.55 (hexane:acetone 3.5:1.5); IR (UATR) vmax cm−Citation1: 3696 (OH), 2920, 1617 (C = O), 1564, 1216 (C-O), 1166, 827; UV (EtOH) λmax, nm (log ԑ): 358 (4.21), 205 (4.17); 1D and 2D NMR spectra data were in good agreement with previously reported data.[Citation13]
Kaempfolienol (4)
White needle; EIMS m/z (rel. intensity): 336 [M-H2O]+ (3), 318 (6), 293 (8), 275 (13), 257 (8), 123 (100), 109 (46), 95 (38), 81 (33); Rf: 0.55 (hexane:acetone 3.5:1.5); IR (UATR) vmax cm−Citation1: 3431 (OH), 2938, 1723, 1463, 1378, 1027 (C-O); UV (EtOH) λmax, nm (log ԑ): 276 (2.27), 204 (3.69); 1D and 2D NMR spectra data were in good agreement with previously reported data.[Citation13]
Zeylenol (5)
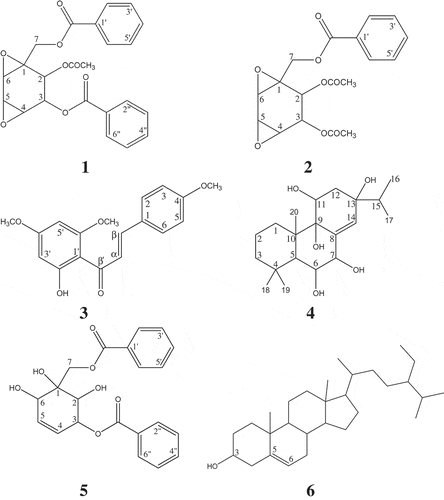
Amorphous white solid; MS (LCMS) m/z (rel. intensity): 791.2348 [2M+Na]+ (79), 407.1118 (79), 385.1297 (50); Rf: 0.52 (hexane:acetone 3.0:2.0); IR (UATR) vmax cm−Citation1: 3443 (OH), 3067, 2928, 1706 (C = O), 1273 (C-O), 1113, 709; UV (EtOH) λmax, nm (log ԑ): 275 (2.93), 226 (4.10), 203 (3.88); 1D and 2D NMR spectrum data were in good agreement with previously reported data.[Citation13] The structures of the chemical constituents isolated from the plant species are shown in .
Antioxidant properties of extracts and isolated compounds
The antioxidant properties of the plant extracts and isolated compounds in this study were evaluated by measuring the abilities of the extracts and compounds to scavenge free radicals, DPPH• and ABTS+• in DPPH and ABTS assays, respectively, and reduce the copper(II) ion (CuCitation2+) and iron(III) ion (FeCitation3+) in CUPRAC and FRAP assays, respectively. This is the first report of the antioxidant properties of extracts and phytochemicals of K. angustifolia against DPPH, ABTS, CUPRAC, and FRAP assays as shown in .
Table 1. Antioxidant activity of Kaempferia angustifolia (rhizomes) and its phytochemicals.
DPPH radical scavenging properties
DPPH is a stable organic nitrogen-centred free radical that can be scavenged by electron or hydrogen atom donation from antioxidant through SET or HAT mechanism.[Citation20] DPPH radical acts as a chromogenic redox reagent to indicate the endpoint of antioxidant reaction. As shown in , both the chloroform and methanol extracts showed similar strengths against DPPH radical with a value of 615.92 mg TE/g. Meanwhile, kaempfolienol (4) is the strongest radical scavenger with a value of 443.92 mg TE/g. The strength of DPPH free radical scavenging activities of the extracts and compounds is in the order of: chloroform and methanol extracts > ethyl acetate extract > kaempfolienol (4) > hexane extract > 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) > zeylenol (5) > crotepoxide (2) > boesenboxide (1).
ABTS radical scavenging properties
ABTS is a decolourization assay based on the scavenging ability of antioxidant against long-life ABTS radical cation which is intensely coloured and generated by oxidation of ABTS with potassium persulfate.[Citation22] ABTS radical cation was chromogenic redox reagent employed in the assay to monitor antioxidant reaction. The methanol extract showed the strongest free radical scavenging activity in ABTS assay with a value of 38.87 mg TE/g of extract (). In ABTS assay, 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) exhibited the strongest activity followed by crotepoxide (2) with values of 42.23 mg TE/g and 41.46 mg TE/g respectively. Interestingly, kaempfolienol (4), which showed good DPPH radical scavenging activity, turns out to be weak in ABTS assay with a value of 28.00 mg TE/g. ABTS free radical scavenging activity of the extracts and compounds is in the order of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) > crotepoxide (2) > methanol extract > boesenboxide (1) > kaempfolienol (4) > chloroform extract > kaempfolienol (4) > ethyl acetate extract > hexane extract.
CUPRAC reducing powers
CUPRAC is an assay based on the reduction of CuCitation2+ to Cu+ by antioxidant as the measurement for the evaluation of antioxidant properties of the sample with copper(II)-neocuproine (Cu(II)-Nc) as the transition metal-based chromophore.[Citation22] In , the CUPRAC assay showed that all the isolated compounds possess stronger antioxidant activities than the extracts with 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) as the most potent reducing agent with the value of 1497.22 mg TE/g. The CUPRAC reducing power of the extracts and compounds is ranked as: 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) > kaempfolienol (4) > boesenboxide (1) > crotepoxide (2) > zeylenol (5) > hexane extract > methanol extract > ethyl acetate extract > chloroform extract.
FRAP reducing powers
FRAP assay measures the reduction of FeCitation3+-TPTZ to FeCitation2+-TPTZ by antioxidants for the evaluation of antioxidant properties of the sample with ferric-tripyridyltriazine (Fe(III)-TPTZ) as the transition metal-based chromophore.[Citation22] As shown in , the ethyl acetate extract showed the strongest reducing power in the FRAP assay with a value of 342.23 mg TE/g. In FRAP assay analysis, the chemical constituents showed good reducing antioxidant power with values more than 80 mg TE/g. Boesenboxide (1), crotepoxide (2), kaempfolienol (4), and zeylenol (5) showed good reducing powers with values of 149.24 mg TE/g, 241.71 mg TE/g, 100.13 mg TE/g, and 89.01 mg TE/g, respectively, whilst 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) showed relatively stronger reducing power than the others with a value of 781.53 mg TE/g. The FRAP reducing power of the extracts and compounds is ranked in the following order: 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) > ethyl acetate extract > chloroform extract > crotepoxide (2) > methanol extract > hexane extract > boesenboxide (1) > kaempfolienol (4) > zeylenol (5).
Discussion
Boesenboxide (1), crotepoxide (2), and zeylenol (5) are cyclohexane oxide derivatives which showed quasimolecular ions in liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS). Boesenboxide (1) showed quasimolecular ions at m/z 871.2221 [2M+Na]+ (calculated for C46H40O16Na, 871.7883 g/mol), 463.0797 [M + K]+ (calculated for C23H20O8 K, 463.4976 g/mol), 447.1055 [M+Na]+ (calculated for C23H20O8Na, 447.3890 g/mol), and 425.1235 [M + H]+ (calculated for C23H21O8, 425.4072 g/mol) which indicated the molecular formula of C23H20O8. Crotepoxide (2) gave quasimolecular ions at m/z 747.1936 [2M+Na]+ (calculated for C36H36O16Na, 747.6497 g/mol), 401.0654 (calculated for C18H18O8K, 401.4283 g/mol), 385.0912 (calculated for C18H18O8Na, 385.3197 g/mol), and 363.1093 (calculated for C18H19O8, 363.3379 g/mol) which indicated the molecular formula of C18H18O8. Zeylenol (5) showed quasimolecular ions, [2M+Na]+, [M+Na]+, and [M + H]+ at m/z 791.2348 (calculated for C42H40O14Na, 791.7467 g/mol), 407.1118 (calculated for C21H20O7Na, 407.3682 g/mol), and 385.1297 (calculated for C21H21O7, 385.3864 g/mol) which indicated the molecular formula of C21H20O7. The Citation1H NMR spectra of boesenboxide (1), crotepoxide (2), and zeylenol (5) showed two doublets at δ 4.70–4.20 (J = 11.9–12.6 Hz) which were the characteristics for the two unequivalent methylene protons (H-7). The Citation1H and Citation13C NMR spectra of boesenboxide (1) and crotepoxide (2) were similar with a slight difference on the number of acetoxyl substituent present. Zeylenol (5) was slightly different from boesenboxide (1) and crotepoxide (2) with the presence of hydroxyl and benzoyloxy groups at C-2 and C-3, respectively, as well as the absence of epoxide group due to epoxide ring opening. Boesenboxide (1) and zeylenol (5) were found previously from K. angustifolia as new compounds.[Citation17] Crotepoxide (2) was first isolated from Croton macrostachyus Hochst. Ex Del. as a novel cyclohexane diepoxide derivative with tumour-inhibitory property.[Citation23]
2ˊ-Hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3), also known as flavokawain A, is an open ring flavonoid widely synthesized in the plant kingdom.[Citation24] It showed a molecular ion M+ peak at m/z 314 in electron impact mass spectrometry (EIMS) which indicated the molecular formula of C18H18O5 with calculated molecular weight of 314.3318 g/mol.[Citation13] The Citation1H and Citation13C NMR spectra of 2ʹ-hydroxy-4,4ʹ,6ʹ-trimethoxychalcone (3) showed three methoxyl groups integrating for three protons each resonating as singlets at δ 3.83 (4ˊ-OCH3), 3.85 (4-OCH3), and 3.91 (6ʹ-OCH3) in the Citation1H NMR spectrum, whilst the signal peaks of the three carbons of these methoxyl groups were seen at δ 55.38, 55.55, and 55.81 in the Citation13C NMR spectrum. A singlet at δ 14.40 in the spectrum was assigned to the proton of the hydroxyl group (2ˊ-OH) in the compound. A pair of AAˊBBˊ type of doublet of doublets was observed in the Citation1H NMR spectrum at δ 6.91 and 7.55 with coupling constants, J values of 2.7 and 9.1 Hz, which represented the para-disubstituted aromatic protons (H-2, H-3, H-5, and H-6). Two meta-coupled aromatic protons (H-3ˊ and H-5ˊ) in the compound were assigned to the doublets at δ 5.96 and 6.10 in the Citation1H NMR spectrum with J = 1.8 Hz. 2ˊ-Hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) had been isolated previously as a new natural chalcone from Piper methysticum Forst. which acts as a yellow pigment.[Citation25]
Kaempfolienol (4) is a diterpene that gave a fragment ion, [M-H2O]+ at m/z 336 in EIMS and indicated a molecular formula of C20H34O5 with calculated molecular weight of 354.4796 g/mol.[Citation13] The Citation1H NMR spectrum of the compound integrated for 29 protons with the presence of five methyl signal peaks at δ 1.26, 1.19, 1.01, 0.95, and 0.91, of which the latter two, due to an isopropyl group, resonated as a doublet, indicating that kaempfolienol (4) is an abietane-type diterpene. Four methylene protons in the compound (H-1, H-2, H-3, and H-12) contribute to two signals for each proton at δ 1.56 (H-1), 1.74 (H-1), 1.43 (H-2), 1.67 (H-2), 1.23 (H-3), 1.31 (H-3), 1.60 (H-12), and 1.64 (H-12), which is due to the nonequivalent methylene protons. In the Citation13C NMR spectra of kaempfolienol (4), a signal peak at δ 45.0, with intensity that is onefold higher than other carbon peaks, was attributed by the overlapping of two carbons, C-3 and C-10, in the compound. Kaempfolienol (4) was a new compound that was isolated and identified first from the rhizomes of K. angustifolia.[Citation26]
Among all the compounds, 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) showed the most potent antioxidant properties in ABTS, CUPRAC, and FRAP assays, whilst kaempfolienol (4) exhibited the strongest antioxidant properties among the compounds in the DPPH assay, suggesting that these compounds fulfil the structural requirements for antioxidant properties. The presence of electron-rich groups such as hydroxy, methoxy, and carbonyl groups in 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) enhances the ability of the compound to donate electrons. Moreover, the α-β unsaturated carbonyl moiety in 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) stabilizes the oxidized or radical state of the compound after the donation of electrons via delocalization of an unpaired electron into the π-conjugated system of the ring, which increases the tendency of the compound to donate electrons (). The presence of hydroxyl and methoxyl groups also enhance the hydrogen-donating ability of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) due to the low O-H and O-CH3 bond dissociation energies of hydroxyl and methoxyl groups, respectively ().[Citation27] However, kaempfolienol (4) showed a relatively stronger antioxidant property than 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) in the DPPH assay, suggesting a lower O-H bond dissociation energy of hydroxyl groups in kaempfolienol (4) as compared to O-CH3 bond in methoxyl groups of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3). The presence of more hydroxyl groups in kaempfolienol (4) as compared to 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) enhances the hydrogen-donating ability of compound 4, thus contributing to a stronger antioxidant properties of kaempfolienol (4) as compared to 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) in the DPPH assay. Hence, the results suggested the antioxidant properties of the compounds in the DPPH assay which were evaluated via HAT mechanism. The proposed chemical reaction between kaempfolienol (4) and DPPH free radical is shown in .[Citation18,Citation27]
Figure 2. The proposed chemical reaction of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) in antioxidant assays: DPPH (A), ABTS (B), CUPRAC (C), and FRAP (D) assays via SET mechanism.
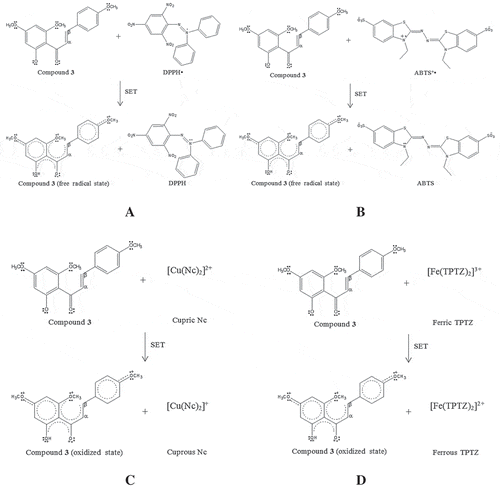
Figure 3. The proposed chemical reaction of 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3) in antioxidant assays: DPPH (A) and ABTS (B) assays via HAT mechanism.
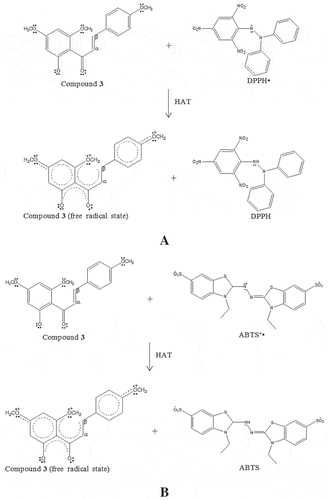
Figure 4. The proposed chemical reaction of kaempfolienol (4) in antioxidant assay DPPH via HAT mechanism.
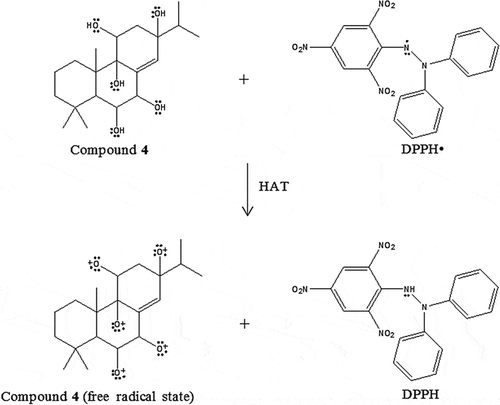
Meanwhile, crotepoxide (2) showed stronger antioxidant property than boesenboxide (1), suggesting that the presence of an electron-releasing group, the methyl group at 3-OCO position of compound 2, improves the electron-donating ability of the compound by increasing the electron density of the oxygen atom at 3-OCO position. In boesenboxide (1), the presence of an electron-withdrawing group, the phenyl group, reduces the electron density of the oxygen atom at 3-OCO position and thus decreases the electron-donating ability of the compound. Furthermore, the presence of the bulky phenyl group in boesenboxide (1) may also attenuate the antioxidant properties of the compound due to the steric hindrance factor (, ). In CUPRAC assay, CuCitation2+ ion has a lower redox potential than FeCitation3+ which makes the CuCitation2+ ion harder to be reduced as compared to FeCitation3+ ion. This is due to the higher stability of CuCitation2+ as compared to Cu+. Therefore, the stability of the oxidized state of the compound may be the main factor that determines the antioxidant strengths of each compound in the CUPRAC assay. In the CUPRAC assay, boesenboxide (1) displayed stronger antioxidant properties than crotepoxide (2), suggesting that the oxidized state of boesenboxide (1) is stable than the oxidized state of crotepoxide (2) (). This phenomenon could be due to the presence of a phenyl group at 3-OCO position of boesenboxide (1), which is absent in crotepoxide (2), which enhances the stability of the oxidized state of the compound by delocalization of unpaired electron into the π-conjugated system of the ring.[Citation22]
Figure 5. The proposed chemical reaction of boesenboxide (1) in antioxidant assays: DPPH (A), ABTS (B), and FRAP (C) via SET mechanism.
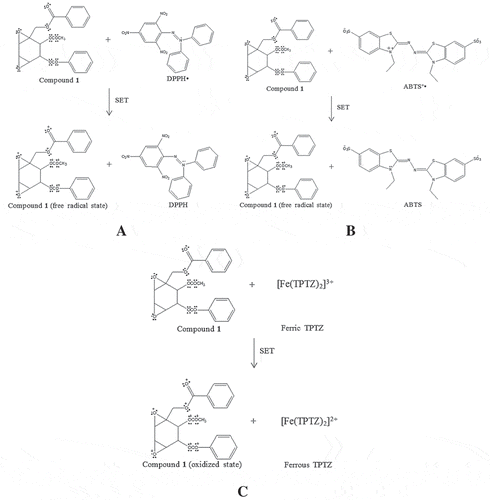
Figure 6. The proposed chemical reaction of crotepoxide (2) in antioxidant assays: DPPH (A), ABTS (B), and FRAP (C) via SET mechanism.
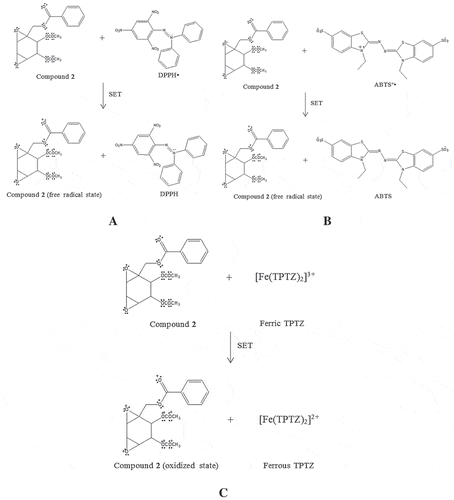
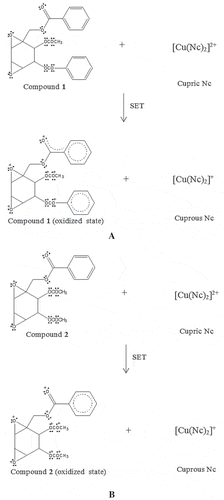
Figure 7. The proposed chemical reaction of boesenboxide (1) (A) and crotepoxide (2) (B) in antioxidant assay CUPRAC via SET mechanism.
Based on the results, zeylenol (5) showed the weakest antioxidant property among the compounds in ABTS, CUPRAC, and FRAP assays, suggesting the possibility of the presence of the least number of oxygen atoms as compared to other compounds in the plant, which reduces the ability of zeylenol (5) to donate electron as compared to other compounds. However, zeylenol (5) displayed moderate antioxidant properties in DPPH assay, which suggested that the presence of hydroxyl group in the compound acts as hydrogen-atom donor and thus enhances the antioxidant properties ().
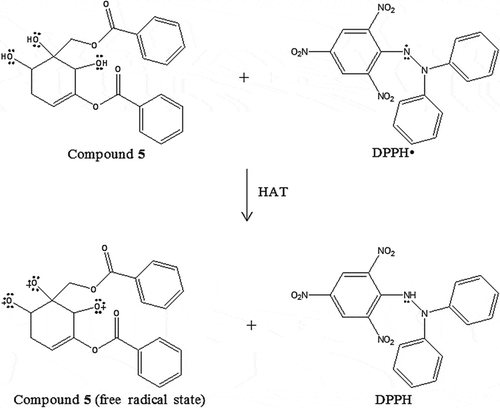
Figure 8. The proposed chemical reaction of zeylenol (5) in antioxidant assay DPPH via HAT mechanism.
On the basis of this result, compounds bearing hydroxyl groups such as 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone (3), kaempfolienol (4), and zeylenol (5) showed relatively strong antioxidant properties in DPPH assay than those without hydroxyl groups such as boesenboxide (1) and crotepoxide (2), suggesting the DPPH assay in the study is HAT mechanism dominant. Contrarily, kaempfolienol (4) and zeylenol (5) showed weaker antioxidant properties among the compounds in the ABTS assay, indicating that the ABTS assay in the study is SET mechanism dominant.[Citation18,Citation27]
In summary, the antioxidant properties of the chemical constituents are closely related to their chemical structures and substituents, in which these criteria define the stability of the oxidized or free radical state of the compound and the ability of the compound to donate an electron or a hydrogen atom. Hence, the high electron density oxygen atom and conjugated π-bond system are suggested to be the main factors that contribute to the strong antioxidant properties of the compound.[Citation28]
Conclusion
Kaempferia angustifolia Rosc. had been used widely by locals as medicine for the treatment of cold, stomach ache, dysentery, and cough as well as masticatory.[Citation13] In this study, the plant extracts exhibited strong antioxidant properties in DPPH, ABTS, CUPRAC, and FRAP assays, which further support its wide applicability for medicine treatments. The chemical constituents afforded from the plant extracts revealed 2ˊ-hydroxy-4,4ˊ,6ˊ-trimethoxychalcone and kaempfolienol as the potent free radical scavengers, suggesting the potential of these compounds to be developed as pharmaceutical drug, particularly for treatment of degenerative diseases. Extended work on the biological effects of these bioactive compounds in vivo such as bioavailability, and temporal and spatial distribution of these compounds in the body will be carried out to provide fundamental knowledge to correlate the health-promoting effects of these compounds.
LJFP_A_1286508_SUPPLEMENTARY_MATERIALS.zip
Download Zip (11.4 KB)Acknowledgment
We would like to extend our sincerest thanks and appreciation to all the science officers of Department of Chemistry (UPM) and Department of Biomedical Science (UPM) as well as School of Chemical Sciences and Food Technology (UKM) for the technical assistance and facilities provided throughout the research study.
Funding
Financial assistances from MyPhD Scholarship of the Ministry of Higher Education and IPS research grant of Universiti Putra Malaysia (No. 9460900) to the first author are gratefully acknowledged.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- López-Alarcón, C.; Denicola, A. Evaluating the Antioxidant Capacity of Natural Products: A Review on Chemical and Cellular-Based Assays. Analytica Chimica Acta 2013, 763, 1–10.
- Sies, H. Oxidative Stress; Academic Press: Orlando, FL, 1985; pp. 1–8.
- Maulik, N.; McFadden, D.; Otani, H.; Thirunavukkarasu, M.; Parinandi, N.L. Antioxidants in Longevity and Medicine. Oxidative Medicine and Cellular Longevity 2013, 2013, 3.
- Toda, S. Polyphenol Content and Antioxidant Effects in Herb Teas. Chinese Medicine 2011, 2(1), 29.
- Simone, C.B.; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and Other Nutrients Do Not Interfere with Chemotherapy or Radiation Therapy and Can Increase Kill and Increase Survival, Part 2. Alternative Therapies in Health and Medicine 2007, 13 (1), 22.
- Kumar, V.; Kaminski, H.J. Treatment of Myasthenia Gravis. Current Neurology and Neuroscience Reports 2011, 11(1), 89–96.
- Roleira, F.M.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant Derived and Dietary Phenolic Antioxidants: Anticancer Properties. Food Chemistry 2015, 183, 235–258.
- Habsah, M.; Amran, M.; Mackeen, M.M.; Lajis, N.H.; Kikuzaki, H.; Nakatani, N.; Rahman, A.A.; Ghafar, A.A.M. Screening of Zingiberaceae Extracts for Antimicrobial and Antioxidant Activities. Journal of Ethnopharmacology 2000, 72, 403–410.
- Pancharoen, O.; Tuntiwachwuttikul, P. Phytochemistry of the Zingiberaceae. Studies in Natural Products Chemistry 2000, 23, 797–865.
- Ficker, C.E.; Smith, M.L.; Susiarti, S.; Leaman, D.J.; Irawati, C.; Arnason, J.T. Inhibition of Human Pathogenic Fungi by Members of Zingiberaceae Used by the Kenyah (Indonesian Borneo). Journal of Ethnopharmacology 2003, 85, 289–293.
- Ashton, H. The Royal Horticultural Society New Encyclopedia of Herbs & Their Uses ( Revised Edition). Reference Reviews 2004, 18(3), 42–43.
- Madaka, F.; Tewtrakul, S. Anti-Allergic Activity of Some Selected Plants in the Genus Boesenbergia and Kaempferia. Songklanakarin Journal of Science and Technology 2011, 33(3), 301–304.
- Tang, S.W.; Sukari, M.A.; Neoh, B.K.; Yeap, Y.S.Y.; Abdul, A.B.; Kifli, N.; Ee, G.C.L. Phytochemicals from Kaempferia angustifolia Rosc. and Their Cytotoxic and Antimicrobial Activities. Biomed Research International 2014, 2014, 6.
- Sulianti, S.B.; Chairul, S.M. Comparison of Volatile Oil Constituent of Two Species of Kunci Pepet (Kaempferia angustifolia Roscue. and K. rotunda Linn.). BioPharm 2005, 3(2), 39–42.
- Seangphakdee, P.; Pompimon, W.; Meepowpan, P.; Panthong, A.; Chiranthanut, N.; Banjerdpongchai, R.; Wudtiwai, B.; Nuntasaen, N.; Pitchuanchom, S. Anti-Inflammatory and Anticancer Activities of (-)-Zeylenol from Stems of Uvaria grandiflora. ScienceAsia 2013, 39(6), 610–614.
- Sukari, M.A.; Neoh, B.K.; Lajis, N.H.; Ee, G.C.L.; Rahmani, M.; Ahmad, F.B.H.; Yusof, U.K. Chemical Constituents of Kaempferia angustifolia (Zingiberaceae). Oriental Journal of Chemistry 2004, 20(3), 451–456.
- Pancharoen, O.; Tuntiwachwuttikul, P.; Taylor, W.C. Cyclohexane Oxide Derivatives from Kaempferia angustifolia and Kaempferia Species. Phytochemistry 1989, 28(4), 1143–1148.
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. Journal of Agricultural and Food Chemistry 2005, 53(6), 1841–1856.
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Analytical Methods 2011, 4(1), 100–107.
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A.; Musa, K.H.; Douqan, E.M. Antioxidant Activity and Physicochemical Properties of Mature Papaya Fruit (Carica papaya L. Cv. Eksotika). Advance Journal of Food Science and Technology 2013, 5, 859–865.
- Özyürek, M.; Bener, M.; Güçlü, K.; Dönmez, A.A.; Süzgeç-Selçuk, S.; Pırıldar, S.; Meriçli, A.H.; Apak, R. Evaluation of Antioxidant Activity of Crataegus Species Collected from Different Regions of Turkey. Records of Natural Products 2012, 6(3), 263–277.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53(10), 4290–4302.
- Kupchan, S.M.; Hemingway, R.J.; Coggon, P.; McPhail, A.T.; Sim, G.A. Tumor Inhibitors. XXIX. Crotepoxide, a Novel Cyclohexane Diepoxide Tumor Inhibitor from Croton macrostachys. Journal of the American Chemical Society 1968, 90(11), 2982–2983.
- Batovska, D.I.; Todorova, I.T. Trends in Utilization of the Pharmacological Potential of Chalcones. Current Clinical Pharmacology 2010, 5, 29.
- Shulgin, A.T. The Narcotic Pepper-The Chemistry and Pharmacology of Piper methysticum and Related Species. Bulletin on Narcotics 1973, 25(2), 59–74.
- Tang, S.W.; Sukari, M.A.; Rahmani, M.; Lajis, N.H.; Ali, A.M. A New Abietene Diterpene and Other Constituents from Kaempferia angustifolia Rosc. Molecules 2011, 16(4), 3018–3028.
- Duroux, P.J.L.; Trouillas, P.; Křen, P.V.; Otyepka, P.M.; Poso, P.A.; Dangles, P.O. New Theoretical Highlighting on the Molecular Interactions of Natural Polyphenols: Penetration in Lipid Membranes and Oxidative Dimerization; School of Pharmacy, University of Limoges: Limoges, French, 2011.
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant Activity of Natural Flavonoids Is Governed by Number and Location of Their Aromatic Hydroxyl Groups. Chemistry and Physics of Lipids 1996, 79(2), 157–163.