ABSTRACT
Pine honey is a honeydew honey that can be produced endemically in eastern Mediterranean. Turkey is the world’s largest producer of pine honey with around 95% export share. Antibiotic residue in honey is still an important problem worldwide. The aim of this study was to determine sulfonamide and tetracycline group antibiotics in pine honey collected from Aegean Region of Turkey. For this purpose, 59 natural pine honey samples were detected by competitive enzyme-linked immunoassay method. Tetracycline group antibiotics were found in 35 honey samples out of 59 between 6 and 42 ppb, whereas in 24 of the samples the levels were below the detection level (< 4 ppb). Sulfonamide group antibiotics were found in 31 honey samples out of 59 between 3 and 32 ppb, while 28 samples were below the detection limit (< 2 ppb). There are no maximum residue limits established for antibiotics in honey according to the European Community regulations, which means honeys should not contain antibiotics.
Introduction
Pine honey is produced by honey bees that collect honeydew from a special insect species called Marchalina hellenica. This species can only live on some certain pine trees such as Pinus brutia and Pinus pinea which are endemic in eastern Mediterranean. M. hellenica feeds by sucking sap from the tree and excretes a viscous honey-like substance which is a white cotton-like fluff. This honeydew protects the insect against predators and attracts bees, so it is collected by bees and converted into pine honey. M. hellenica has one generation per year, because of this honey derived from honeydew has a considerable economic value.[Citation1] Turkey is the world’s largest producer of pine honey with a 90% rate; also pine honey is a significant national export product of Turkey (around 95% export share). Within Turkey, Muğla province (Eagen region of Turkey) is the most productive area of pine honey.[Citation2]
The most important feature of pine honey is that it can be preserved for a long time without texture deterioration or crystallization. Its colour is darker, taste is less sweet, and is less bitter than flower honey. So it can be consumed easily by all ages. Also, pine honey has a wider range of usage area in various products in medicine and food industries.[Citation3]
Besides being healthy and natural, honey is exposed to many kinds of contaminations. The contamination sources can be environmental or apicultural. The most important contaminants from beekeeping are acaricides, pesticides, and antibiotics. Antibiotics have widely been used for preventive and therapeutic measures to diseases. Residues that occur, especially, as a result of unconscious use of antibiotics in order to prevent bee diseases cause food safety issues. Antibiotic residues are also potentially dangerous in terms of public health.[Citation4,Citation5]
In addition to increasing the antibiotic resistance of pathogens, antibiotic residues are known to produce adverse effects on human health. Sulfonamide derivatives consumed with food lead to renal dysfunctions including crystalluria. For both treatment purposes and food pollution, there are reports of possible sulfonamide allergies and anaphylaxis developments defined as 1–2% skin, mucosal lesions, and serum diseases in people due to sulfonamide intake.[Citation6–Citation8] It was reported that penicillin derivatives cause urticaria and advanced anaphylactic shocks, chloramphenicol causes irreversible blood abnormalities, nitrofuran has teratogenic effects, aminoglycosides cause nephrotoxicity, and sulfamethazine causes thyroid hyperplasias.[Citation9,Citation10]
The most important public health protection measures against the potential danger of veterinary drug residues in honey are the legislations regarding this subject. In Turkish Food Codex Honey Communique (2012/53) harmonized with European Commission Honey Directive (2001/110/EC), regulations related to residues in honey and the honeycomb are given. According to maximum residue limits (MRLs) of Veterinary Medicinal Products in Animal Food Regulation referred in Turkish Food Codex Honey Communique, honey should not contain antibiotics. The use of antibiotics in beekeeping is not legal even in EU countries. Moreover, there are no MRLs established for antibiotics in honey according to the European Community regulations, which means that honey containing antibiotic residues is not permitted to be sold. For regulating residues of antibiotics in imported honey, the Union has set Reference Points for Action (RPAs). RPAs are residue concentrations which are technically feasible to detect by food control laboratories. When an RPA is exceeded, the member state is obliged to reject the consignment. Till date, RPAs have been established in honey for substances such as chloramphenicol and nitrofurans. Also, in the CRL guidance paper of 7 December 2007, concentrations for antibiotics were recommended. The purpose of the technical guidance is to improve and harmonize the performance of analytical methods used for the substances for which MRLs have not been established according to the regulations.[Citation11–Citation14]
Determining the presence and level of antibiotic residues in honey produced in Turkey is important both in terms of the public health and honey exports. The aim of this study is to determine the presence and levels of sulfonamide and tetracycline group antibiotics in Aegean Region pine honey.
Material and methods
Sampling
A total of 59 natural pine honey samples collected from honey beekeepers that produce honey from 3 different cities and 13 districts of Aegean Region in Turkey were used as materials. shows the pine tree distribution and pine honey production areas of Turkey. According to the regions production capacities, 16 honey samples from Aydın, 36 from Muğla, and 7 from Balıkesir were obtained. Samples were transferred to the laboratory at 4°C and were kept indoors in glass containers, protected from temperature, light, and moisture until the analysis stage. Samples were taken directly from the beekeepers after harvest without any treatment in order to minimize the preservation and processing-related decrease in the amount of antibiotic residues.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) method was used to determine the antibiotic residues. ELISA technique was performed according to the manufacturer’s instruction by using Ridascreen test kit, R3505 for tetracycline, R3004 for sulfonamide, and R3001 for sulfamethazine. For the tetracycline group antibiotics analysis, 1 g of samples were taken and pretreated with 50 ml 20 nM PBS buffer. ELISA standards were prepared daily prior to analysis. The well in which the standards and samples will be placed was determined and 50 µl was transferred to each well by using a micropipette. A measure of 50 µl anti-tetracycline antibody was added to each well and left to incubation at room temperature for 1 h. Wells were then washed 3 times with approximately 250 μl washing solution and 100 µl conjugate was added to each well and left to incubation for 15 min at room temperature in the dark. Then, 100 µl substrate was added to each well and left to incubation for 15 min in the dark. Following the incubation, 100 µl stop solution was added to the wells and the result was read in 450 nm ELISA reader (Awareness Technology, USA).
For sulfonamide group antibiotic analysis, 1 g of samples were taken to the centrifuge tubes and 6 ml of 50 mM sodium acetate buffer was added. The solution was set to 5 pH. Honey samples were stirred until being completely dissolved at 20–25°C and centrifuged at 3000 g for 10 min. The extract was passed through a C18 column and readied for analysis. Standards were prepared daily prior to analysis. The well in which the standards and samples will be placed was determined and 50 µl was transferred to each well by using a micropipette. A measure of 50 μl conjugate and 50 μl antibody were added to each well and left to incubation at room temperature for 1 h. Wells were then washed 3 times with approximately 300 μl distilled water. A measure of 1000 µl substrate-chromogen was injected to each well for 30 min and left to incubation at room temperature in the dark. Following the incubation, 100 µl stop solution was added to the wells and the result was read in 450 nm ELISA reader in 60 min (Awareness Technology). By the ELISA test kit used for the study, sulfamethoxypyridazine, sulfapyridine, sulfamethoxydiazine, sulfamethoxazole, sulfadimethoxine, sulfaquinoxaline, sulfachloropyridazine, sulfamerazine, sulfadiazine, sulfamethizole, sulfadoxine, sulfachloropyrazine, sulfaguanidine, sulfaphenazole, sulfisoxazole, sulfanilamide, sulfamethazine, and sulfacetamide antibiotics could be detected with a decreasing sensitivity in the specified order. Since sulfamethazine antibiotic is frequently used in beekeeping and the test kit’s sensitivity is low (2%) for this antibiotic, sulfamethazine was detected separately. Sulfamethazine analyses of the samples were carried out with the same method, by using anti-sulfamethazine antibody (R3001 test kit).
Statistical analyses
Statistical analyses were conducted with one-way ANOVA test for the difference between three regions, and p < 0.05 was applied as the minimum level of significance. Statistical software SPSS 14.01 was used for the analyses.
Results and discussion
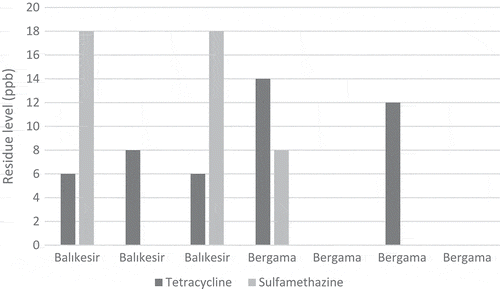
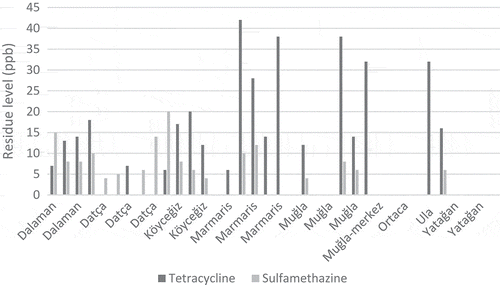
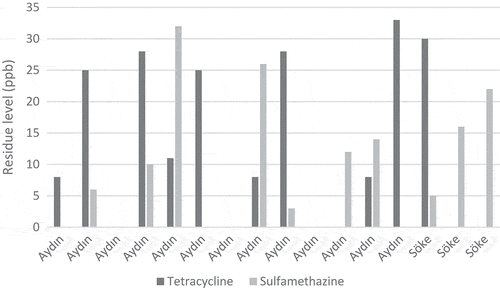
In the study, 59 natural pine honey samples obtained directly from beekeepers producing honey in Aegean region were examined. Tetracycline group antibiotics were found in 35 honey samples between 6 and 42 ppb, whereas in 24 of the samples the levels were below the detection level (< 4 ppb). Sulfamethazine antibiotic was found in 31 honey samples between 3 and 32 ppb, while 28 samples were below the detection limit (< 1 ppb). Sulfonamide group antibiotics were below the limits (< 2 ppb) in all of the samples. Accordingly, sulfamethazine was detected in 59.3% and tetracycline group was detected in 52.5% of the honey samples. – show the tetracycline group and sulfamethazine antibiotic levels in the honey samples taken from the three different regions. The highest amount of tetracycline group antibiotics was 42 and 38 ppb, while the highest amount of sulfamethazine antibiotic was 32 and 26 ppb. There were no statistically significant differences between the three regions results (p > 0.05).
Antibiotic residues are important for the public health, so it is essential to monitor them in food. There are many studies on the different antibiotic residues in honey produced in the world and in Turkey but still not enough information is available in the literature. Saridaki-Papakonstadinou et al.[Citation15] reported that 29% of the honey samples they examined had drug residues of at least one of the tetracycline group (tetracycline, oxytetracycline, chlortetracycline, and doxycycline). Of them 17 (20.3%) samples had residues of more than one antibiotic in the tetracycline group. Güneş et al.,[Citation16] in their study on 50 honey samples collected from bee producers in Marmara region, analysed the samples for oxytetracycline and sulfonamide antibiotic groups with LC-MS/MS and found no residues. The results show that the beekeepers in the region are not using sulfonamide group antibiotics. Erdoğdu et al.[Citation17] carried out the analysis for sulfonamide derivative antibiotics for 536 honey samples with LC-MS/MS. Although the ratio of the samples in which residues were detected (23%) was lower than our study, residue levels were found to be very high (> 200 mg/kg).
In another study on the honeys produced in Turkey by Sunay,[Citation18] it was determined that 25% of the samples contained sulfonamide and tetracycline group antibiotics. This result shows that the residue levels determined were similar to the results obtained in our study. In a study carried out in Belgium, in 72 honey samples obtained from producers, 4.2% sulfonamide and 2.8% tetracycline group antibiotics were determined.[Citation6] In another study carried out in the United States, residues were detected in 38% of the honey samples tested for sulfonamide group and chloramphenicol. This result is lower than the example ratio in which antibiotic was detected in this study (68.75%). Mahmoudi et al.[Citation19] investigated antibiotic residues in honey samples collected randomly from Qazvin Province, Iran, by ELISA. Result shows that the highest percentage of antibiotic residues in honey samples was the enrofloxacin (20.7%) followed by gentamicin (18.15%), sulfonamide (14.81%), and tetracycline (14.07%) residues.
The differences between the results of the researches are obviously dependent on the method used in the detection of antimicrobials. Two main approaches are the screening tests and chromatographic methods for determination of antibiotic residues. Both methods have advantages and disadvantages. Screening tests are useful in detecting the group of analytes also, they have short analysis time, and good selectivity. Their simplicity and cost-effectiveness make them particularly useful in routine work. Chromatographic methods are useful for detection of residues of antimicrobial drugs in honey at trace levels. However, most of them are limited to one class of compounds, have a long analysis time, and are expensive. Also advanced sample-preparation techniques have to be applied especially to honey because of the complex composition of the matrix.[Citation20]
Conclusion
Today, the potential hazard of antibiotic resistance to the public health is well known. Because of the uncontrolled sale of veterinary drugs and their unconscious use, antibiotic residues in Turkish pine honey cause significant problems in export. In this study, it was found that significant part of the honey samples examined in the study contained sulfonamide and/or tetracycline group antibiotic residues. The findings are evidence of a rising problem with possible health consequences to honey consumers. It was concluded that supervision of sale and use of veterinary drugs within the framework of national residue monitoring plan for public health is of great importance.
References
- Bacandritsos, N. Establishment and Honeydew Honey Production of Marchalina hellenica (Coccoidea Margarodidae) on Fir Tree (Abies cephalonica). Bulletin of Insectology 2004, 57 (2), 127–130.
- Miguel, S.; Pukkala, T.; Yesil, A. Integrating Pine Honeydew Honey Production into Forest Management Optimization. European Journal of Forest Research 2014, 133, 423–432.
- Tananaki, C.; Thrasyvoulou, A.; Giraudel, J.L.; Montury, M. Determination of Volatile Characteristics of Greek and Turkish Pine Honey Samples and Their Classification by Using Kohonen Self Organising Maps. Food Chemistry 2007, 101, 1687–1693.
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Science World Journal 2012, 2012, 1–9.
- Bogdanov, S. Contaminants of Bee Products. Apidologie 2006, 37, 1–18.
- Reybroeck, W. Residues of Antibiotics and Sulphonamides in Honey on the Belgian Market. Apiacta 2003, 38, 23–30.
- Sheridan, R.; Policastro, B.; Thomas, S.; Rice, S. Analysis and Occurrence of Sulfonamide Antibacterials and Chloramphenicol in Honey by Solid-phase Extraction Followed by LC-MS/MS Analysis. Journal of Agricultural and Food Chemistry 2008, 56 (10), 3509–3516.
- Tittlemier, S.A.; Riet, J.V.D.; Burns, G.; Potter, R.; Murohy, C.; Rourke, W.; Pearce, H.; Dufresne, G. Analysis of Veterinary Drug Residues in Fish and Shrimp Composites Collected during the Canadian Total Diet Study, 1993–2004. Food Additives and Contaminants 2007, 24, 14–20.
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecological Indicators 2008, 8 (1), 1–13.
- Wang, S.; Xu, B.; Zhang, Y.; He, J.X. Development of Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Neomycin Residues in Pig Muscle, Chicken Muscle, Egg, Fish, Milk and Kidney. Meat Science 2009, 82, 53–58.
- Anon. 2002/657/EC Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Official Journal of The European Communities, Brussels.
- Anon. Turkish Food Codex Communiqué on Honey (No:2005/49). Official Journal of Turkish Republic, Ankara.
- Anon. Turkish Food Codex, Regulation on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (No:2012/28282). Official Journal of Turkish Republic, Ankara.
- Anon. CRL Guidance Paper (7 December 2007). CRLs View on State of the Art Analytical Methods for National Residue Control Plans. Community Reference Laboratories (CRLs) for Residues According to Council Directive96/23/EC.
- Saridaki-Papakonstadinou, M.; Andredakis, S.; Burriel, A.; Tsachev, I. Determination of Tetracycline Residues in Trakia Journal of Sciences 2006, 4 (1), 33–36.
- Güneş, M.E.; Güneş, E.; Cıbık, R. Oxytetracycline and Sulphonamide Residues Analysis of Honey Samples from Southern Marmara Region in Turkey. Bulgarian Journal of Agricultural Science 2009, 15,163–167.
- Erdoğdu, A.T.; Coşkun, Y.; Güven, S.İ. Determination of Sulphonamide Antibiotics Residues in Honey Presented for Consumption. The Journal of Bornova Veterinary Science 2011, 33, 37–44.
- Sunay, A.E. Antibiotic Residue problem in Honey. Uludag Bee Journal 2006, 4, 43–148.
- Mahmoudi, R.; Reza, N.; Mohammadreza, P.A. Antibiotic Residues in Iranian Honey by Elisa. International Journal of Food Properties 2014, 17 (10), 2367–2373.
- Barganska, Z.; Slebioda, M.; Namiesnik, J. Determination of Antibiotic Residues in Honey. Trends in Analytical Chemistry 2011, 30 (7), 1035–1041.