ABSTRACT
Lathyrus sativus L. is considered one of the most promising calorie and protein sources for the vast, expanding populations living in drought-prone and marginalized areas of the world. This study was conducted to determine the β-N-oxalyl-L-2,3-diaminopropionic acid (β-ODAP), L-homoarginine, and asparagine content of a total of 173 Lathyrus sativus L. genotypes, of which 93 were collected under natural conditions in Antalya. The β-ODAP, L-homoarginine, and asparagine content of Lathyrus sativus L. genotypes ranged from 1.55 to 20.8 mg/g seeds, 1.35 to 11.64 mg/g seeds, and 0.59 to 5.22 mg/g seeds, respectively.
Introduction
At the present time, many countries are interested in sustainable increases in agricultural production to feed a growing population and meet the challenges of climate change. To accomplish this, research centered on developing and improving underutilized or neglected plants is increasing. Lathyrus sativus L. (grass pea), an orphan legume of the arid areas is a good alternative and “insurance crop” in areas that are prone to abiotic stresses. In the face of climate change challenges, researchers recently started to pay attention to the grass pea, which is considered a model crop for sustainable agriculture.[Citation1–Citation4] Lathyrus sativus L. is a legume crop from the genus Lathyrus that constitutes 187 species and subspecies. Lathyrus sativus L. is widely cultivated as food and feed in Central, Southern, and Eastern Europe, North Africa the Mediterranean, and across Asia.[Citation5]
Grass pea has a number of advantages over other plants, such as its high protein content, a high-yield potential, low-fertilization requirements, floods tolerance, salinity tolerance, and drought survival. The hardy, penetrating root system allows cultivation of grass peas in a broad spectrum of soil types, including very poor soil.[Citation6–Citation8] Grass pea fixes nitrogen through its roots. The nitrogen fixed by grass pea is able to both to meet its own nitrogen requirements and positively affects the soil nitrogen balance to meet the demand of subsequent crops.[Citation9,Citation10]
Grass pea which is environmentally resistant is used not only for human food but also for cattle feed and green forage.[Citation1] However, heavy consumption of these species can lead to lathyrism, caused by the non-protein amino-acid β-N-oxalyl-L-α, β-diaminopropionic acid (β-ODAP). The β-ODAP content in grass pea seeds is highly influenced by environmental factors.[Citation1] The β-ODAP content of grass pea varies widely, among both genotypes and environments.[Citation11] Hanbury et al.[Citation12] reported that genotype was the most important determinant of β-ODAP concentrations, while the environment had less influence on Lathyrus sativus L. and Lathyrus cicera L. However, Wuletaw[Citation13] studied the stability of β-ODAP content in L. sativus and reported a significant interaction between genotype and environment.
Amino acids such as homoarginine and asparagine specify what roles in grass pea. Homoarginine, a non-protein amino acid, was the first to be characterized from L. sativus seeds but had been ignored because of its lack of toxic attributes.[Citation12,Citation14,Citation15] Homoarginine is now recognized as a normal metabolite in humans and its role in human health is becoming increasingly important. Homoarginine in L. sativus contributes to cardiovascular health.[Citation16–Citation18] Homoarginine, which also play an important role in physiological and biochemical processes has been proposed to modulate the toxicity of β-ODAP.[Citation19] L. sativus seeds are also rich in L-homoarginine, which acts as a precursor for lysine in higher animals.[Citation20]
Asparagine is essential for healthy brain development in children and affects some body functions. Asparagine is the main form of transported nitrogen in many legume plants. In developing soybean seeds, asparagine content reaches 33–49% of the free amino-acid pool and may be a determinant of storage protein content. Also, it has been confirmed to be a positive correlation between asparagine and storage protein content in soybean seeds.[Citation21,Citation22]
The present study was conducted to determine the β-ODAP, L-homoarginine, and asparagine contents of a total of 173 grass pea genotypes. These data identified grass pea genotypes with low β-ODAP content and high yield that are capable of adapting to the hot and arid climatic condition, creating a database that would be useful in selecting genotypes.
Materials and methods
Plant materials
In this study, a total 173 Lathyrus sativus L. genotypes were used as plant material. Among these genotypes, 93 were collected by the Batı Akdeniz Agricultural Research Institute (BATEM) from Antalya natural flora; 10 were ensured by the Republic of Yemen Ministry of Agriculture and Irrigation, 4 were provided by the GAP International Agricultural Research and Training Center, 20 were obtained from the Aegean Agricultural Research Institute from Genebank, and 43 were provided by the Bahri Dagdas International Agricultural Research Bahri Dagdas. The Ceora cultivar was ensured by Prof. Dr. Kadambot Siddique (The University of Western Australia), and İptaş, Karadağ and Eren cultivars were obtained from the Gaziosmanpasa University Faculty of the Agriculture Department of Field Crops.
Seeds of each genotypes were planted and harvested in the BATEM fields under Antalya land conditions. The seeds obtained from these plants were used for content analysis. Analysis was carried out in Akdeniz University Center for Food and Agricultural Research Laboratory.
Extraction
The dried seed samples were powdered using a blender. Homogeneous samples were divided into 1-g portions in individual 50-ml sample tubes and extracted with 25 ml of extraction solution, 0.1% (v/v) formic acid in water:methanol (50:50) (v/v).[Citation23] For recovery studies (), a standard was added to the tube at this stage. The mixture was extracted using ultra-turrax for 2 min at 10000 rpm. To control temperature changes, the sample tubes were placed in an ice bath during ultra-turrax extraction. Extracted samples were centrifuged at 4°C at 4000 rpm for 10 min. The supernatant was passed through a 0.2-µm PTFE membrane filter. Filtered samples were diluted with a mobile phase and injected at 10 µl volumes to LC-MS/MS.
Table 1. Transitions, collisions energies, retention times of β-ODAP, L-homoarginine, and asparagine.
Table 2. Recovery (%), RSD (%), and LOQ (mg/L) of β-ODAP, L-homoarginine, and asparagine.
Chemicals
All reagents and standards were analytical grade. The amino-acid standard mixture, L-homoarginine hydrochloride, and L-asparagine were purchased from Sigma-Aldrich in high purity (>98%). β-ODAP was purchased from Chemfaces, China, in high purity (>98%). Individual stock standard solutions of chemicals (approximately 1000 mg/L) were prepared in methanol and stored at −18°C. From these stock standard solutions, a working standard mixture was prepared for calibration and recovery experiments. LC-MS grade methanol was purchased from Merck.
Methodology of UHPLC-MS/MS
These methods (quadrupole, time of flight, quadrupole ion trap, etc.) are used for analyzing the active substance, content, and residues, found in foods. In recent studies, one of the most powerful and versatile analysis methods used for the detection of analytes is tandem mass spectrometry (MS/MS). Tandem mass spectrometers are the preferred method to develop efficient and fast quantitation from the same injection of multiple analytes in different product groups.[Citation24,Citation37] The UHPLC-MS/MS system consisted of a Thermo Accela UHPLC (pump, degasser, autosampler) coupled to a Thermo Quantum Access Max triple quadrupole mass spectrometer. An electro spray ionization (ESI) probe was used for atmospheric pressure ionization. A Thermo Hypersil Gold (100 mm × 2.1 mm, 1.9 µm particulate size) C18 column was used for analytical separation of the analytes. The UHPLC column oven temperature was set to 40°C, and injection volume was 10 µl.
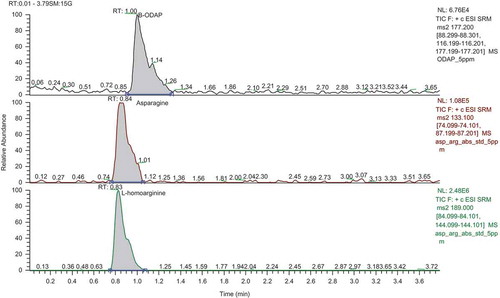
Reverse-phase chromatography was implemented to separate the analytes. The mobile phase consisted of 0.1% (v/v) formic acid and 4 mM ammonium acetate in water:methanol (95:5) (eluent A) and methanol (eluent B). The analysis started with 100% mobile phase A and was held for 2 min at this composition. After 2 min, the mobile phase A percentage was linearly decreased to 0% over 1.5 min and held at 0% for 3 min. The flow was then changed to 100% mobile phase A with a re-equilibration time of 0.5 min. The total run time was 7 min, and the flow rate was 0.4 ml/min. By means of a gradient program, the apolar characteristics of the column and polar structure of the analytes were detected. The retention times of β-ODAP, asparagine, and L-homoarginine were 1.0, 0.84, and 0.83 min, respectively ().
Compared to other chromatographic detectors, sensitivity, specificity and wide range applicability properties make LC-MS/MS systems more favorable. The determined analytes, β-ODAP, asparagine, and L-homoarginine were moderately polar and prone to positive ionization techniques due to their chemical structure. The analytes were charged with addition of a proton (M + H+). These m/z values and the transition to fragmented ions make the system more selective to the analytes.[Citation25]
The optimum mass spectrometer parameters for identification and quantification were investigated. Transitions, collision energy, capillary temperature, and gas pressures parameters were optimized during infusion of the compounds. For the infusion parameters, diluted single stock standards were used. Dilutions were performed using mobile phase A. As a result, the optimized capillary temperature was set to 325°C, the electrospray voltage was set to +3.2 kV, the sheath gas pressure used was 30 L/min, the aux gas pressure used was 10 L/min, and the collision gas pressure was set to 1.5 m Torr. Multiple reaction monitoring (MRM) mode was used for detection. The total MS acquire time was 7 min.
Calibration curves
A 100 mg/L working standard mixture was used for preparing the calibration points. This standard mixture was obtained from individual stock standard solutions of chemicals diluted with methanol. Six different calibration points for asparagine, L-homoarginine, (0–10 mg/L), and β-ODAP (0–50 mg/L) were prepared using working standard mixture dilutions with mobile phase A. The linearity values (rCitation2) of β-ODAP, asparagine, and L-homoarginine were 0.9956, 0.9976, and 0.9984, respectively.
Results and discussion
UHPLC-MS/MS is a new method for reliable sample analysis based on recovery studies with blank samples as shown in . LOQ values were obtained from UHPLC-MS/MS β-ODAP, L-homoarginine, and asparagine as 1.51 mg/L, 0.41 mg/L, and 0.45 mg/L, respectively. The reproducibility criterion for β-ODAP, L-homoarginine, and asparagine concentrations is 17% RSD. RSD values for β-ODAP, L-homoarginine, and asparagine were found to be 9.61%, 3.25%, and 3.66%, respectively. RSD values below 17% indicate reliable analysis results. Also, chromatograms of β-ODAP, asparagine, and L-homoarginine of grass pea are presented in .
The β-ODAP, L-homoarginine, and asparagine contents for 173 grass pea genotypes were detected using UHPLC-MS/MS, as shown in The β-ODAP content of grass pea genotypes ranged from 1.55 mg/g to 20.8 mg/g, with the lowest β-ODAP content found in LS-106 (1.55), LS-165 (2.24) Eren (2.81) and LS-166 (2.93) genotypes. In addition, the β-ODAP content of the Ceora variety, which has been registered with low β-ODAP content, was found to be 3.52 mg/g. The seeds analyzed under Austria conditions using the Ceora variety had a β-ODAP content of 0.1 mg/g.[Citation2,Citation26] However, when this variety was grown under Antalya conditions, the β-ODAP content was 3.52 mg/g. This situation is thought to result from changing environmental conditions or genetic segregation, caused by foreign pollination which exists at a rate of 2–5% in Lathyrus species. Vaz Patto et al.[Citation3] and Jiao et al.[Citation27] previously reported that the L. sativus plant has a wide range of variability in β-ODAP content. The same variety of L. Sativus can show the differences in β-ODAP content when grown in different locations. Additionally, Siddique et al.[Citation28] indicated that the interaction between genotype and environment was minimal, but environment effects were large consideration for the variation in β-ODAP concentrations.
Table 3. Contents of β-ODAP, L-homoarginine, and asparagine of 173 grass pea genotypes.
The L-homoarginine content ranged from 1.35 mg/g to 11.64 mg/g. The L-homoarginine content was low in the LS-49 (1.35), LS-74 (1.45), LS-83 (1.63), and LS-82 (1.66) genotypes. Meanwhile, the LS-147 (11.64), LS-135 (11:22), LS-129 (10.86), and LS-106 (10.83) genotypes had high levels. Grass pea genotype asparagine content ranged from 0.59 mg/g to 5.22 mg/g. Our results indicate that LS-97 (0.59), LS-124 (0.76), and LS-169 (0.85) had the lowest asparagine contents, while the LS-151 (5.22), LS-144 (5.08), and LS-143 (5.02) genotypes had the highest asparagine contents.
Onar et al.[Citation29] determined in their research with local grass pea varieties, grown in Turkey that the concentrations of β-ODAP, homoarginine, and asparagine ranged from 0.10% to 0.87% (w/w), from 0.21% to 1.27% (w/w), and from 0.006% (w/w) to 0.47% (w/w), respectively. These researchers reported a positive correlation between β-ODAP and homoarginine. Karadag et al.[Citation30] in their research on 27 grass pea genotypes, detected β-ODAP contents between 27.60 mg/kg and 1235.85 mg/kg in winter planting, while the β-ODAP content was between 68.97 mg/kg and 1028.01 mg/kg in summer planting. Basaran et al.[Citation31] determined that the β-ODAP content of 52 grass pea populations grown under Samsun conditions in Turkey was 1.40 mg/g and 3.05 mg/g. In their study on the Lathyrus species, Sacristan et al.[Citation32] determined that the β-ODAP content was between 0.79 mg/g and 5.05 mg/g, and the homoarginine content was between 7.49 and 12.44 mg/g. Zhao et al.[Citation33] determined that the β-ODAP content in grass pea seeds was between 0.16% and 0.58%, while the homoarginine content was between 0.32% and 1.06%. Fikre et al.[Citation23] analyzed grass peas from multiple genotypes that were collected from different regions and found that β-ODAP, homoarginine, and asparagines content ranged from 0.02% to 0.54%, from 0.68% to 0.86%, and from 0.03% to 0.08%, respectively. Our results were similar to earlier studies.
The β-ODAP content of grass pea seeds ranges from 0.2 to 7.2 mg/g due to genetic and environmental conditions but this needs to be lower than 0.22% to be safe for human and animal consumption.[Citation34] β-ODAP content is largely controlled by genetic cues but is also affected by environmental factors.[Citation2] Grass pea lines and varieties with low β-ODAP contents developed through varied breeding method are unable to maintain these low levels under different environmental conditions.[Citation35]
Homoarginine was characterized as one of the first unusual non-protein amino acids from L. sativus seeds and 30 other species of Lathyrus before the discovery of β-ODAP. Similar to asparagine, homoarginine is a natural substrate for nitric oxide which emerged as important signaling molecule in both the cardiovascular system and cerebral metabolism. Therefore, grass pea is an important plant species as a valuable natural dietary source of homoarginine (Rao et al., 1963; Yun et al., 2011).[Citation17,Citation36]
When the β-ODAP, L-homoarginine, and asparagine contents of 173 grass pea genotypes were evaluated, a broad range was observed in each case. One of the 173 total grass pea genotypes was found to have a total content <0.2% (LS-106: 0.155%), which is within the confidence limits interval for human and animal consumption.
In our analysis on 173 grass pea samples, the β-ODAP, L-homoarginine, and asparagine contents varied from 1.550 to 8.200, from 1.350 to 11.640, and from 0.590 to 5.220 mg/g, respectively. The averages were 5.047 (β-ODAP), 5.645 (L-homoarginine), and 2.786 (asparagine). Standard deviations of β-ODAP, L-homoarginine, and asparagine contents were found to be 1.359, 1.979, and 0.835, respectively; and the variance was 1.848 (β-ODAP), 3.919 (L-homoarginine), and 0.697 (asparagine). Histograms and normal curves of contents are shown in .
Figure 2. Histogram of β-ODAP L-homoarginine and asparagine with normal curve, and normal probability plot for β-ODAP L-homoarginine and asparagine.
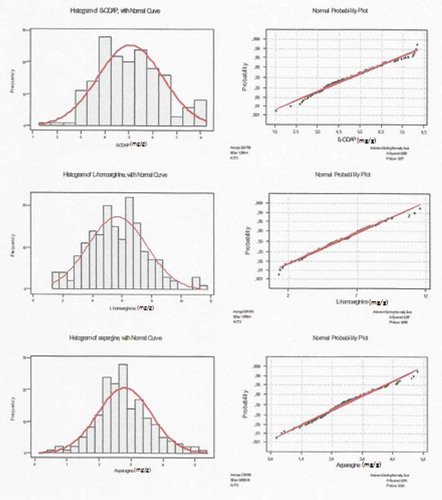
According to Anderson–Darling normality test results, the p-values were 0.071, 0.618, and 0.020 for β-ODAP, L-homoarginine, and asparagines, respectively. As β-ODAP and L-homoarginine showed normal distributions, with p-values >0.05, this situation is not valid for asparagine. Regression analysis of the relationship between β-ODAP and L-homoarginine content and between β-ODAP and asparagine content are as follows:
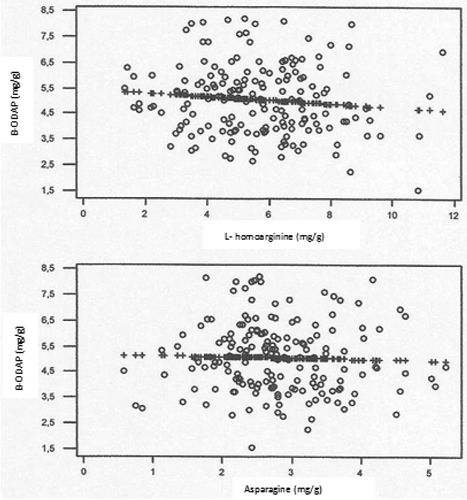
The best linear and disperse graphics are indicated in . Pearson correlation values between β-ODAP and either L-homoarginine or asparagine from the 173 grass pea samples were calculated to be −0.103 and −0.027, respectively, and showed p-values of 0.179 and 0.725, respectively. These values have negative and weak associations. Onar et al.[Citation29] reported a positive correlation between β-ODAP and homoarginine, and a correlation between asparagines and β-ODAP levels was observed in some accessions.
Conclusions
The β-ODAP, L-homoarginine, and asparagine contents of a total of 173 grass pea genotypes, a large proportion of which were collected from natural flora of Antalya, were determined using UHPLC-MS/MS. The obtained data indicate that there are large variations in content among genotypes that are caused by both genetic features and environmental conditions. Correlation analysis determined a weakly negative correlation not only between the L-homoarginine and β-ODAP but also between asparagine and β-ODAP. Assembling a rich gene pool in terms of the genetic material is of great importance for future breeding aimed toward obtaining grass pea varieties, with low β-ODAP content that are highly efficient and adapted to local areas.
Funding
This study was financially supported by the Akdeniz University Scientific Research Projects Coordination Unit. [grant number FBA-2015-1135].
Additional information
Funding
References
- Campbell, C.G.;. Grass Pea, Lathyrus Sativus L. Promoting the Conservation and Use of Underutilized and Neglected Crops. Nr 18. Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany/International Plant Genetic Resources Institute: Rome, Italy, 1997; 92 pp.
- Hanbury, C.D.; Siddique, K.H.M.; Galwey, N.W.; Cocks, P.S. Genotype-Environment Interaction for Seed Yield and ODAP Concentration of Lathyrus Sativus L. and L. Cicera L. in Mediterranean-Type Environments. Euphytica 1999;110:445–460.
- VazPatto, M.C.; Skiba, B.; Pang, E.C.K.; Ochatt, S.J.; Lambein, F.; Rubiales, D. Lathyrus Improvement for Resistance against Biotic and Abiotic Stresses: From Classical Breeding to Marker Assisted Selection. Euphytica 2006;147:133–147.
- Yang, T.; Jiang, J.; Burlyaeva, M.; Hu, J.; Coyne, C.J.; Kumar, S.; Redden, R.; Sun, X.; Wang, F.; Chang, J.; Hao, X.; Guan, J.; Zong, X. Large-Scale Microsatellite Development in Grasspea (Lathyrus Sativus L.), an Orphan Legume of the Arid Areas. BMC Plant Biology 2014;17(14):65.
- Smartt, J.; Kaul, A.K.; Araya, W.A.; Rahman, M.M.; Kearney, J. Grass Pea (Lathyrus Sativus L.) as a Potential Safe Legume Food Crop. In Expanding the Production and Use of Cool Season Food Legumes. Muehlbauer, F.J.; Kaiser, W.J.; Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; 144–155.
- Campbell, C.G.; Mehra, R.B.; Agrawal, S.K.; Chen, Y.Z.; Abd-El-Moneim, A.M.; Khawaja, H.I.T.; Yadav, C.R.; Tay, J.U.; Araya, W.A. Current Status and Future Strategy in Breeding Grass Pea (Lathyrus Sativus). Euphytica 1994;73:167–175.
- Grela, E.R.; Rybinski, W.; Klebaniuk, R.; Matras, J. Morphological Characteristics of Some Accessions of Grass Pea (Lathyrus Sativus L.) Grown in Europe and Nutritional Traits of Their Seeds. Genetic Resource and Crop Evoluation 2010;57:693–701.
- Arslan, M.;. Importance and Current Situation of Grass Pea (Lathyrus Sativus L.) in Forage Crops Production of Turkey. Turkish Journal of Agricultural And Natural Sciences 2016;3(1):17–23.
- Ahlawat, I.P.S.; Singh, A.; Saraf, C.S. Effects of Winter Legumes on the Nitrogen Economy and Productivity of Succeeding Cereals. Experimental Agriculture 1981;17:57–62.
- Muehlbauer, F.J.; Tullu, A. Newcrop Factsheet – Lathyrus Sativus L. http://www.hort.purdue.edu/newcrop/CropFactSheets/grasspea.html. 1997 (Accessed July 25, 2016)
- Dahiya, B.S.; Jeswani, L.M. Genotype & Environment Interactions for Neurotoxic Principle (BOAA) in Grass Pea. Indian Journal of Agricultural Sciences 1975;45(9):437–439.
- Hanbury, C.D.; White, C.L.; Mullan, ., B.P.; Siddique, K.H.M. A Review of the Use and Potential of Lathyrus Sativus L. and Lathyrus Cicera L. Grain for Animal Feed. Animal Feed Science and Technology 2000;87:1–27.
- Wuletaw, T.;. Stability of Grass Pea (Lathyrus Sativus L.) Varieties for ODAP Content and Grain Yield in Ethiopia. Lathyrus Lathyrism Newsletter 2003;3:32–34.
- Akalu, G.; Johansson, G.; Nair, B.M. Effect of Processing on the Content Β-N-Oxalyl-L-2,3-Diaminopropionic Acid (Β-Odap) in Grass Pea (Lathyrus Sativus) Seeds and Flour as Determined by Flow Injection Analysis. Food Chemistry 1998;62:233–237.
- Aletor, V.A.; Abd EI Moneim, A.M.; Goodchild, A.V. Evaluation of the Seeds of the Selected Lines of Three Lathyrus Spp. for BOAA, Tannins, Trypsin Inhibitor Activity and Certain in Vitro Characteristics. Journal of Science and Food Agriculture 1994;65:143–151.
- Piergiovanni, A.R.; Damascelli, A. L-Homoarginine Accumulation in Grass Pea (Lathyrus Sativus L.) Dry Seeds. A Preliminary Survey. Food and Nutrition Sciences 2011;2:207–213.
- Rao, S.L.N.; Ramachandran, L.K.; Adiga, P.R. The Isolation and Characterization of L-Homoarginine from the Seeds of Lathyrus Sativus. Biochemistry 1963;2:298–300.
- Singh, S.S.; Rao, S.L.N. Lessons from Neurolathyrism: A Disease of the past & the Future of Lathyrus Sativus (Khesari Dal). Indian Journal of Medical Research 2013;138:32–37.
- Shamin, M.Z.; Hossain, M.S.; Islam, K.; Yusuf, H.K.M.; Lambein, F. Mechanism of ODAP Toxicity in One-Day-Old Chicks. Dhaka University Journal of Biology Science 2002;11(1):1–7.
- Quereshi, M.Y.; Pilbean, D.J.; Evans, C.S.; Bell, E.A. The Neurolathyrogen of α Amino-β-Oxalylaminopropionic Acid in Legume Seeds. Phytochemistry 1977;16:477–479.
- Ratajczak, W. Asparagine Metabolism in Developing Seeds of Lupinus Luteus L. Biochemie und Physiologie der Pflanzen 1986;181:17–22.
- Borek, S.; Galor, A.; Paluch, E. Asparagine Enhances Starch Accumulation in Developing and Germinating Lupin Seeds. Journal of Plant Growth Regulation 2013;32:471–482.
- Fikre, A.; Korbu, L.; Kuo, Y.H.; Lambein, F. The Contents of the Neuro-Excitatory Amino Acid Β-Odap (B-N-Oxalyl-L-A,B-Diaminopropionic Acid), and Other Free and Protein Amino Acids in the Seeds of Different Genotypes of Grass Pea (Lathyrus Sativus L.). Food Chemistry 2008;110:422–427.
- Klein, J.; Alder, L. Applicability of Gradient Liquid Chromatography with Tandem Mass Spectrometry to the Simultaneous Screening for about 100 Pesticides in Crops. The Journal of AOAC International 2003;86:1015–1037.
- Pitt, J.J.;. Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry. Clinical Biochemistry Review 2009;30(1):19–34.
- Kumar, S.; Bejiga, G.; Ahmed, S.; Nakkoul, H.; Sarker, A. Genetic Improvement of Grass Pea for Low Neurotoxin (Β-Odap) Content. Food and Chemical Toxicology 2011;49:589–600.
- Jiao, C.J.; Jiang, J.L.; Ke, L.M.; Cheng, W.F.; Li, M.Z.; Li, X.; Wanga, C.Y. Factors Affecting Β-Odap Content in Lathyrus Sativus and Their Possible Physiological Mechanisms. Food and Chemical Toxicology 2011;49:543–549.
- Siddique, K.H.M.; Loss, S.P.; Herwig, S.P.; Wilson, J.M. Growth, Yield and Neurotoxin (ODAP) Concentration of Three Lathyrus Species in Mediterranean-Type Environments of Western Australia. Australian Journal of Experimental Agriculture 1996;36:209–218.
- Onar, A.N.; Erdoğan, B.Y.; Ayan, I.; Acar, Z. Homoarginine, Β-Odap, and Asparagine Contents of Grass Pea Landraces Cultivated in Turkey. Food Chemistry 2014;143:277–281.
- Karadag, Y.; Isıldak, O.; Elmastas, M.; Yavuz, M. Comparison of Α, Β and Total ODAP (Β-N-Oxalyl-L-Ά,Β-Diamino Propionic Acid) Contents in Winter- and Springsown Grasspea (Lathyrus Sativus L.) Genotypes. African Journal of Biotechnology 2010;9 (49):8339–8342.
- Basaran, U.; Acar, Z.; Karacan, M.; Onar, A.T. Variation and Correlation of Morpho-Agronomic Traits and Biochemical Contents (Protein and Β-Odap) in Turkish Grass Pea (Lathyrus Sativus L.) Landraces. Turkish Journal of Field Crops 2013;18(2):166–173.
- Sacristán, M.; Varela, A.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Legaz, M.; Muzquiz, M. Determination of β-N-Oxalyl-L-α, β-Diaminopropionic Acid and Homoarginine in Lathyrus Sativus and Lathyrus Cicera by Capillary Zone Electrophoresis. Journal of Science and Food Agriculture 2014;95:1414–1420.
- Zhao, L.; Chen, X.G.; Hu, Z.D.; Li, Q.F.; Chen, Q.; Li, Z.X. Analysis of Β-N-Oxalyl-L-2,3-Diaminopropionic Acid and Homoarginine in Lathyrus Sativus by Capillary Zone Electrophoresis. Journal of Chromatography 1999;857:295–302.
- Abd El Moneim, A.M.; Van Dorrestein, B.; Baum, M.; Mulugeta, W. Role of ICARDA in Improving the Nutritional Quality and Yield Potential of Grass Pea (Lathyrus Sativus) for Subsistence Farmers in Developing Countries. CGIAR-wide conference on Agriculture Nutrition, 1999; pp. 5–6.
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrussativus (Grass Pea) and Its Neurotoxin ODAP. Phtytochemistry 2006;67:107–121.
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Nitric Oxide in Health and Disease of the Nervous System. Molecular Physiology 1997;2:300–310.
- Mol, H.G.; Van Dam, R.C.; Steijger, O.M. Determination of Polar Organophosphorus Pesticides in Vegetables and Fruits Using Liquid Chromatography with Tandem Mass Spectrometry: Selection of Extraction Solvent. Journal of Chromatography A 2003;1015:119–127.