ABSTRACT
Proteolytic changes in Suanyu, a traditional fermented fish product of China, were determined during 6 weeks of fermentation. The gradual decrease of pH value and aw was detected, accompanied with an increasing trend detected in trichloroacetic acid–soluble C-1: peptides concentration. Protein composition changed throughout the fermentation. A decrease in sarcoplasmic and myofibrillar protein fractions was accompanied by an increase in the insoluble fraction. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed most bands of sarcoplasmic, myofibrillar, and insoluble proteins lightened or disappeared due to proteolysis or denaturation. New bands emerged especially in insoluble proteins. Fluctuation of molecular weight distribution of the water-soluble nitrogen in Suanyu was found during fermentation. The highest percentage in Suanyu end-products was observed in the fraction of <0.18 kDa, followed by 0.18–0.5 kDa, 66.02 ± 0.38% and 10.79 ± 0.27%, respectively. Free amino acids analysis showed that nearly all amino acids increased, especially for Asp, Glu, Met, Phe, Leu, Val, and Ile. Overall, intense proteolysis in Suanyu brought numerous of small peptides and free amino acids, which contribute to the taste and flavor of Suanyu.
Introduction
Suanyu is one of the traditional fermented fish products in South China. It is mainly produced and consumed in a few provinces, such as Hunan, Guizhou, and Guangxi.[Citation1] It is mainly made with whole or pieces of freshwater fish, common carps (Cyprinus carpio) or grass carps (Ctenopharyngodon idellus), mixed with cooked rice or other grains, salt, and spice. Suanyu is usually manufactured in small family workshops in winter. Slow spontaneous fermentation proceeds in a sealed environment for about 1 month or longer. Meanwhile, Suanyu can be preserved for a long time. People could consume it directly without cooking or after simple frying. Similar fermented fish products are found in East and Southeast Asia, such as Narezushi and Nukazuke in Japan,[Citation2,Citation3] as well as Plaa-som, Som-fak, and Som-fug in Thailand.[Citation4–Citation6]
Fermentation is one of the oldest techniques in food preservation as it not only extends the shelf-life but also enhances the flavor and nutritional quality of the product.[Citation7] Proteolysis is an extremely important biochemical change in fermented meats, such as sausages and hams. It influences both texture and flavor of meat due to the generation of several low-molecular-weight compounds, including peptides, amino acids, aldehydes, organic acids, and amine.[Citation8,Citation9] Many reports proved proteolysis is caused by endogenous enzymes in muscle together with microbial enzymes of autochthonous microorganisms or starter cultures.[Citation10–Citation12] Protein degradation of fermented meat products has been deeply studied by many reporters.[Citation13–Citation16]
Suanyu is an excellent protein food source, with a unique umami and sour taste which generated during fermentation. However, little research has been done on protein degradation of Suanyu. The objective of this study was to investigate the protein degradation pattern of Suanyu during spontaneous fermentation, including changes in protein composition and molecular weight distribution of the water-soluble nitrogen (WSN), as well as the content and composition of free amino acids (FAA).
Materials and methods
Sanyu preparation
Carps (Cyprinus carpio L.) were purchased from a local market (Wuxi, Jiangsu, China). The fish was gutted, scaled, and cut into 3–5 cm pieces. Subsequently, the pieces were washed with tap water several times and then pre-cured with sucrose (2% w/w) and salt (3% w/w) at 4°C for 48 h, before dried at 60°C for 2 h. The pre-cured fish pieces mixed with ground roasted corn powder (200 g/kg) were put into a ceramic or glass jar for the fermentation process. These products were fermented at ambient temperature (24°C) for 6 weeks. Samples were taken for proteolysis analysis on the weeks of 0, 1 2, 3, 4, 5, and 6.
Determination of pH and aw
Ten gram of sample collected from each sampling point were homogenized with 90 mL deionized water. The pH was measured with a digital pH meter (Mettler Toledo 320-s). Water activity (aw) was determined using a digital water activity meter (Rotronic Hygroskop DT).
Determination of protein and TCA-soluble peptide content
Protein content was determined according to the Kjeldahl method (ISO 937:1978). TCA-soluble peptides were monitored by the modified method of Visessanguan et al.[Citation7] Samples (3 g) were added to 27 mL of cold 5% (w/v) TCA. The mixture was homogenized at 11,000 rpm for 2 min and kept on ice for 1 h followed by centrifugation at 12,000g for 15 min. TCA-soluble peptide content in the supernatant was measured by the method of Lowry et al.[Citation17] and expressed as μmol tyrosine/g.
Determination of protein composition
The protein components in Suanyu were fractionated according to their solubility. Sarcoplasmic and myofibrillar proteins were obtained as reported by Chaves-López et al.[Citation13] Then, 20 g of sample was diluted (1:10 w/v) with 20 mM phosphate buffer (pH 6.5), homogenized, and centrifuged (13,000g at 4°C for 20 min). The supernatant was the sarcoplasmic fraction. The pellet was resuspended in 200 mL of 30 mM sterilized phosphate buffer (pH 7.4). The suspension was homogenized and centrifuged (10,000g at 4°C for 20 min). The pellet was washed three times in the same buffer to remove muscle proteinases. After washing, the pellet was weighed and resuspended in nine volumes of 100 mM phosphate buffer with 0.7 M KI (pH 6.5). After homogenization and centrifugation, the supernatant (myofibrillar fraction) was obtained. The pellet was washed three times, and then resuspended in 10 mL of 5% SDS (sodium dodecyl sulfate). The mixture was incubated at 85°C for 60 min, and then centrifuged at 12,000g for 30 min. The supernatant was insoluble fraction. The protein contents of all fractions were determined by the biuret method.[Citation18]
Analysis of protein component degradation by SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in a vertical gel electrophoresis unit (Mini-Protean-3Cell, Bio-Rad, Richmond, CA, USA) according to the method of Laemmli.[Citation19] A 10% separating gel with 5% stacking gel was used. Electrophoresis was performed at 100–120 V until the front marker reached the bottom of the gels. After electrophoresis, the gels were stained using Coomassie Brilliant Blue R-250, and destained using 40% methanol and 10% acetic acid. Protein molecular weight markers (Precision Plus Protein Dual Xtra Standard, Bio-Rad), from 2 to 250 kDa, were used as standards.
Molecular weight distribution of WSN
The extraction of WSN fraction was determined according to the method of Sun.[Citation20] Samples (2 g) were added to 10 mL of 50 mM citric acid–sodium citrate buffer solution (pH 6.0). The mixture was homogenized and left for 2 h at 4°C before centrifuged at 12,000g for 15 min at 4°C. The supernatant (WSN fraction) was filtered through rapid filter paper and ultrafiltration membrane (0.22 μm) successively. The molecular weight distribution of WSN was determined by gel permeation chromatography. A high-performance liquid chromatography (HPLC) (Waters 600, Milford, MA, USA), equipped with a TSK gel 2000 SWXL (300 mm × 7.8 mm) column, was used for the analysis. The mobile phase was acetonitrile–water (45:55) with 0.1% (v/v) trifluoroacetic acid. The elution was performed at a 0.5 mL/min flow rate (30°C). Four molecular weight standards, cytochrome C (MW 12,384), bacitracin (MW 1450), Gly–Gly–Tyr–Arg (MW 451), and Gly–Gly–Gly (MW 189), were used to make reference curve. The molecular weight of peptides was calculated by the elution volume.
FAA analysis
Five gram of each sample was diluted with isopyknic trichloroacetic acids (TCA) (10 g/100 mL) separately, and centrifuged (12,000g at 4°C for 10 min). FAA contents were determined by HPLC (Agilent 1100 Series, Palo Alto, CA, USA). Peak identification and quantification were accomplished by determining the retention time and recoveries of FAA standards (Sigma Chemical, St. Louis, MO, USA).
Statistical analyses
All tests were repeated three times. Analysis of variance (ANOVA) was performed, and comparisons between means were analyzed by Duncan’s multiple range test. Statistical analysis was performed using the SPSS statistic program (version 16.0) for Windows (SPSS, Inc., Chicago, IL, USA).
Results and discussion
pH and aw analysis
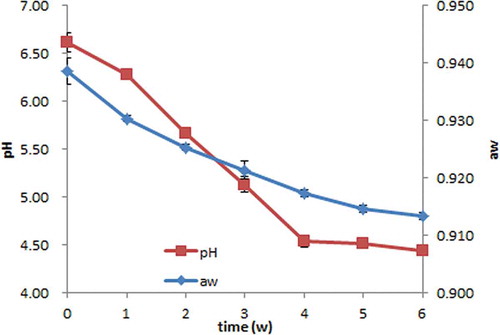
Results for analysis of pH and aw are shown in . The pH of Suanyu generally decreased with increasing fermentation time. A continuous decrease in pH (from 6.62 ± 0.10 to 4.53 ± 0.06) was observed in the first 4 weeks, and then the pH value remained around 4.5 by the end of the fermentation. Zeng et al.[Citation21] found lactic acid bacteria (LAB) in Suanyu increased to 8.31 log cfu/g in 3 weeks of traditional fermentation, and then remained during the later ripening. The pH decrease is probably due to acid accumulation, especially lactic acid, produced by LAB.[Citation7] The low pH is generally regarded as an important factor to ensure the safety of fermented products.[Citation4,Citation22] Previous findings revealed that acid has a clear physicochemical effect on proteins, such as gel producing and denaturation,[Citation23,Citation24] and also proteolytic enzymes of meat or LAB would be both affected.[Citation25,Citation26] Water activity decreased significantly from an initial value of 0.939 ± 0.002 to a final value of 0.913 ± 0.001 during the whole fermentation period, following the typical trend in similar products reported by other authors.[Citation27–Citation29] The initial value (0.939 ± 0.002) is much lower than that of the fresh fish, because it was determined after 48 h of pre-curing (with salt and sugar) and 2 h of drying (60°C).
Protein and TCA-soluble peptides
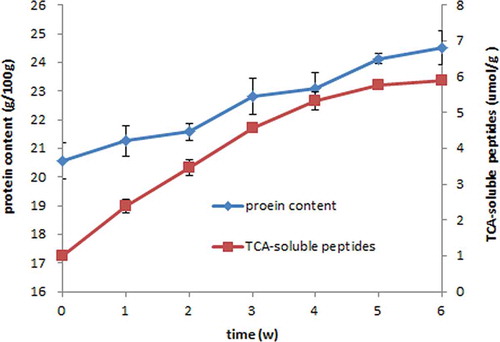
Changes of fish protein content and TCA-soluble peptides throughout fermentation are displayed in . The protein content continually increased from 20.57 ± 0.62 to 24.51 ± 0.61 g/100 g sample after 6 weeks of fermentation. On the other hand, TCA-soluble peptides content dramatically increased from 1.01 ± 0.06 to 5.89 ± 0.09 μmol tyrosine/g. Similar results have been reported by some authors.[Citation22,Citation24,Citation30] The increasing TCA-soluble peptide content indicated a great hydrolysis of muscle proteins during fermentation.[Citation22,Citation23] Degradation of proteins resulting in an increase in peptides and FAA has been already detected in many fermented products, such as sausages, hams, cheeses, and so on.[Citation12,Citation31,Citation32]
Changes of protein composition
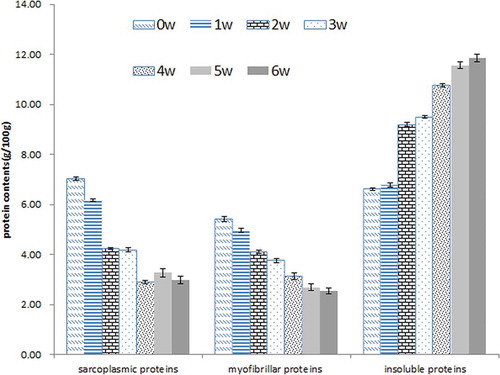
Suanyu proteins were classified into three fractions based on solubility. The amount of each fraction varied with fermentation time (). As fermentation went on, a dramatic decrease was found in both sarcoplasmic and myofibrillar protein fractions. Meanwhile, a continuous increase in the insoluble fraction was detected. There may be two reasons which could explain the decrease of both sarcoplasmic and myofibrillar proteins contents. First, the declined pH led to denaturation and subsequent aggregation of protein, which prevented their extractions from the corresponding solutions. Therefore, the original soluble fraction turned into insoluble proteins. The increasing insoluble proteins confirmed this point. Second, the decline of pH during the fermentation deeply promoted the proteolysis caused by endogenous cathepsins B, H, L, and D in muscle together with microbial enzymes of autochthonous microorganisms.[Citation10,Citation11,Citation26] Proteolysis generated large number of peptides and FAA. The increase in TCA-soluble peptides proved this point. Indeed, these results agreed with other reports.[Citation7,Citation23]
SDS-PAGE analysis of protein components
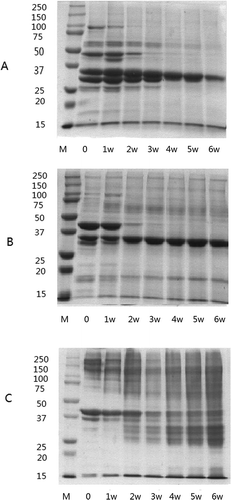
Electrophoretic profiles of each protein fraction during the fermentation are shown in . SDS-PAGE electrophoretograms of sarcoplasmic proteins showed a dramatic change throughout the whole fermentation period (). Nearly all bands of sarcoplasmic proteins disappeared within the first 3 weeks of fermentation except for band of ~35 kDa, due to proteolysis or acid and salt-induced denaturation.[Citation23,Citation33] Band of ~35 kDa increased in the first few weeks and then decreased until the end of the fermentation. Electrophoretograms of myofibrillar protein fractions are given in . Notable changes occurred in composition of myofibrillar proteins during fermentation. Myosin and actin are the main myofibrillar proteins. The intensity of myosin heavy chain (MHC) was found to be very low during the whole fermentation period. The reason was probably that MHC had already denatured into insoluble proteins during the 48 h of curing period and 2 h of drying before fermentation. Protein band of 100 kDa (α-actinin) disappeared after the first week of processing, accompanied with the appearance of degradation products with molecular weight of 50–75 kDa. There was a sharp decrease in the intensity of the band of 43 kDa (actin) and 30 kDa in the first or second week, while the band of 35 kDa (tropomyosin) became more intensely stained as fermentation progressed. In addition, bands of 15–25 kDa disappeared rapidly except 18 kDa, which increased continuously. There are lots of reports on hydrolysis of sarcoplasmic and myofibrillar proteins in many fermented meat products.[Citation8,Citation34] However, studies on hydrolysis of insoluble proteins of such products are rare. Proteins in the insoluble fraction consist of stromal proteins and denatured proteins.[Citation33] The pattern of the insoluble fraction (Fig. 4C) at the beginning of fermentation is similar to that of the myofibrillar protein fraction, which was thought to be denatured actomyosin,[Citation35] had proved this. As fermentation went on, the intensity of the main bands, ~100–220, 43, and 35 kDa, decreased or even disappeared. As degradation proceeded, numerous polypeptides with molecular weights in the range of 50–150, 25–30, and 15–18 kDa were detected. Denaturation due to the declined pH resulted in the three-dimensional structure alteration of protein. Proteins are more readily attached by proteolytic enzymes after denaturation than they are in their native state. Meanwhile, endogenous cathepsins B, H, L, and D in fish muscle are mostly active at acidic pH values. According to Fig. 4C, our results verify that proteins after denaturation are more easily hydrolyzed.
Molecular weight distribution of WSN analysis
Molecular weight distribution of the WSN was monitored over the fermentation and the results are shown in . In the beginning of the fermentation, higher molecular weight of more than 10 kDa and lower less than 0.18 kDa were the main two fractions in the WSN, which occupied nearly one-third and half, respectively. Next fractions were 5–10 and 0.18–0.5 kDa. Fraction of 0.5–5 kDa occupied the least part of the WSN, around 2.33%.
Table 1. Molecular weight distribution of water-soluble nitrogen (WSN) during processing.
Peptides with molecular weights more than 10 kDa increased in the first 2 weeks, and then decreased to a very low percentage of 8.20 ± 0.13%. On the contrary, the fraction with molecular weights less than 0.18 kDa thought to be FAA decreased to about 32.36 ± 1.34% in the first 2 weeks, and then increased to a high percentage of 66.02 ± 0.38%. The possible reason was as follows. Microorganisms in Suanyu need to consume lots of FAA for fast growth and generation in the first 2 weeks of fermentation, which resulted in a continuous decline of the fraction of <0.18 kDa. Two weeks later, high–molecular-weight proteins hydrolyzed largely due to the combined action of microbial enzymes and endogenous cathepsins of fish muscle,[Citation26,Citation36] hence the percent of proteins >10 kDa declined sharply till the end. Meanwhile, accumulation of small molecular substances originally from protein degradation resulted in a steady increase of the fraction of <0.18 kDa.
Throughout the fermentation, the fraction of 5–10 kDa decreased in the first 2 weeks, then increased in the third week, whereas decreased again in the last few weeks. For the fractions of 0.18–5 kDa, it increased first and then decreased slightly. It suggested that production and degradation of peptides occurred constantly in these fractions during the fermentation. Reports indicated that oligopeptides with molecular mass less than 3 kDa, produced by amino acid synthesis and enzymatic hydrolysis, are related to flavor.[Citation15,Citation37]
At the end of the fermentation, the percentage of <0.18 kDa fraction reached 66.02 ± 0.38%, followed by the fraction of 0.18–0.5 kDa at the percentage of 10.79 ± 0.27%. Therefore, the predominant fraction of WSN in Suanyu is FAA, as well as small peptides. A few reports showed the molecular weight distribution of the WSN in sausages. Henriksen and Stahnke[Citation38] extracted low-molecular-weight water-soluble compounds from dry sausages. The extracts were fractionated by gel filtration chromatography and revealed the molecular weight of the majority of the peptides to be less than 4.2 kDa. Sun[Citation33] found two fractions of molecular weight of >10 and 3–5 kDa were predominant in Cantonese sausage during the whole processing. By contrast, the molecular weight of the majority of the peptides in Suanyu was lower, which indicated proteolysis during fermentation was more complete than in fermented meat products, such as sausages and hams.
FAA analysis
The changes in the concentration of FAA during fermentation are shown in . A significant increase in the concentration of total amino acid (TAA) concentration of Suanyu, from 1675.19 ± 45.64 to 4550.27 ± 101.33 mg/100 g of dry matter before and after fermentation, was detected. The predominant amino acids in the beginning were, in decreasing order, His, Gly, Lys, Ala, and Arg, at concentrations higher than 100 mg/100 g of dry matter, respectively. And above all, the concentration of His, 599.36 ± 16.53 mg/100 g of dry matter, accounted for about one-third of the total. During the fermentation, nearly all amino acids increased except for Trp, Arg, and His. In particular, the levels of most of the amino acids increased more than fivefold as much as that of the initial, such as Asp, Glu, Met, Phe, Leu, Val, and Ile. Among them, Glu and Asp, as typical umami taste amino acids, gave Suanyu a unique umami taste. Besides, aromatic amino acids (Phe), branched-chain amino acids (Leu, Ile, and Val), and Met are the major precursors of many important aroma compounds in fermented meats and cheeses. Substantial accumulation of these FAA would contribute to the formation of Suanyu’s flavor. Meanwhile, the increasing trend of the majority of amino acid during meat fermentation was reported by many reporters.[Citation28,Citation39]
Table 2. Changes in free amino acid (FAA, mg/100 g dm) during Suanyu processing.
After 6 weeks of fermentation, the predominant amino acids changed to be Glu, His, Lys, Ala, Leu, and Asp, at concentrations higher than 400 mg/100 g of dry matter, respectively. Other reporters studied the main amino acids in other fermented fish or meat products. Liu et al.[Citation40] found that Glu, Asp, Leu, Gly, and Ala were the main amino acids in salted fish from Southeast of Guizhou. Kuda et al.[Citation3] found the predominant FAA were Ala, Lys, Leu, Glu, and Thr in aji-no-susu, a traditional fermented fish with rice produced in the Noto Peninsula of Japan. Zhou et al.[Citation29] found that Glu, Asp, and Leu were the main ones in sour meat of China. In general, there was agreement for some of the main FAA in these products, such as Glu, Leu, Lys, and Ala.
Conclusion
Protein degradation during traditional fermentation of Suanyu, a Chinese fermented fish, was investigated in this study. Findings show that proteolysis induced by gradually decreased pH changed the protein compositions. Protein denaturation induced by acidification made the proteolysis easier. Moreover, FAA became the predominant fraction of WSN, as well as small peptides, due to sufficient proteolysis. In particular, a large increase was detected in total amino acid. The two most rapidly growing ones were Glu and Asp which gave Suanyu a very umami taste.
Funding
This research was financially supported by the earmarked fund for China Agricultures Research System (number CARS-46-22), National Natural Science Foundation of China (number NFSC 31371823), and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Additional information
Funding
References
- Zeng, X.; Xia, W.; Jiang, Q.; et al. Chemical and Microbial Properties of Chinese Traditional Low-Salt Fermented Whole Fish Product Suan Yu. Food Control 2013, 30, 590–595. doi:10.1016/j.foodcont.2012.07.037.
- An, C.; Takahashi, H.; Kimura, B.; et al. Comparison of PCR-DGGE and PCR-SSCP Analysis for Bacterial Flora of Japanese Traditional Fermented Fish Products, Aji-Narezushi and Iwashi-Nukazuke. Journal Sciences Food Agricultural 2010, 90, 1796–1801.
- Kuda, T.; Tanibe, R.; Mori, M.; Take, H.; Michihata, T.; Yano, T.; Takahashi, H.; Kimura, B. Microbial and Chemical Properties of Aji-No-Susu, a Traditional Fermented Fish with Rice Product in the Noto Peninsula, Japan. Fisheries Science 2009, 75, 1499–1506. doi:10.1007/s12562-009-0175-0.
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Kijrongrojana, K.; Tanaka, M. Some Characteristics of Commercial Som-Fug Produced in Thailand. Food Chemistry 2004, 88, 527–535. doi:10.1016/j.foodchem.2004.01.067.
- Paludan-Muller, C.; Huss, H.H.; Gram, L. Characterization of Lactic Acid Bacteria Isolated from a Thai Low-Salt Fermented Fish Product and the Role of Garlic. International Journal of Food Microbiology 1999, 46, 219–229. doi:10.1016/S0168-1605(98)00204-9.
- Saithong, P.; Panthavee, W.; Boonyaratanakornkit, M.; Sikkhamondhol, C. Use of a Starter Culture of Lactic Acid Bacteria in Plaa-Som, a Thai Fermented Fish. Journal of Bioscience and Bioengineering 2010, 110, 553–557. doi:10.1016/j.jbiosc.2010.06.004.
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Thepkasikul, P. Changes in Composition and Functional Properties of Proteins and Their Contributions to Nham Characteristics. Meat Science 2004, 66, 579–588. doi:10.1016/S0309-1740(03)00172-4.
- Martín-Sánchez, A.M.; Chaves-López, C.; Sendra, E.; Sayas, E.; Fenández-López, J.; Pérez-Álvarez, J.Á. Lipolysis, Proteolysis and Sensory Characteristics of a Spanish Fermented Dry-Cured Meat Product (Salchichón) with Oregano Essential Oil Used as Surface Mold Inhibitor. Meat Science 2011, 89, 35–44. doi:10.1016/j.meatsci.2011.03.018.
- Ikonić, P.M.; Tasić, T.A.; Petrović, L.S.; et al. Proteolysis in Serbian-Traditional Dry Fermented Sausage Petrovská Klobása as Influenced by Different Ripening Processes. International Scholarly and Scientific Research & Innovation 2014, 8, 993–996.
- Toldra, F. Biotechnology of Flavor Generation in Fermented Meats. In Meat Biotechnology, Ed.; Toldra, F.; Springer: New York, USA, 2008, 199–215.
- Toldra, F. Proteolysis and Lipolysis in Flavour Development of Dry-Cured Meat Products. Meat Science 1998, 49, SS101–SS110. doi:10.1016/S0309-1740(98)90041-9.
- Chen, Q.; Liu, Q.; Sun, Q.; Kong, B.; Xiong, Y. Flavour Formation from Hydrolysis of Pork Sarcoplasmic Protein Extract by a Unique LAB Culture Isolated from Harbin Dry Sausage. Meat Science 2015, 100, 110–117. doi:10.1016/j.meatsci.2014.10.001.
- Chaves-López, C.; Paparella, A.; Tofalo, R.; Suzzi, G. Proteolytic Activity of Saccharomyces Cerevisiae Strains Associated with Italian Dry-Fermented Sausages in a Model System. International Journal of Food Microbiology 2011, 150, 50–58. doi:10.1016/j.ijfoodmicro.2011.07.017.
- Gallego, M.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Degradation of LIM Domain-Binding Protein Three during Processing of Spanish Dry-Cured Ham. Food Chemistry 2014, 149, 121–128. doi:10.1016/j.foodchem.2013.10.076.
- López, C.M.; Bru, E.; Vignolo, G.M.; Fadda, S.G. Identification of Small Peptides Arising from Hydrolysis of Meat Proteins in Dry Fermented Sausages. Meat Science 2015, 104, 20–29. doi:10.1016/j.meatsci.2015.01.013.
- Ikonić, P.; Tasić, T.; Petrović, L.; Škaljac, S.; Jokanović, M.; Mandić, A.; Ikonić, B. Proteolysis and Biogenic Amines Formation during the Ripening of Petrovská Klobása, Traditional Dry-Fermented Sausage from Northern Serbia. Food Control 2013, 30, 69–75. doi:10.1016/j.foodcont.2012.06.021.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; et al. Protein Measurement with the Folin Phenol Reagent. The Journal of Biological Chemistry 1951, 193, 265–275.
- Salwin, H. Biuret Method for Soluble Whey Proteins in Nonfat Dry Milk Solids. Journal of Food Science 1954, 19, 235–245. doi:10.1111/j.1365-2621.1954.tb17445.x.
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. doi:10.1038/227680a0.
- Sun, W.; Zhao, H.; Zhao, Q.; Zhao, M.; Yang, B.; Wu, N.; Qian, Y. Structural Characteristics of Peptides Extracted from Cantonese Sausage during Drying and Their Antioxidant Activities. Innovative Food Science & Emerging Technologies 2009, 10, 558–563. doi:10.1016/j.ifset.2009.07.006.
- Zeng, X.; Xia, W.; Jiang, Q.; Yang, F. Effect of Autochthonous Starter Cultures on Microbiological and Physico-Chemical Characteristics of Suan Yu, a Traditional Chinese Low Salt Fermented Fish. Food Control 2013, 33, 344–351. doi:10.1016/j.foodcont.2013.03.001.
- Chadong, K.; Yunchalard, S.; Piyatheerawong, W. Physicochemical Characteristics and Protein Degradation during Fermentation of Plaa-Som, A Traditional Fermented Fish Product of North-Eastern Thailand. Indian Journal of Traditional Knowledge 2015, 14, 220–225.
- Xu, Y.; Xia, W.; Yang, F.; Nie, X. Physical and Chemical Changes of Silver Carp Sausages during Fermentation with Pediococcus Pentosaceus. Food Chemistry 2010, 122, 633–637. doi:10.1016/j.foodchem.2010.03.023.
- Nie, X.; Lin, S.; Zhang, Q. Proteolytic Characterisation in Grass Carp Sausage Inoculated with Lactobacillus plantarum and Pediococcus pentosaceus. Food Chemistry 2014, 145, 840–844. doi:10.1016/j.foodchem.2013.08.096.
- Fadda, S.; Vildoza, M.J.; Vignolo, G. The Acidogenic Metabolism of Lactobacillus Plantarum Crl 681 Improves Sarcoplasmic Protein Hydrolysis during Meat Fermentation. Journal of Muscle Foods 2010, 21, 545–556. doi:10.1111/(ISSN)1745-4573.
- Sriket, C. Proteases in Fish and Shellfish: Role on Muscle Softening and Prevention. International Food Research Journal 2014, 21, 433–445.
- Díaz, O.; Fernandez, M.; De Fernando, G.D.G.; De La Hoz, L.; Ordoñez, J.A. Proteolysis in Dry Fermented Sausages: The Effect of Selected Exogenous Proteases. Meat Science 1997, 46, 115–128. doi:10.1016/S0309-1740(97)00013-2.
- Casaburi, A.; Aristoy, M.-C.; Cavella, S.; Di Monaco, R.; Ercolini, D.; Toldrá, F.; Villani, F. Biochemical and Sensory Characteristics of Traditional Fermented Sausages of Vallo Di Diano (Southern Italy) as Affected by the Use of Starter Cultures. Meat Science 2007, 76, 295–307. doi:10.1016/j.meatsci.2006.11.011.
- Zhou, C.Q.; Cheng, D.H.; Mu-Ying, D.U. Study on Protein Degradation and Its Affecting Factors in Fermentation Process of Sour Meat. Food Science 2009, 30, 127–130.
- Qian, Z.; Xiao-Yun, G.; Hai-Song, Z.; et al. Analysis of Components and Fermented Flavor Substances in Libo Sour Meat from Guizhou during Fermentation Period. Food Science 2013, 34, 173–177.
- Leroy, F.; Verluyten, J.; Vuyst, L.D. Functional Meat Starter Cultures for Improved Sausage Fermentation. International Journal of Food Microbiology 2006, 106, 270–285. doi:10.1016/j.ijfoodmicro.2005.06.027.
- Flores, M.; Toldrá, F. Microbial Enzymatic Activities for Improved Fermented Meats. Trends in Food Science & Technology 2011, 22, 81–90. doi:10.1016/j.tifs.2010.09.007.
- Sun, W. Sudies on Lipolysis, Protelysis and Flavor Compounds during Processing of Cantonese Sausage; South China University of Technology: Guangzhou, China, 2011.
- Hu, Y.; Xia, W.; Ge, C. Effect of Mixed Starter Cultures Fermentation on the Characteristics of Silver Carp Sausages. World Journal of Microbiology and Biotechnology 2006, 23, 1021–1031. doi:10.1007/s11274-006-9330-2.
- Hashimoto, K.; Watabe, S.; Kono, M.; Shiro, K. Muscle Protein Composition of Sardine and Mackerel. Nippon Suisan Gakkaishi 1979, 45, 1435–1441. doi:10.2331/suisan.45.1435.
- Montel, M.C.; Masson, F.; Talon, R. Bacterial Role in Flavour Development. Meat Science 1998, 49, SS111–SS123. doi:10.1016/S0309-1740(98)90042-0.
- Zhang, M.-X.; Wang, X.-C.; Liu, Y.; Xu, X.-L.; Zhou, G.-H. Isolation and Identification of Flavour Peptides from Puffer Fish (Takifugu Obscurus) Muscle Using an Electronic Tongue and MALDI-TOF/TOF MS/MS. Food Chemistry 2012, 135, 1463–1470. doi:10.1016/j.foodchem.2012.06.026.
- Henriksen, A.P.; Stahnke, L.H. Sensory and Chromatographic Evaluations of Water Soluble Fractions from Dried Sausages. Journal of Agricultural & Food Chemistry 1997, 45, 2679–2684.
- Bolumar, T.; Nieto, P.; Flores, J. Acidity, Proteolysis and Lipolysis Changes in Rapid-Cured Fermented Sausage Dried at Different Temperatures. Food Science & Technology International 2001, 7, 269–276. doi:10.1106/FPV5-Q8LM-XCWK-BC4T.
- Liu, Y.; Lishan, W.; Li, L.; et al. Analysi and Evaluation on Nutritional Compoents of Salted Fish from Southeast of Guizhou. Food Science 2008, 29, 420–422.