ABSTRACT
The occurrence of malachite green (MG) residue was determined in 177 farmed fish samples (i.e., 85 carp, Cyprinus carpio, and 92 rainbow trout, Oncorhynchus mykiss) purchased from north-west part of Iran from 2014 to 2015. Competitive enzyme immunoassay method validated according to the criteria of Commission Decision 2002/657/EC was used to determine the MG residue. The mean recoveries were in the range of 79.1–86.2% and the limit of detection of the method was 0.30 µg/kg. The value of detection capability for MG in fish muscle was less than 1 µg/kg, which was below the minimum required performance limit of 2 µg/kg. MG was detected in 108 fish samples, corresponding to 61.0% of the total examined samples, ranging between 0.35 and 7.12 µg/kg. Most of the contaminated samples (28.8%) contained MG at the level of 0.3–1 µg/kg. Altogether, 18 out of 177 samples contained residue of MG higher than 2 µg/kg. The results indicated that the usage of MG in Iranian fish farms is frequent. It is alarming and could be a potential hazard for public health.
Introduction
Malachite green (MG) is a cationic triphenylmethane dye that has been used to colour materials such as silk, wool, leather, cotton, and paper products.[Citation1,Citation2] MG is also used as a veterinary drug to control fungal infections in the fish farming industry around the world.[Citation3–Citation5] It is very effective for the treatment of external parasites and protozoan, e.g., Ichthyophthirius, Chilodonella, and Trichodina in aquaculture.[Citation6–Citation8]
MG causes damage to liver, spleen, and kidney, decreases food intake, produces teratogenic and carcinogenic effects, and is highly cytotoxic to mammalian cells. During waterborne exposure, MG is easily absorbed into fish tissues and transformed to the reduced, colourless compound, leucomalachite green (LMG), which is lipophilic and remains in fatty tissues for extended periods of time.[Citation3,Citation9–Citation11]
The use of MG in aquaculture has become a serious hazard to consumers due to its teratogenic, genotoxic, and carcinogenic properties.[Citation12] Therefore, the use of MG in food producing animals is not authorized in many countries.[Citation13,Citation14] At present, illegal use of MG in the fish farms is expanded due to its easy availability, low cost, and effectiveness. So, monitoring of MG residues in aquaculture products is necessary for safety assessment and human health protection.[Citation15,Citation16]
Several analytical methods have been reported for determination of MG and LMG residues in fish tissue. According to the European Commission, methods for determining MG in fish tissues should meet the minimum required performance limit (MRPL) of 2 µg/kg for the sum of MG and LMG.[Citation17] Enzyme-linked immunosorbent assay (ELISA),[Citation3,Citation16,Citation18] high-performance liquid chromatography coupled to visible or fluorescence detectors,[Citation19,Citation20] and mass spectrometry[Citation3,Citation21–Citation23] are the methods used for determination of MG and LMG. However, ELISA method is the common analytical technique for screening of MG residue in aquaculture products due to its high sensitivity, rapidity, and cheapness.[Citation24] This study aimed to determine the occurrence of MG in farmed fish purchased from the north-west part of Iran from 2014 to 2015.
Materials and methods
Sample collection
A total of 177 samples consisting of 85 common carp (Cyprinus carpio) and 92 rainbow trout (Oncorhynchus mykiss) samples were purchased from local markets in north-west part of Iran over a 2-year period (83 samples in 2014 and 94 samples in 2015). The samples were transported to the laboratory inside an icebox. After arrival, the fish were eviscerated, beheaded, deboned, and homogenized with a blender and stored at 20°C until analyses.
Chemicals and reagents
The MG residue in the samples was determined by competitive enzyme immunoassay using EuroProxima B.V. (Arnhem, The Netherlands) test kit. Most of the used reagents were provided by the kit manufacturer. For recovery study, MG oxalate and LMG reference standards were obtained from Sigma-Aldrich (St. Louis, MO). Methanol, acetonitrile, perchloric acid, and dichloromethane were obtained from Merck (Darmstadt, Germany). The stock standard solutions (1000 ng/ml) were prepared by dissolving 10 mg standard in 10 ml methanol and stored at 20°C. Working solutions (10 ng/ml) were prepared daily by diluting stock solutions with methanol and stored at 4°C.
Instruments
The following instruments were used in sample preparation: T25 homogenizer (IKA WERKE GMBH & CO.KG, Germany), 2–16 P centrifuge (Sigma labor zentrifugen GMBH, Germany), nitrogen evaporation system N-EVAP model 11250 (Orgamonation Associates Inc., Berlin, USA), and BioTek ELx800 Absorbance Microplate Readers (BioTek Instruments Inc., USA).
Sample extraction
A portion (2 g) of the ground sample was weighed into 50 ml polypropylene tube. An amount of 8 ml of perchloric acid–acetonitrile mixture was added and vortexed for 1 min. Then, 4.5 ml of dichloromethane was added and vortexed for 10 min. Following centrifugation for 5 min at 4000 rpm, 5 ml of the supernatant was pipetted into a glass tube and evaporated to dryness under mild stream of nitrogen at 50°C. Residues were dissolved in 25 µl methanol and 215 µl sample dilution buffer. After centrifugation (4000 rpm, 10 min), 50 µl of the extract was used for ELISA test.
Test procedure
An amount of 50 µl of the test samples and MG standard solutions in duplicate were added to the wells of microtiter plates precoated with MG antibodies. Then, 25 µl of enzyme conjugate and 25 µl of antibody solution were added to the wells and incubated for 30 min at 4°C in the dark place. In the next stage, the wells were washed three times with washing buffer. Afterward, 100 µl of substrate was added to the wells and incubated for 15 min at room temperature. Finally, the reaction was stopped by adding 100 µl of stop solution to each well, and the absorbance was read at 450 nm in ELISA plate reader.
Method validation
Validation of the method was determined according to the criteria laid down by Commission Decision 2002/657/EC for qualitative screening methods.[Citation25] Accuracy of the method was checked by analysing blank samples of trout and carp fortified with MG and LMG at levels of 2, 3, and 4 µg/kg. At each level, analyses were performed with six replicates. The mentioned analyses were carried out on three different days. The recovery was calculated by the following formula: (the measured content/the fortified level) ×100. The precision (repeatability and within-laboratory reproducibility) was expressed as the relative standard deviation (RSD) of the recovery.
The limit of detection (LOD) was calculated from the mean value of 20 blank samples plus three times the standard deviation. To determine the matrix effects, detection capability (CCβ) was determined by analysing 20 blank samples fortified at a level of 1 µg/kg with MG. To evaluate the selectivity/specificity of the method, blank samples of rainbow trout and carp muscles (n = 20) obtained from different specimens were analysed. The samples were spiked with known amounts (10 µg/kg) of crystal violet and leucocrystal violet to evaluate possible interference that may occur in the method.
Statistical analyses
The statistical analyses were performed using t-test and chi-square test of the SPSS software version 20 for windows (SPSS Inc., Chicago, IL, USA) to compare the detection rates and concentrations of MG.
Results and discussion
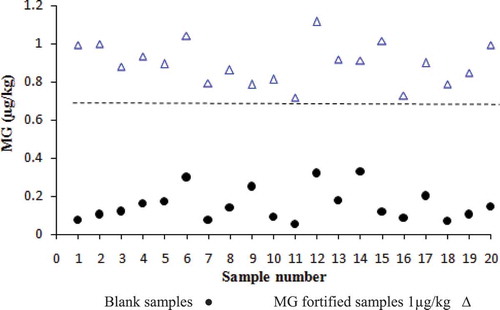
According to the validation procedure, the mean recoveries for MG were in the range of 79.1–86.2% and the RSDs were less than 9.3% and 10.8% for repeatability and within-laboratory reproducibility, respectively. The LOD of the method was 0.30 µg/kg (). However, the recovery values of LMG were in the range of 15–18% which is not in accordance with regulations set by European Commission Decision 2002/657/EC for required recovery of 70%.[Citation25] The validation parameters indicated that the method is not appropriate for the detection of LMG. Also in the validation study, as shown in , there was no overlap between the blank and fortified samples so the value of CCβ for MG was less than 1 µg/kg, which is below the MRPL of 2 µg/kg. The selectivity/specificity of the method for crystal violet and leucocrystal violet was less than 10%. The overall results indicate that the method is only suitable for determination of MG in fish muscle samples.
Table 1. Accuracy and precision of the method for malachite green (MG) and leucomalachite green (LMG) in spiked samples (n = 6).
The concentrations of MG in the farmed fish samples over the 2-year period are shown in . MG was detected in 108 samples, corresponding to 61.0% of the total examined samples, ranging between 0.35 and 7.12 µg/kg. As shown in , most of the contaminated samples (28.8%) contained MG at the level of 0.3–1 µg/kg. The mean concentration of MG was significantly higher (p < 0.05) in the samples obtained in the year 2015 (1.99 µg/kg) than those obtained in the year 2014 (1.04 µg/kg). Altogether, 18 out of 177 samples contained residue of MG higher than 2 µg/kg. The improper administration of veterinary drugs (especially antibiotics) in Iranian fish farms has caused the emergence of resistant microorganisms specifically resistant bacteria and fungi. To solve this problem, farmers have found MG highly effective and also attractive because of its low cost.
Table 2. Malachite green concentrations in farmed fish in north-west part of Iran.
The results of the present study are comparable to those reported in some other surveys on MG residue in fish samples. MG residue was reported from various food products in India; and it was more prevalent in foodstuffs from rural markets than those from urban markets.[Citation26] In a survey conducted in Slovenia, 7 out of 33 trout samples (21.2%) obtained from fish farms and markets contained residues of MG.[Citation19] In Croatia, a 3-year period survey showed the MG residue in 13 out of 72 farmed fish samples (18.1%) ranging between 0.0004 and 1.07 µg/kg.[Citation24] In a previous survey conducted in Iran, 70 out of 144 rainbow trout samples (48.6%) contained residues of MG, ranging between 0.3 and 146.1 µg/kg.[Citation27] In a recent study performed in Iran, it was found that 70 out of 120 samples (58.4%) obtained from fish markets contained detectable residues of MG.[Citation6]
In order to determine the residues of MG in farmed fish samples in the United Kingdom, monitoring and inspection programs were continuously performed from 2001 to 2011.[Citation28] In 2001, 2002, and 2003, 16.1%, 9.9%, and 4.2% of tested farmed salmon and trout samples contained MG and LMG residues, respectively. The fish farms which use MG dye were restricted, and their products did not enter the food chain. The Rapid Alert System for Food and Feed in the European Union recorded 123 cases of high concentrations of MG and LMG in fish and fish products in the period from 2002 to 2015.[Citation29] Most of the reported cases (more than 50%) were the residues of MG in catfish, pangasius fillets, tilapia, and milkfish originating from the Asian countries.[Citation29] The European Commission has requested the Member States to perform national surveillance programs for monitoring of certain substances and residues in live animals and animal products based on Council Directive 96/23/EC.[Citation30] According to its last report, 1.8% of the analysed aquaculture samples were contaminated with unauthorized dyestuffs such as MG, LMG, crystal violet, or leucocrystal violet.[Citation31] Despite the fact that triphenylmethane dyes such as MG and crystal violet are not permitted as a veterinary medicine in many countries, they are still used in aquaculture industry. Therefore, monitoring and inspection programs in various aquaculture products must be well conducted regarding the harmful potential effects of these residues on human health.
Conclusion
This study showed a high incidence of MG residue in farmed fish samples obtained from north-west part of Iran. It is also indicated that the usage of MG dye in this part of Iran is increasing per year. Although farmed fish are not exported from Iran, long-term consumption of contaminated fish can cause health hazards, including effects on the immune system and reproductive system as well as genotoxic and carcinogenic potentials. Also if enough attention is not paid to the problem, MG contained in farmed waste water might enter the aquatic environment and cause serious environmental hazards. Since this study is limited to MG residue, further investigations should be carried out to determine the LMG residue in farmed fish.
Declaration of interest
All authors declare no conflicts of interest.
References
- Šafařı́k, I.; Šafařı́ková, M. Detection of Low Concentrations of Malachite Green and Crystal Violet in Water. Water Research 2002, 36, 196–200.
- Stammati, A.; Nebbia, C.; De Angelis, I.; Albo, A.G.; Carletti, M.; Rebecchi, C.; Zampaglioni, F.; Dacasto, M. Effects of Malachite Green (MG) and Its Major Metabolite, Leucomalachite Green (LMG), in Two Human Cell Lines. Toxicology In Vitro 2005, 19, 853–858.
- Alderman, D.J. Malachite Green: A Review. Journal of Fish Diseases 1985, 8, 289–298.
- Chen, R.C.; Wei, K.J.; Wang, T.M.; Yu, Y.M.; Li, J.Y.; Lee, S.H.; Wang, W.H.; Ren, T.J.; Tsai, C.W. Simultaneous Quantification of Antibiotic Dyes in Aquatic Products and Feeds by Liquid Chromatography–Tandem Mass Spectrometry. Journal of Food and Drug Analysis 2013, 21, 339–346.
- Stolker, A.A.M.; Brinkman, U.T. Analytical Strategies for Residue Analysis of Veterinary Drugs and Growth-Promoting Agents in Food-Producing Animals—A Review. Journal of Chromatography Part A 2005, 1067, 15–53.
- Adel, M.; Dadar, M.; Oliveri Conti, G. Antibiotics and Malachite Green Residues in Farmed Rainbow Trout (Oncorhynchus Mykiss) from the Iranian Markets: A Risk Assessment. International Journal of Food Properties 2017, 20, 402–408.
- Ekanem, A.P.; Obiekezie, A.; Kloas, W.; Knopf, K. Effects of Crude Extracts of Mucuna pruriens (Fabaceae) and Carica papaya (Caricaceae) against the Protozoan Fish Parasite Ichthyophthirius multifiliis. Parasitology Research 2004, 92, 361–366.
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological Effects of Malachite Green. Aquatic Toxicology 2004, 66, 319–329.
- Mitrowska, K.; Posyniak, A.; Zmudzki, J. Determination of Malachite Green and Leucomalachite Green Residues in Water Using Liquid Chromatography with Visible and Fluorescence Detection and Confirmation by Tandem Mass Spectrometry. Journal of Chromatography Part A 2008, 1207, 94–100.
- Plakas, S.; El Said, K.; Stehly, G.; Gingerich, W.; Uptake, A.J. Tissue Distribution, and Metabolism of Malachite Green in the Channel Catfish (Ictalurus punctatus). Canadian Journal of Fisheries and Aquatic Sciences 1996, 53, 1427–1433.
- Robaina, N.F.; Reis, L.G.T.D.; Cassella, R.J. Diffuse Reflectance Determination of Malachite Green Using Polyurethane Foam as Solid Support and Sodium Dodecylsulfate as Counter Ion. Talanta 2011, 85, 749–753.
- Gouranchat, C. Malachite Green in Fish Culture (State of the Art and Perspectives). Bibliographic Study. Ecole Natl Veterinaire ENVT, Nantes (France) 2000, 142, 2000.
- López-Gutiérrez, N.; Romero-González, R.; Plaza-Bolaños, P.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Simultaneous and Fast Determination of Malachite Green, Leucomalachite Green, Crystal Violet, and Brilliant Green in Seafood by Ultrahigh Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Anal Method 2013, 6, 406–414.
- Sudova, E.; Machova, J.; Svobodova, Z.; Vesely, T. Negative Effects of Malachite Green and Possibilities of Its Replacement in the Treatment of Fish Eggs and Fish: A Review. Veterinarni Medicina 2007, 52, 527–539.
- Schnick, R.A. The Impetus to Register New Therapeutants for Aquaculture. The Progressive Fish-Culturist 1988, 50, 190–196.
- Yang, M.C.; Fang, J.M.; Kuo, T.F.; Wang, D.M.; Huang, Y.L.; Liu, L.Y.; Chen, P.H.; Chang, T.H. Production of Antibodies for Selective Detection of Malachite Green and the Related Triphenylmethane Dyes in Fish and Fishpond Water. Journal of Agricultural and Food Chemistry 2007, 55, 8851–8856.
- European Communities. Commission Decision 2004/25/EC, Amending Decision 2002/657/EC as Regards the Setting of Minimum Required Performance Limits (MRPLs) for Certain Residues in Food of Animal Origin. Official. Journal of European Community 2004, L6, 38–39.
- Xing, W.; He, L.; Yang, H.; Sun, C.; Li, D.; Yang, X.; Li, Y.; Deng, A. Development of a Sensitive and Group-Specific Polyclonal Antibody-Based Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Malachite Green and Leucomalachite Green in Water and Fish Samples. Journal of the Science of Food and Agriculture 2009, 89, 2165–2173.
- Bajc, Z.; Doganoc, D.Z.; Gačnik, K.Š. Determination of Malachite Green and Leucomalachite Green in Trout and Carp Muscle by Liquid Chromatography with Visible and Fluorescence Detection. Slovenian Veterinary Research 2007, 44, 81–90.
- Mitrowska, K.; Posyniak, A.; Zmudzki, J. Determination of Malachite Green and Leucomalachite Green in Carp Muscle by Liquid Chromatography with Visible and Fluorescence Detection. Journal of Chromatography Part A 2005, 1089, 187–192.
- Nebot, C.; Iglesias, A.; Barreiro, R.; Miranda, J.M.; Vázquez, B.; Franco, C.M.; Cepeda, A. A Simple and Rapid Method for the Identification and Quantification of Malachite Green and Its Metabolite in Hake by HPLC–MS/MS. Food Control 2013, 31, 102–107.
- Tao, Y.; Chen, D.; Chao, X.; Yu, H.; Yuanhu, P.; Liu, Z.; Huang, L.; Wang, Y.; Yuan, Z. Simultaneous Determination of Malachite Green, Gentian Violet and Their Leuco-Metabolites in Shrimp and Salmon by Liquid Chromatography–Tandem Mass Spectrometry with Accelerated Solvent Extraction and Auto Solid-Phase Clean-Up. Food Control 2011, 22, 1246–1252.
- Valle, L.; Díaz, C.; Zanocco, A.L.; Richter, P. Determination of the Sum of Malachite Green and Leucomalachite Green in Salmon Muscle by Liquid Chromatography–Atmospheric Pressure Chemical Ionisation-Mass Spectrometry. Journal of Chromatography Part A 2005, 1067, 101–105.
- Bilandžić, N.; Varenina, I.; Kolanović, B.S.; Oraić, D.; Zrnčić, S. Malachite Green Residues in Farmed Fish in Croatia. Food Control 2012, 26, 393–396.
- European Communities. Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Official. Journal of European Community 2002, L221, 8–36.
- Tripathi, M.; Khanna, S.K.; Das, M. Surveillance on Use of Synthetic Colours in Eatables Vis a Vis Prevention of Food Adulteration Act of India. Food Control 2007, 18, 211–219.
- Fallah, A.A.; Barani, A. Determination of Malachite Green Residues in Farmed Rainbow Trout in Iran. Food Control 2014, 40, 100–105.
- Veterinary Residues Committee’s. Annual Report on Surveillance for Veterinary Residues in Food in UK for 2001 to 2011. http://www.vmd.defra.gov.uk/VRC/reports/annual.html
- Rapid alert system for food and feed. https://webgate.ec.europa.eu/rasff-window/portal/index.cfm/event/searchResultList 2013.
- European Commission. Council Directive 96/23/EC of 29 April 1996 on Measures to Monitor Certain Substances and Residues Thereof in Live Animals and Animal Products and Repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Official Journal of the European Communities 1996, L125, 1e28.
- European Commission. Commission Staff Working Document on the Implementation of National Residue Monitoring Plans in the Member States in 2010 (Council Directive 96/23/EC). https://ec.europa.eu/food/food/chemicalsafety/residues/control_en.htm 2010.