ABSTRACT
This study was designed to evaluate and compare the interactions of essential oils from orange (Citrus sinensis [L.] Osbeck) and lemon (Citrus limon) peels on enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin-I-converting enzyme [ACE]). The essential oils were obtained from the peels by hydrodistillation and were passed over anhydrous sodium sulphate. The interaction of the oil on α-amylase, α-glucosidase, and ACE activities was subsequently determined. The results revealed that the essential oils inhibited α-amylase and α-glucosidase activities. However, lemon peel (IC50 = 8.16 µg/mL and 7.56 µg/mL) essential oil exhibited stronger inhibitory activity on α-amylase and α-glucosidase activities compared to orange peels (IC50 = 11.51 µg/mL and 11.53 µg/mL), respectively. Similarly, both essential oils reduced the activity of ACE. Moreover, lemon peel (IC50 = 26.17 µg/mL) essential oil showed higher inhibitory effect compared to orange peel (IC50 = 31.79 µg/mL). Our findings revealed that essential oils from lemon and orange peels are potential antidiabetic and antihypertensive agents. Moreover, essential oil from lemon peels was more potent than orange peels.
Introduction
Essential oils are aromatic oily liquids which are volatile in nature and are present in different parts of plants. These volatile liquids are known to contain different classes of compounds such as monoterpenes, sesquiterpenes, diterpenes, triterpenes, and terpenoids. Research into the use of essential oils has gained much interest recently and many of these oils are used as food preservatives, flavourants, and alternative therapy in traditional medicine for the treatment of some diseases.[Citation1,Citation2] Essential oils have been reported to possess several biological activities which include antioxidative, antimicrobial, anti-inflammatory, antiaflatoxigenic, anticancer, and antidiabetic activities.[Citation3–Citation5] Furthermore, essential oils have been extracted from the leaves, stem, roots, and peels of different plants.
Citrus sp. have been identified as excellent sources of essential oils. Citrus essential oil is largely present in the peels compared to other parts and it has a wide application in food industries as additives in the formulation of syrup, drinks, cakes, and ice cream.[Citation6,Citation7] The yield of essential oils and their chemical constituents vary between species and their origin. The oils are present within the glands of the external layer of the fruit skin.[Citation7] Several reports have revealed the chemical composition of essential oils from some Citrus sp.[Citation8–Citation10] Previous reports from our laboratory have shown the chemical composition, anticholinesterase, and antioxidant activities of essential oils from orange and lemon peels.[Citation11,Citation12] However, we observed that there is dearth of information on the antidiabetic and antihypertensive activities of essential oils from orange and lemon peels.
Diabetes mellitus is a major public health problem affecting millions of people all over the world.[Citation13,Citation14] The onset and development of type-2 diabetes is characterized by consistent hyperglycaemia due to enzymatic degradation of carbohydrates and lipids in the cell which affects glucose metabolism.[Citation15] Common therapeutic strategy to reduce hyperglycaemia in diabetic patients involves the inhibition of carbohydrate hydrolysing enzymes. These enzymes (α-amylase and α-glucosidase) break down complex carbohydrates to glucose which is rapidly absorbed into the bloodstream leading to hyperglycaemia.[Citation16] Previous reports have revealed that diabetes and hypertension are interrelated as 75% of diabetic patients are known to be hypertensive.[Citation17,Citation18] Type-2 diabetic patients are more prone to develop hypertension which is a major risk factor of cardiac, renal, and cerebral dysfunction.[Citation19] Angiotensin-converting enzyme (ACE) plays a major role in the development of hypertension. Increase in ACE activity leads to elevated levels of angiotensin II which is a potent vasoconstrictor that can raise blood pressure.[Citation20] However, ACE inhibitors can reduce blood pressure in hypertensive patients by limiting the conversion of angiotensin I to angiotensin II. Therefore, in this study, we report for the first time a comparative study on the modulatory effects of essential oils from orange peels on α-amylase, α-glucosidase, and ACE activities.
Materials and methods
Sample collection and preparation
Orange fruits were purchased from Akure main market in Ondo State, while lemon fruits were obtained in a farm in Akute Ogun State both in Southwest Nigeria. The fruits were identified and authenticated at the Biology department, Federal University of Technology of Akure, Nigeria. The peels were sun dried to constant weight and pulverised into powder using a laboratory blender.
Extraction of essential oil
Essential oil was extracted from the powder of the orange peels (100 g) by hydrodistillation via a Clevenger apparatus.[Citation21] The essential oil layer was collected from the apparatus via the outlet and passed over anhydrous sodium sulphate. The oils were stored separately in sealed vials at 4°C for further analysis.
Gas chromatography analysis
Essential oils from orange and lemon peels were analysed using Hewlett-Packard 5890 gas chromatograph equipped with flame ionization detectors (FID) and DB-5 column. The sample 1 µL solution in hexane (1:100) was injected into the system. The following conditions were applied: (1) injection temperature: 290°C; (2) injection volume: 1.0 µL; (3) injection mode: split (1:50); (4) temperature program: 50°C for 4 min, rising at 3°C/min to 240°C, then rising at 15°C/min to 300°C, held at 300°C for 3 min; and (5) FID (290°C): (i) H2 flow: 50 mL/min and (ii) air flow: 400 mL/min. The individual constituents of the essential oils were identified via Mass spectrometry (MS) and their identity was confirmed in comparison with mass spectra of authentic standards and that of the data that are already available in National institute for standards (NIST) and Wiley mass spectral libraries. The per cent composition of the essential oils was computed from the area of the peaks of the Gas Chromatography (GC). Kovats retention index (KI) was calculated using the KI formula.
α-Amylase inhibition assay
The reaction mixture that contains different concentration of each essential oil (0–16 µg/mL) and 500 µL of Hog pancreatic α-amylase (EC 3.2.1.1) (0.5 mg/mL) was incubated at 25°C for 10 min. In total, 500 µL of starch (1%) solution prepared with 0.02 M sodium phosphate buffer was added to the previous mixture and was incubated for 10 min at 25°C. Dinitrosalicyclic acid (1.0 mL) was added to the mixture to stop the reaction. The resultant solution was incubated for 5 min at 100°C. The solution was allowed to cool and distilled water was added to the mixture before the absorbance was measured at 540 nm.[Citation22] The α-amylase activity was calculated and expressed as percentage inhibition using the following formula:
α-Glucosidase inhibition assay
The enzyme (1.0 U/mL) was prepared in phosphate buffer (0.1 M). Different concentration of the essential oil (0–16 µg/mL) was added to 100 µL of the enzyme solution which was incubated at 25°C for 10 min. Moreover, 50 µL of p-nitrophenyl-α-D-glucopyranoside solution (5 mM) which was prepared in 0.1 M phosphate buffer (pH 6.9) was added. The solution was incubated at room temperature for 5 min. After the incubation, the absorbance was measured at 405 nm.[Citation13] The α-glucosidase inhibitory activity was expressed as percentage inhibition using Eq. (1).
Angiotensin-I-converting enzyme inhibition assay
The effect of the essential oils on ACE activity was evaluated using the method of Golbidi et al.[Citation19] ACE solution (50 µL, 4 mU) and the essential oils (0–60 µg/mL) were incubated at 37°C for 15 min. After the incubation, 150 μL of 8.33 mM of Bz–Gly–His–Leu in 125 mM Tris–HCl buffer (pH 8.3) was added to the solution. The mixture was incubated at 37°C for 30 min and 250 µL of 1 M HCl was added to stop the reaction. The Bz–Gly formed in the reaction was extracted with 1.5 mL ethyl acetate. After the extraction, the ethyl acetate fraction was separated by centrifugation and 1 mL of the fraction was allowed to evaporate in a test tube. The residue that was formed was dissolved with distilled water and the absorbance was measured at 228 nm.[Citation23] The ACE inhibitory activity was calculated and expressed as percentage inhibition using Eq. (1).
Data analysis
All the experiments were performed in triplicates and the data were expressed as mean ± standard deviation (SD). Statistical analysis was conducted by one-way analysis of variance using Graph pad prism 5.0. The concentration of the samples that caused 50% inhibition was calculated using nonlinear regression analysis.
Results and discussion
Chemical constituents of essential oils
presents the components of essential oils from orange and lemon peels which were identified via GC–MS. The essential oil constituents comprise of monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpene hydrocarbons and were listed in their order of their elution with their KI. The most abundant compound in orange peel essential oil is limonene (92.14%). Moreover, β-myrcene (2.70%) was also present in appreciable levels compared to other compounds. However, lemon peel had lower levels of limonene (53.07%) compared to orange peels. Other constituents such as β-pinene (9.53), borneol (5.57%), neral (4.70%), sabinene (4.18%), α-pinene (3.82%), linalool (3.7), 1,8-cineole (3.38%), β-myrcene (3.33%), geranial (3.33%), β-caryophyllene (1.49%), and linalyl acetate (1.48%) were present in appreciable levels compared to orange peels.
Table 1. Chemical composition of essential oil.
Effect of essential oil on α-amylase and α-glucosidase activities
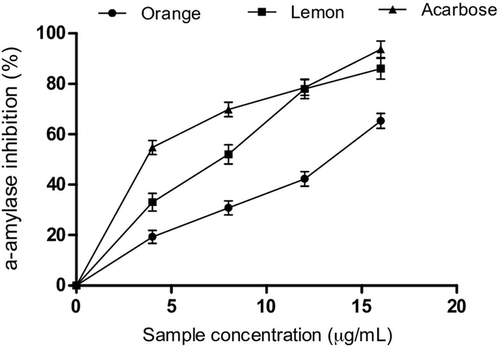
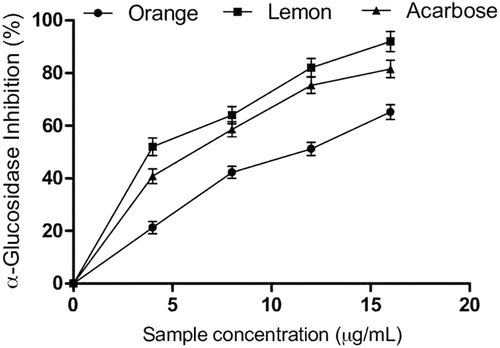
The antidiabetic activity of essential oils from orange and lemon peels was evaluated through different methods. The result obtained from the enzyme assays revealed that essential oils from orange peels (IC50 = 11.51 µg/mL) exhibited lower capacity to inhibit α-amylase activity than lemon peels (IC50 = 8.16 µg/mL) as shown in and the IC50 values in . Moreover, both essential oils showed lower inhibitory effects on α-amylase activity compared to acarbose (IC50 = 7.45 µg/mL). Similarly, orange peel (IC50 = 11.53 µg/mL) essential oils exhibited lower inhibitory effects on α-glucosidase activity than lemon peel oil (IC50 = 7.56 µg/mL) as shown in and the IC50 values in . However, essential oils from lemon peels exhibited higher inhibitory capacity on α-glucosidase activity than acarbose (IC50 = 8.44 µg/mL). Previous experimental investigation has shown that α-amylase and α-glucosidase are important therapeutic targets in the management and/or treatment of type-2 diabetes.[Citation24] α-amylase hydrolyses starch by degrading the internal glycosidic bonds to release oligosaccharides which are further hydrolysed by α-glucosidase into monosaccharides and are absorbed into the bloodstream.[Citation25] The accumulation of these monosaccharides especially glucose in the bloodstream leads to postprandial hyperglycaemia, a major risk factor in the pathogenesis of diabetes mellitus.[Citation26] The essential oils from orange and lemon peels which were used in this study inhibited α-amylase and α-glucosidase activities. Our findings revealed that lemon peel essential oil exhibited higher inhibitory effects on α-amylase and α-glucosidase activities compared to orange peels. More importantly, our findings revealed that lemon peel essential oil showed higher inhibitory effects on acarbose. Moreover, the α-glucosidase inhibitory activity of lemon peels was higher than that of α-amylase inhibitory activity. Previous report has shown that high α-glucosidase inhibition is more effective in the management of type-2 diabetes.[Citation25] This is due to the fact that antidiabetic drugs such as acarbose inhibits α-amylase excessively and this may lead to several side effects. The inhibition of α-amylase activity will limit the hydrolysis of the internal glycosidic linkages of polysaccharides, while decrease in α-glucosidase activity will limit the breakdown of disaccharides and delay glucose absorption into the blood from the small intestine.[Citation27] The observed inhibitory effects of the essential oils give credence to the fact that both essential oils could be promising antidiabetic agents. However, our findings suggest that lemon peel essential oil is more potent than orange peel oil. The observed higher inhibitory capacity of essential oil from lemon is not fully understood; however, our findings suggest that the antidiabetic activities of both essential oils could be due to the synergistic effects of monoterpenes and sesquiterpene hydrocarbons which were identified in the oils. An in vivo investigation on the antidiabetic effect of limonene revealed that this monoterpene exhibited hypoglycaemic effects in streptozotocin-induced diabetic rats.[Citation28] Furthermore, previous report from our laboratory on the antidiabetic activity of clove bud showed that its essential oil inhibited α-amylase and α-glucosidase activities in vitro and the inhibitory effects were attributed to the chemical components.[Citation29] Similarly, some reports have also revealed that terpenes are capable of exerting blood glucose lowering effects and monoterpenes such as α-pinene, β-pinene, myrcene, 1,8-cineole, and sabinene have been identified as potent inhibitors of α-amylase and α-glucosidase activities.[Citation28–Citation31]
Table 2. IC50 values of α-amylase, α-glucosidase, and ACE inhibition.
Table 3. R2 values of α-amylase, α-glucosidase, and ACE inhibition.
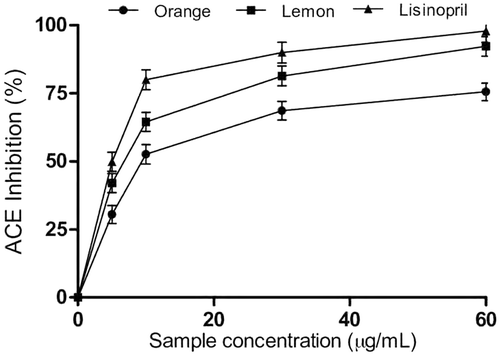
Antihypertensive activity of essential oils from orange and lemon peels
shows the interaction of orange and lemon peel essential oils and lisinopril on ACE activity. Lemon peel oil (IC50 = 26.17 µg/mL) showed significant inhibitory capacity on ACE activity than orange peel oil (IC50 = 31.79 µg/mL). The IC50 in also revealed that lisinopril (IC50 = 24.03 µg/mL) was more potent than essential oils from orange and lemon peels. The inhibition of ACE is an important therapeutic intervention in the management/treatment of hypertension. The decrease in ACE activity hinders the conversion of angiotensin I to angiotensin II, a vasoconstrictor which has been implicated in elevated blood pressure.[Citation32] In addition, inhibition of angiotensin-II production is could also prevent the progression of type-2 diabetes as elevated levels angiotensin II may induce insulin insensitivity in the peripheral tissues, which is critical in the development of this degenerative condition.[Citation33] In this study, we observed that the essential oil from orange and lemon peels reduced ACE activity. Moreover, the ACE inhibitory of the essential oils agrees with previous studies which revealed that essential oils from Periploca laevigata, Thymus algeriensis, and Aframomum danielli inhibited ACE activity in vitro.[Citation10,Citation34,Citation35] Lemon peels exhibited stronger inhibitory capacity on ACE compared to orange peels. However, lisinopril exhibited stronger inhibitory effects than the essential oils from lemon and orange peels. Previous studies have linked the ACE inhibitory activities of bioactive compounds such as terpenoids and flavonoids to their ability to compete with the substrate of the enzyme and chelation of zinc metal ions at the active site.[Citation10] Furthermore, the ACE inhibitory activity of the essential oils can also prevent the generation of reactive oxygen species which is induced by elevated levels of angiotensin II. The terpenoids, monoterpenes, and sesquiterpenes which are present in the essential oils could contribute to their inhibitory effect on ACE. Our findings revealed that essential oils from orange and lemon peels are potential antihypertensive agents, although essential oil from lemon peels was more potent than orange peels.
Conclusion
This study revealed that essential oils from orange and lemon peels show in vitro inhibitory effects on enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (ACE). Moreover, lemon peel essential oil exhibited higher antidiabetic and antihypertensive activities compared to orange peels. Our findings suggest that these essential oils are potential antidiabetic and antihypertensive agents.
References
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287.
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. Journal of Food Science 2014, 79, R1231–1249.
- Odeh, F.; Rahmo, A.; Alnori, A.S.; Chaty, M.E. The Cytotoxic Effect of Essential Oils Citrus Aurantium Peels on Human Colorectal Carcinoma Cell Line (LIM1863). Jourrnal of Microbiology Biotechnology and Food Sciences 2012, 1(6), 1476–1487.
- Mohamed, A.; Emary, G.; Ali, H. Influence of Some Citrus Essential Oils on Cell Viability, Glutathione-S-Transferase and Lipid Peroxidation in Ehrlich Ascites Carcinoma Cells. Journal of American Science 2010, 6(10), 820–826.
- Siddique, S.; Shafique, M.; Parveen, Z.; Khan, S.; Khanum, R. Volatile Components, Antioxidant and Antimicrobial Activity of Citrus Aurantiumvar Bitter Orange Peel Oil. Pharmacology Online 2011, 2, 499–507.
- Hosni, K.; Kerkenni, A.; Medfei, W.; Brahim, N.; Sebei, H. Volatile Oil Constituents of Rosa Caninal.: Quality as Affected by the Distillation Method. Organic Chemistry International 2010, 2010, 7.
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Natural Product Research 2016. DOI:10.1080/14786419.2016.1219860
- Tao, N.; Liu, Y.; Zhang, M. Chemical Composition and Antimicrobial Activities of Essential Oil from the Peel of Bingtang Sweet Orange (Citrus Sinensis [L.] Osbeck). International Journal of Food Science and Technology 2009, 44, 1281–1285.
- Abu-Darwisha, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical Composition and Biological Activities Ofartemisia Judaica Essential Oil from Southern Desert of Jordan. Journal of Ethnopharmacology 2016, 191, 161–168.
- Zouari, N.; Fakhfakh, N.; Zouari, S.; Bougatef, A.; Karray, A.; Neffati, M.; Ayadi, M.A. Chemical Composition, Angiotensin I-Converting Enzyme Inhibitory, Antioxidant and Antimicrobial Activities of Essential Oil of Tunisian Thymus Algeriensis Boiss. Et Reut. (Lamiaceae). Food and Bioproducts Processing 2011, 89(4), 257–265.
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential Oil from Lemon Peels Inhibit Key Enzymes Linked to Neurodegenerative Conditions and Pro-Oxidant Induced Lipid Peroxidation. Journal of Oleo Science 2014, 63(4), 373–381.
- Ademosun, A.O.; Oboh, G.; Olupona, A.J.; Oyeleye, S.I.; Adewuni, T.M.; Nwanna, E.E. Comparative Study of Chemical Composition, In Vitro Inhibition of Cholinergic and Monoaminergic Enzymes, and Antioxidant Potentials of Essential Oil from Peels and Seeds of Sweet Orange (Citrus Sinensis [L.] Osbeck) Fruits. Journal of Food Biochemistry 2016, 40(1), 53–60.
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innovative Food Science and Emerging Technologies 2007, 8, 46–54.
- Manaharan, T.; Palanisamy, U.D.; Ming, C.H. Tropical Plant Extracts as Potential Antihyperglycemic Agents. Molecules 2012, 17, 5915–5923.
- Bild, D.; Teutsch, S.M. The Control of Hypertension in Persons with Diabetes: A Public Health Approach. Public Health Reports 1987, 102, 522–529.
- Epstein, M.; Sowers, J.R. Diabetes Mellitus and Hypertension. Hypertension 1992, 19, 403–418.
- Lago, R.M.; Singh, P.P.; Nesto, R.W. Diabetes and Hypertension. Nature Clinical Practice Endocrinology and Metabolism 2007, 3, 667.
- Bereketoglu, C.; Kasap, M.; Pazarbas, A. Studies on Angiotensin-Converting Enzyme Insertion/Deletion Polymorphism and Genotype Distributions in Turkish Preeclampsia Patients. Journal of Pregnancy 2012. DOI:10.1155/2012/108206.
- Golbidi, S.; Ebadi, S.A.; Laher, I. Antioxidants in the Treatment of Diabetes. Current Diabetes Review 2011, 7(2), 106–125.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazura, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. International Journal Biochemistry and Cell Biology 2007, 39, 44–84.
- Council of Europe. European Pharmacopoeia, 3rd Ed., Council of Europe Press: Strasbourg, 1997, 121–122.
- Worthington Biochemical Corp. Worthington Enzyme and Related Biochemicals; Worthington Biochemical Corp: Freehold, NJ, 2001.
- Cushman, W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin I-Converting Enzyme of Rabbit Lung. Biochemical Pharmacology 1971, 20, 1637–1648.
- Nazaruk, J.; Borzym-Kluczyk, M. The Role of Triterpenes in the Management of Diabetes Mellitus and Its Complications. Phytochemistry Review 2015, 14, 675–690.
- Ademiluyi, A.O.; Oboh, G. Soybean Phenolic-Rich Extracts Inhibit Key-Enzymes Linked to Type 2 Diabetes (α-Amylase and α-Glucosidase) and Hypertension (Angiotensin I Converting Enzyme) In Vitro. Experimental Toxicology Pathology 2013, 65(3), 305–359.
- Aryangat, A.V.; Gerich, J.E. Type 2 Diabetes: Postprandial Hyperglycemia and Increased Cardiovascular Risk. Vascular Health and Risk Management 2010, 6, 145–155.
- Adedayo, B.C.; Oboh, G.; Oyeleye, S.I.; Olasehinde, T.A. Antioxidant and Antihyperglycemic Properties of Three Banana Cultivars (Musa Spp.). Scientifica (Cairo) 2016, 2016, 8391398.
- Murali, R.; Saravanan, R. Antidiabetic Effect of D-Limonene, a Monoterpene in Streptozotocin-Induced Diabetic Rats. Biomedicine and Preventive Nutrition 2012, 2, 269–275.
- Oboh, G.; Akinbola, A.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential Oil from Clove Bud (Eugenia Aromatic Kuntze) Inhibit Key Enzymes Relevant to the Management of Type-2 Diabetes and Some Pro-Oxidant Induced Lipid Peroxidation in Rats Pancreas In Vitro. Journal Oleo Science 2015, 65, 775–782.
- Hamde, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Feki, A.; Allouche, N. Inhibitory Potential of Omega-3 Fatty and Fenugreek Essential Oil on Key Enzymes of Carbohydrate-Digestion and Hypertension in Diabetes Rats. Lipids in Health and Disease 2011, 10, 226.
- Basak, S.; Candan, F. Chemical Composition and In Vitro Antioxidant and Antidiabetic Activities of Eucalyptus Camaldulensis Dehnh. Essential Oil. Journal of the Iranian Chemical Society 2010, 7, 216–226.
- Shodehinde, S.A.; Adefegha, S.A.; Oboh, G.; Oyeleye, S.I.; Olasehinde, T.A.; Nwanna, E.E.; Adedayo, B.C.; Boligon, A.A. Phenolic Composition and Evaluation of Methanol and Aqueous Extracts of Bitter Gourd (Momordica Charantia L) Leaves on Angiotensin-I-Converting Enzyme and Some Pro-Oxidant-Induced Lipid Peroxidation In Vitro. Journal of Evidence Based Complementary and Alternative Medicine 2016, 21(4), NP67–NP76.
- Chu, K.Y.; Leung, P.S. Angiotensin II in Type 2 Diabetes Mellitus. Current Protein and Peptide Science 2009, 10(1), 75–84.
- Hajji, M.; Masmoudi, O.; Souissi, N.; Triki, Y.; Kammoun, S.; Nasri, M. Chemical Composition, Angiotensin I-Converting Enzyme (ACE) Inhibitory, Antioxidant and Antimicrobial Activities of the Essential Oil from Periploca Laevigata Root Barks. Food Chemistry 2010, 121(3), 724–731.
- Adefegha, S.A.; Olasehinde, T.A.; Oboh, G. Essential Oil Composition, Antioxidant, Antidiabetic and Antihypertensive Properties of Two Afromomum Species. Journal of Oleo Science 2017, 66(1), 51–63.