?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effects of curdlan (2%, 3% and 4%) on the gel properties of Alaska surimi using high-temperature treatment were examined in this study. Curdlan treatment improved the gel strength of surimi in a concentration-dependent manner. Based on the results of dynamic rheology and differential scanning calorimetry (DSC), curdlan promoted the stability of proteins in the gel. Moreover, curdlan facilitated the interaction between gel proteins and actomyosin, thereby preventing the aggregation and denaturation of protein and increased the thermal transition temperature. The results of scanning electron microscopy showed that the addition of curdlan induced the formation of a more ordered and denser gel matrix and the fibrils in the three-dimensional network became more delicate. Therefore, surimi samples with curdlan may hold more moisture and exhibit improved transfer of free water to bound water, leading to a higher water-holding capacity (WHC).
Introduction
In recent years, consumers have demanded more convenient foods, including ready-to-eat (RTE) products, which do not require any preparation before consumption.[Citation1] Surimi has distinct gelling properties and can be used as a food base in RTE seafood products because it is a concentrate of salt-soluble myofibrillar proteins.[Citation2] Surimi gel, prepared from deboned and washed fish paste, has been used to produce restructured seafood analogs or gel-based food products, such as Japanese traditional gels, called ‘Kamaboko’.[Citation3] Additionally, surimi-based products have become common due to their unique textural properties and high nutritional value.
Myosin and actomyosin are the main components responsible for gelation, the most important functional property of surimi.[Citation4] Solubilized proteins first expand to create a continuous matrix and then undergo thermal aggregation and cross-linking, finally forming solid and elastic surimi gels with a three-dimensional muscle protein network.[Citation5,Citation6] Rearrangement of the hydrogen bonds, covalent bonds and hydrophobic interactions within proteins affects the formation of the network.[Citation7]
To produce instant surimi products, the components must have a long shelf life, which can be realized by sterilization.[Citation8] After sterilizing at 120°C, the products can be stored at a normal temperature and exhibit a much longer shelf life than the untreated products. Therefore, studies on the influence of high temperature on surimi gels are needed. The gel properties of surimi-based foods are particularly affected by the heat-processing steps used during the conversion of raw surimi paste to cooked gel.[Citation9] Previous studies demonstrated that high-temperature treatment (≥100°C) could markedly decrease the breaking force, breaking deformation and gel strength, thus damaging the texture of the products.[Citation10] Therefore, because consumers are more likely to purchase high-quality surimi products, new methods are needed to produce surimi-based products with a long shelf life and good textural properties in order to meet this public demand.
Many different components, including non-muscle protein[Citation11] and various hydrocolloid polysaccharides, have been used to improve the gel properties. Non-muscle proteins, e.g. protein isolates from black beans, can increase the water-holding capacity (WHC) and gel strength of sardine surimi and the gel becomes denser with a more ordered structure.[Citation12] Food hydrocolloids, such as polysaccharides and proteins, can contribute to the structure and functional properties of many processed foods. Leloup and others[Citation13] introduced complex gel systems made up of protein and polysaccharide. They showed that the length of the polysaccharide chain exceeded a certain range during the heating process; thus, the steric effects of long-chain polysaccharides block the aggregation of protein. The protein–water–polysaccharide complex is quite different from that of each absolute system and some changes in the composition, structure and functional properties of the protein particles are observed. Thus, the addition of polysaccharides may influence protein gelation properties, thereby affecting surimi gelation. Several hydrocolloids, including starch, carrageenan, xanthan gum and konjac, are used as gel binders, texture stabilizers and fat substitutes in the production of surimi gel products to improve the mechanical properties of surimi gels.[Citation14–Citation18] Additionally, Herranz and others[Citation19] showed that deacetylated konjac glucomannan (KGM) improved the thermostability of gels, and Barrera and others[Citation20] found that the LM35 pectin could decrease expressible water and increase the levels of shears stress and hardness.
Curdlan is a linear glucan, interconnected by β-(1→3)-d-glucans without branching. As a water-insoluble microbial exopolysaccharide, curdlan is generated by microorganisms, such as Alcaligenes faecalis.[Citation21–Citation23] Because of its distinct thermal gel properties, curdlan has extensive applications in the food industry, particularly in meat products. Curdlan suspensions may form both thermally reversible and thermally irreversible gels under different heating temperatures. The former (low-set gel) appeared when heated to 60°C and subsequently cooled. In contrast, a firm, resilient, high-set gel is formed at temperatures higher than 80°C. In the low-set gel, the curdlan micelles are cross-linked with molecules of a single helix through hydrogen bonds; however, cross-linking is also observed in curdlan micelles containing triple-stranded helixes with hydrophobic interactions in high-set gels.[Citation24] Owing to variations in the gelation mechanisms, these two types of gels have been added to different types of foods, such as noodles, bean curd and low-fat meat products.[Citation18,Citation25,Citation26] However, few studies have investigated the applications of curdlan in fish meat gel-based seafood products.
In our previous work,[Citation2] we showed that the deacetylation of KGM has an active effect on gel strength by retarding the protein denaturation of Alaska surimi significantly under high-temperature treatment. Thus, the purpose of the present study was to investigate the effects of curdlan at different concentrations on the physicochemical properties of surimi gels treated at a high temperature (120°C). We also aimed to clarify the recombination mechanism of the surimi gel–curdlan complex system.
Materials and methods
Materials
Frozen Alaska Pollock surimi (grade AAA) was purchased from JINCAN Foods Co., Ltd. (Qingdao, Shandong, China). Surimi was stored at −20°C until use. Food-grade curdlan was provided by Zhongke Biotechnology Co., Ltd. (Shandong, China). All of the chemicals used in this experiment were analytical grade and purchased from Sigma (St. Louis, MO, USA) or Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Preparation of curdlan and surimi gel
Curdlan powder was added to water and stirred for 10 min. The suspension was then kept until further use. Frozen Alaska Pollock surimi (250 g) was first semi-thawed for 3–4 h at 4°C and cut into small strips (approximately 3 cm cubes). Surimi cubes were then chopped in a Stephan vertical vacuum cut mixer (Model UM 5; Stephan Machinery Co., Hameln, Germany) at a speed of 1500 rpm for 4 min. A chilling medium (ethanol:water, 95:5) was continuously circulated to keep the sample temperature below 4°C during chopping. Sodium chloride (3 wt%) was added and mixed with the surimi at a speed of 1800 rpm for 2.5 min. Subsequently, the mixture was chopped for an additional 3 min under 0.5 bar pressure to remove air pockets with or without the curdlan suspension. The moisture level was adjusted to 75%. The final curdlan concentrations were 0%, 2%, 3% and 4% (w/w, based on the surimi gel). The surimi sol was squeezed into plastic casings (3 cm i.d.), in which both ends were fastened tightly.
High-temperature treatment
Surimi samples were sterilized at 120°C. An electric/steam amphibious sterilization retort (JINDING Food Machinery Co., Ltd., Zhucheng, Shandong, China) was used for high-temperature treatment. The desired temperature was preset to ensure the accuracy of the sterilization temperature. During the heating process, counter-pressure was applied by adding compressed air into the retort in order to prevent casings from bulging. This counter-pressure was also preset. After sterilization, the temperature was cooled down via closing the steam source and adding cooled water into the spray tube. The surimi gels were heated under the same counter-pressure (0.12 MPa) until their central temperature reached 120 ± 1°C and then held for 15 min. The temperature was monitored using an Ellab TrackSense-Pro (Henglv Engineering Co., Ltd., Hubei, China). The samples were arranged in ice water for chilling and finally kept at 4°C until analysis.
Instrumental texture analysis
A TMS-PRO texture analyser (Food Technology Co., USA) was used to measure the textural properties of surimi gels. The gels were equilibrated at room temperature (25°C) before analysis. Cylindrical samples (2.5 cm in length) were prepared, placed in a texture analyser and tested by a spherical plunger (5 mm in diameter, 60 mm/min depression speed). The choices of breaking force and deformation were based on the first force peak.[Citation27]
Determination of WHC
Surimi gels were cut into several pieces, one of which was randomly chosen to analyse its water content (X1 %). Three pieces of Whatman filter paper and two pieces of filter paper were each placed under and above the surimi gels. The samples were subjected to a pressure of 5 kg for 2 min and the water content (X2 %) was then measured again. The WHC was calculated using the following equation:
Rheological measurements
The dynamic viscoelasticity, namely the storage modulus (G′) of curdlan-containing surimi samples, was measured using an MCR101 rheometer (Anton Paar Ltd., Austria) with a parallel plate (50 mm in diameter) and having a 1.0-mm gap. Dynamic temperature sweep measurements were conducted at a heating rate of 1°C/min and a frequency of 1.0 Hz, maintaining the amplitude strain at a constant value (2%) within the LVE region, which was determined by stress sweep tests.
Differential scanning calorimetry
DSC measurements were performed using a Netzsch DSC 200PC (Netzsch, Bavaria, Germany). About 10 mg of surimi paste was sealed in an aluminium pan and an empty pan was used as a reference. We set the scan temperature from 25°C to 120°C, at a heating rate of 5°C/min.
Nuclear magnetic resonance spin-spin relaxation (T2) measurements and post-processing
NMR relaxation measurements were performed using a Niumag Benchtop Pulsed NMR Analyzer PQ001 (Niumag Electric Corporation, Shanghai, China) operating at a resonance frequency for protons of 22.6 MHz. The 3-cm-long sample was placed in a glass tube (15 mm, i.d.) and inserted in the NMR probe. The spin-spin relaxation time (T2) measurements were performed by the Carr–Purcell–Meiboom–Gill (CPMG) sequence with a τ-value (time between 100°C and 180°C pulse) of 100 μs and 8 scan repetitions. The interval time was set to acquire 5000 echoes for each 2.5 s.
MultiExp Inv Analysis software (Niumag Electric Corporation, Shanghai, China) was used to perform the distributed exponential fitting of the CPMG decay curves. Time constants of each process were calculated from the peak position and the area under each peak (corresponding to the proportion of water molecules exhibiting that relaxation time) was calculated through cumulative integration. Additionally, the width of the relaxation population was determined by the standard deviation of the observed relaxation times for the given peak.
Scanning electron microscopy
The microstructures of the surimi gels were analysed using SEM.[Citation28] The surimi gels were cut into pieces with 2–3 mm thickness and fixed with a 3% glutaraldehyde solution. Before dehydration in a gradient ethanol series of 50%, 70%, 80%, 90% and 100% (v/v), samples were washed for 1 h in distilled water and then mounted on bronze stubs and sputter-coated with gold. Finally, SEM (JEOLJSM-5800 LV; Tokyo, Japan) was applied to observe the specimens at an acceleration voltage of 15 kV.
Statistical analysis
The experiments were run in triplicate and the data were calculated as means ± standard deviations. Differences were compared by the least significant difference (p < 0.05). Statistical analysis was performed using ORIGIN software (Version 8.0; Microcal Software Inc., Northampton, MA, USA).
Results and discussion
Breaking force and deformation
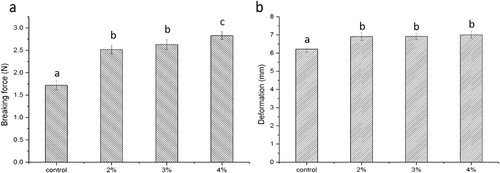
Gel strength is a key parameter used to determine the quality of fish meat gel-based seafood products. The breaking force () and deformation () of the surimi gels with curdlan (2%, 3% and 4%) are depicted in . According to previous studies, surimi gels deteriorated rapidly as the heating temperature rose to 120°C.[Citation2,Citation10] High-temperature treatment may mitigate the gel strength and disrupt the gel structure of surimi. However, in the presence of curdlan, the properties of the surimi gel were improved compared with those of the control. As shown in , the breaking force increased as the concentration of curdlan increased and the highest value was obtained at 4% curdlan. However, deformation of the surimi samples containing curdlan increased with a slight gradient compared with that of the control.
Surimi gels are sensitive to heat-induced gelation.[Citation29] According to a previous analysis using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE),[Citation10] high-temperature treatment (120°C) abolished myosin heavy chain (MHC) levels and reduced the actin content, producing gels with poor properties. As shown in , curdlan improved the breaking force and deformation of the surimi gels compared with those of the control. Wu and others[Citation30] studied the effects of curdlan on the rheological properties of restructured Ribbonfish meat gel and found that curdlan has positive effects on increasing the cross-linking density in the complex gel networks when combined with fish meat, resulting in an ordered and stable three-dimensional gel structure, thus improving its properties. The good gel properties of samples with curdlan could be attributed to the interaction between curdlan gel and fish meat protein in the complex matrix during the gelation heating process. Curdlan in meat products exhibits strong thermo-irreversibility because it can inhibit the formation of hydrogen bonds during cross-linking in junction zones and strongly entrap released or free water within its gel structure when heating.[Citation31]
Dynamic rheology
G′ is the stored energy caused by elastic deformation in a viscoelastic material during the process of deformation. We tested the storage modulus to study the effects of curdlan on the dynamic rheological properties of surimi gel. Temperature curves of G′ during the heating process were compared to determine the dynamic viscoelasticity after the addition of 0%, 2%, 3% and 4% curdlan (). In the control group, we found that G′ reached a first peak at 29.8°C and dropped to the minimum G′ value rapidly when the temperature reached 54°C. With the rise of temperature, slight fluctuations in G′ were observed. G′ reached a second peak at 86.8°C and then decreased thereafter. These changes in G′ were caused by denaturation and cross-linking of several compositions in fish meat proteins at different heating temperatures. The initial increase in G′ at low temperatures could be related to the molecular interactions of actomyosin, which formed a weaker three-dimensional gel network,[Citation32] and the second peak of G′ could be explained by the formation of a highly elastic protein gel through denaturation of myosin and actomyosin.[Citation33] When the heating temperature was above 100°C, the G′ decreased in all samples because high-temperature treatment destroyed the non-covalent interactions of protein and polymerized non-enzymatic myosin in the surimi gel. Zhang and others[Citation10] suggested that this polymerization might prevent fish meat proteins from forming high-quality network during the process of thermal gelation.
Figure 2. Changes in the storage modulus (G’) of surimi with or without curdlan as a function of temperature.
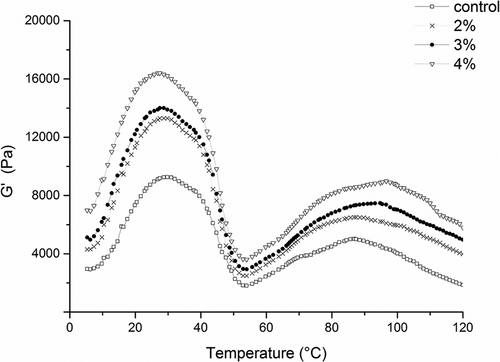
As shown in , similar trends for the storage modulus G′ curves were observed for surimi samples with curdlan. As the temperature increased, the G′ first increased and then decreased, forming the first peak. When the temperature reached 55°C, a nadir was observed. The second peak was obtained between 85°C and 95°C. However, compared with the control group, the values of G′ increased as the concentration of curdlan increased. Although curdlan increased the values of G′, as shown in , the overall profiles of the storage modulus were not altered. Thus, the protein network still predominated in the polysaccharide and protein complex system when the gel formed and curdlan functioned by improving or promoting the formation of surimi gels. The reason for the increase in the first peak may be due to the onset of the swelling of curdlan molecules,[Citation34,Citation35] accompanied by breaks in some inter- or intramolecular hydrogen bonds.[Citation36] Additionally, as the temperature increased, the second peak of G′ values increased compared with the control, indicating the formation of thermo-irreversible gels through hydrophobic interactions between curdlan molecules[Citation37] and synergy with proteins.
Effects of curdlan on the endothermic transitions of surimi protein by DSC
The formation of surimi gels involves the heat-induced denaturation of different components of myofibrillar protein and the denaturation temperatures of different proteins can vary widely. Thus, heat uptake of the various proteins represented by endothermic peaks was detected using DSC; these heat uptake peaks were expected to represent the points at which the hydrogen bonds broke and the ordered network structure of the protein gradually unfolded during the denaturation process.[Citation38] shows the different DSC thermograms of surimi pastes with or without curdlan. In the control group, the first peak corresponded to the endothermic transition of myosin, the second peak was related to the endothermic transition of actin and the third peak in meat systems corresponded to sarcoplasmic proteins.[Citation39] However, since the sarcoplasmic proteins were removed during the washing process, its transition temperature was not observed in our thermograms.
Figure 3. Differential scanning calorimetry (DSC) thermograms of surimi pastes with and without curdlan.
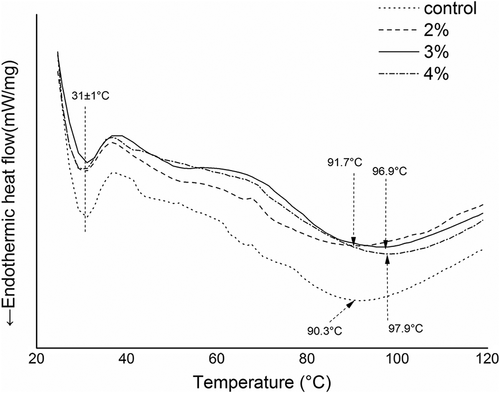
In the DSC thermograms of surimi samples with or without curdlan, we could clearly distinguish two thermal denaturation transitions. The first peak, appearing at approximately 31 ± 1°C, corresponded to myosin and was not altered following the addition of curdlan. However, as the concentration of curdlan increased, the value of the second peak gradually became higher. The transition temperatures were 90.3°C for the control and 91.7°C, 96.9°C and 97.9°C for 2%, 3% and 4% curdlan, respectively. Based on these results, curdlan perhaps did not alter the thermal transition temperature of myosin, but could interact with actin, thereby improving the transition temperature. Because the molecular weight of actin is much lower than that of myosin and because curdlan can form a stable thermo-irreversible gel above 70°C, the added curdlan may bind with actin easily and form a stable gel when the heating temperature reaches 80°C, contributing to the improvement of the thermal transition temperature of actin.[Citation2] Murray and others[Citation40] considered the transition temperature as an indication of the rupture of hydrogen bonds and protein aggregation based on hydrophobic interactions; hence, we speculated that curdlan may react with actin through hydrogen bonding, thereby increasing the transition temperature.
Microstructure of surimi gels with curdlan
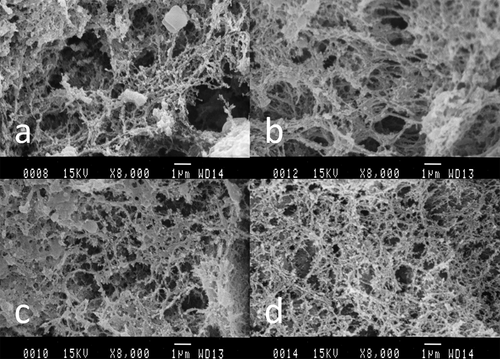
To confirm the effects of curdlan on the formation of surimi gels, the microstructures of four surimi samples were observed. As shown in , all samples formed a three-dimensional network structure, which contributed to the elastic characteristics of the gel. However, surimi gel containing curdlan exhibited a more compact gel matrix than the control, for which the protein gel was much rougher and looser with larger cavities. The addition of curdlan produced smaller pores and a more compact network. Moreover, as the curdlan content increased, fibrils in the three-dimensional protein network became more delicate, inducing a more ordered and denser restructured gel network. Thus, combined with DSC and rheology results, our findings suggested that curdlan could interact with actomyosin, effectively preventing protein aggregation and improving the gel structure.
Water-holding capacity
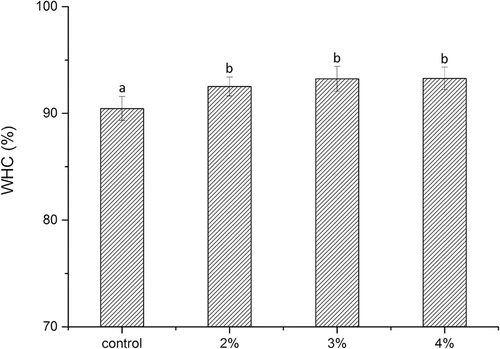
WHC is an important parameter associated with the stability of surimi gel-based restructured seafood. Because of its high WHC, fish meat protein can bind with abundant amounts of water and therefore preserve moisture during the heating process.[Citation26] The effects of curdlan on the WHCs of surimi gels are shown in . The WHCs of gels increased as the content of curdlan increased, suggesting that more water was bound to or reserved in the network.[Citation41] Funami and others[Citation31,Citation42] reported that curdlan in meat absorbed free or released water during the heating process and held water well within the gel structures. The water-binding capacity of protein gels is affected by several factors, including hydration, hydrogen bonds and hydrophobic interactions.[Citation25] The addition of curdlan may lead to the formation of more hydrogen bonds and hydrophobic interactions. Moreover, according to SEM analysis, curdlan could make the gel structure become dense and uniform, which may increase the WHC of the gel.[Citation43,Citation44] However, an obvious increase in WHC between surimi gels containing 3% or 4% curdlan under heat treatment at 120°C was observed in this study. Hu and others[Citation45] speculated that the dilution effect of curdlan at higher levels may lower the number of water-binding sites in proteins to some extent, thus reducing the ability of binding and interaction between muscle proteins and water molecules.
The T2 time of surimi gels with curdlan under high-temperature treatment
Low-field NMR (LF-NMR) technology can be used to assay the moisture distribution and state of products during the storage process at the molecular level, which can accurately reflect the moisture migration state in foods. Choi and Kerr[Citation46] studied the moisture content and molecular liquidity of wheat starch through[Citation1] H-NMR, which has been widely used in the study of moisture content in meat muscle.[Citation47] NMR transverse relaxation is related to WHC and can be used as an important indicator to assess the WHC of surimi gel.[Citation48] NMR transverse relaxation can easily distinguish different WHCs of the gel samples. Therefore, it is necessary to analyse the T2 to investigate the water distribution in surimi gels.
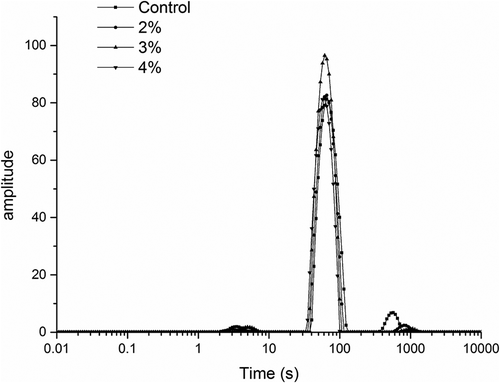
The distributed T2 relaxation times of the surimi gels with or without curdlan under high-temperature treatment are shown in . The T2 was characterized by a small population with a relaxation time of a few milliseconds; additionally, a major population was observed at around 30–130 ms and a minor population was observed at about 300–1500 ms. The first peak T21 was in the range of 0–10 ms. This result could be explained by the close association of the bound water with the large molecules, like proteins in the gel system or protons located on macromolecular structures plasticized by water.[Citation49] T22, the second peak, represented the immobilized water, whereas the third peak T23 represented free water. As shown in , most water in surimi gels was immobilized; as curdlan increased, the peak of T22 became higher and the position of each peak of T2 moved to the left, indicating that free water transformed to bound water. Additionally, the relaxation time of T22 became shorter with the addition of curdlan, clarifying that water became less movable in the surimi gels, consistent with the WHC results.
Conclusion
Curdlan affected the gel properties of surimi samples under high-temperature treatment. Curdlan could form thermo-irreversible gels at high temperatures and interact with surimi protein, thereby increasing the gel strength. According to the results of dynamic rheology and DSC, during the heating process, the addition of curdlan did not influence the denaturation of myosin but did affect the interaction with actin and increased its thermal transition temperature. In surimi products, curdlan promoted the cross-linking density of the complex gel network, resulting in a more uniform and stable three-dimensional network, thus allowing the surimi gel to hold more free or released water. Moreover, as the curdlan content increased, the effect on the protein gels was more significant. Based on the above-mentioned results, curdlan could be considered an alternative to enhance the gel properties or improve the quality of surimi products because it could markedly strengthen the qualities of Alaska surimi treated with high temperature (120°C).
Funding
This study was supported by the National Natural Science Funds (31571865 and 31371791).
Additional information
Funding
References
- Lorentzen, G.; Ytterstad, E.; Olsen, R.L.; Skjerdal, T. Thermal Inactivation and Growth Potential of Listeriainnocua in Rehydrated Salt-Cured Cod Prepared for Ready-To-Eat Products. Food Control 2010, 21(8), 1121-6%@ 0956-7135.
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of Deacetylation of Konjac Glucomannan on Alaska Pollock Surimi Gels Subjected to High-Temperature (120 C) Treatment. Food Hydrocolloids 2015, 43, 125–131.
- Park, J.W. Surimi Seafood: Products, Market, and Manufacturing. Surimi and Surimi Seafood 2005, 375–433.
- Paker, I.; Matak, K.E. Impact of Sarcoplasmic Proteins on Texture and Color of Silver Carp and Alaska Pollock Protein Gels. LWT-Food Science and Technology 2015, 63(2), 985–991.
- Benjakul, S.; Visessanguan, W.; Chantarasuwan, C. Effect of High‐Temperature Setting on Gelling Characteristic of Surimi from Some Tropical Fish. International Journal of Food Science & Technology 2004, 39(6), 671–680.
- Gordon, A.; Barbut, S. Effect of Chloride Salts on Protein Extraction and Interfacial Protein Film Formation in Meat Batters. Journal of the Science of Food and Agriculture 1992, 58(2), 227–238.
- Sánchez-González, I.; Carmona, P.; Moreno, P.; Borderías, J.; Sanchez-Alonso, I.; Rodríguez-Casado, A.; Careche, M. Protein and Water Structural Changes in Fish Surimi during Gelation as Revealed by Isotopic H/D Exchange and Raman Spectroscopy. Food Chemistry 2008, 106(1), 56–64.
- Zhang, L.; Zhang, F.; Wang, X. Changes of Protein Secondary Structures of Pollock Surimi Gels under High-Temperature (100°C and 120°C) Treatment. Journal of Food Engineering 2016, 171, 159–163.
- Zhu, Z.; Lanier, T.C.; Farkas, B.E.; Li, B. Transglutaminase and High Pressure Effects on Heat-Induced Gelation of Alaska Pollock (Theragra chalcogramma) Surimi. Journal of Food Engineering 2014, 131, 154–160.
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z.; Xue, C. Effects of High-Temperature Treatment (⩾100°C) on Alaska Pollock (Theragra chalcogramma) Surimi Gels. Journal of Food Engineering 2013, 115(1), 115–120.
- Ramírez-Suárez, J.; Addo, K.; Xiong, Y. Gelation of Mixed Myofibrillar/Wheat Gluten Proteins Treated with Microbial Transglutaminase. Food Research International 2005, 38(10), 1143–1149.
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of Protein Isolates from Black Bean and Mungbean on Proteolysis and Gel Properties of Surimi from Sardine (Sardinella albella). LWT - Food Science and Technology 2013, 50(2), 511–518.
- Leloup, V.; Colonna, P.; Ring, S. Diffusion of a Globular Protein in Amylose and Amylopectin Gels. Food Hydrocolloids 1987, 1(5), 465–469.
- Park, J.W. Ingredient Technology and Formulation Development. In: Park JW (ed) Surimi and surimi seafood. Food Science and Technology-New York-Marcel Dekker 2000, 343–392.
- Debusca, A.; Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Physicochemical Properties of Surimi Gels Fortified with Dietary Fiber. Food Chemistry 2014, 148, 70–76.
- Pietrowski, B.N.; Tahergorabi, R.; Matak, K.E.; Tou, J.C.; Jaczynski, J. Chemical Properties of Surimi Seafood Nutrified with Ω-3 Rich Oils. Food Chemistry 2011, 129(3), 912–919.
- Ramírez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food Hydrocolloids as Additives to Improve the Mechanical and Functional Properties of Fish Products: A Review. Food Hydrocolloids 2011, 25(8), 1842–1852.
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. Journal of Food Science and Technology 2010, 47(6), 587–597.
- Herranz, B.; Tovar, C.A.; Solo-de-Zaldívar, B.; Borderias, A.J. Effect of Alkalis on Konjac Glucomannan Gels for Use as Potential Gelling Agents in Restructured Seafood Products. Food Hydrocolloids 2012, 27(1), 145–153.
- Barrera, A.; Ramırez, J.; González-Cabriales, J.; Vázquez, M. Effect of Pectins on the Gelling Properties of Surimi from Silver Carp. Food Hydrocolloids 2002, 16(5), 441–447.
- Harada, T. Production, Properties, and Application of Curdlan [With Applications to the Food Industry]; ACS Symposium Series American Chemical Society, 1977.
- Lee, K.B.; Bae, J.H.; Kim, J.S.; Yoo, Y.C.; Kim, B.S.; Kwak, S.T.; Kim, Y.S. Anticoagulant Activity of Sulfoalkyl Derivatives of Curdlan. Archives of Pharmacal Research 2001, 24(2), 109–113.
- McIntosh, M.; Stone, B.; Stanisich, V. Curdlan and Other Bacterial (1→ 3)-Β-D-Glucans. Applied Microbiology and Biotechnology 2005, 68(2), 163–173.
- Kanzawa, Y.; Harada, T.; Koreeda, A.; Harada, A.; Okuyama, K. Difference of Molecular Association in Two Types of Curdlan Gel. Carbohydrate Polymers 1989, 10(4), 299–313.
- Chen, C.; Wang, R.; Sun, G.; Fang, H.; Ma, D.; Yi, S. Effects of High Pressure Level and Holding Time on Properties of Duck Muscle Gels Containing 1% Curdlan. Innovative Food Science & Emerging Technologies 2010, 11(4), 538–542.
- Wang, M.; Chen, C.; Sun, G.; Wang, W.; Fang, H. Effects of Curdlan on the Color, Syneresis, Cooking Qualities, and Textural Properties of Potato Starch Noodles. Starch‐Stärke 2010, 62(8), 429–434.
- Lanier, T.C. Measurement of Surimi Composition and Functional Properties. Surimi Technology 1992, 12, 123-163.
- Liu, R.; Zhao, S.-M.; Xiong, S.-B.; B-J, X.; L-H, Q. Role of Secondary Structures in the Gelation of Porcine Myosin at Different Ph Values. Meat Science 2008, 80(3), 632–639.
- Ogawa, M.; Ehara, T.; Tamiya, T.; Tsuchiya, T. Thermal Stability of Fish Myosin. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1993, 106(3), 517–521.
- Wu, C.; Yuan, C.; Chen, S.; Liu, D.; Ye, X.; Hu, Y. The Effect of Curdlan on the Rheological Properties of Restructured Ribbonfish (Trichiurus Spp.) Meat Gel. Food Chemistry 2015, 179, 222–231.
- Funami, T.; Yada, H.; Nakao, Y. Thermal and Rheological Properties of Curdlan Gel in Minced Pork Gel. Food Hydrocolloids 1998, 12(1), 55–64.
- Lefèvre, F.; Fauconneau, B.; Ouali, A.; Culioli, J. Thermal Gelation of Brown Trout Myofibrils: Effect of Muscle Type, Heating Rate and Protein Concentration. Journal of Food Science 2008, 63(2), 299–304.
- Mleko, S.; Foegeding, E.A. Ph Induced Aggregation and Weak Gel Formation of Whey Protein Polymers. Journal Food Sciences 2000, 65(1), 139–143.
- Hirashima, M.; Takaya, T.; Nishinari, K. DSC and Rheological Studies on Aqueous Dispersions of Curdlan. Thermochimica Acta 1997, 306(1), 109–114.
- Konno, A.; Harada, T. Thermal Properties of Curdlan in Aqueous Suspension and Curdlan Gel. Food Hydrocolloids 1991, 5(5), 427–434.
- Konno, A.; Okuyama, K.; Koreeda, A.; Harada, A.; Kanzawa, Y.; Harada, T. Molecular Association and Dissociation in Formation of Curdlan Gels; Food Hydrocolloids: Springer: 1994, 30(2), 113–118.
- Funami, T.; Funami, M.; Yada, H.; Nakao, Y. A Rheological Study on the Effects of Heating Rate and Dispersing Method on the Gelling Characteristics of Curdlan Aqueous Dispersions. Food Hydrocolloids 2000, 14(5), 509–518.
- Spink, C.H. Differential Scanning Calorimetry. Methods in Cell Biology 2008, 84, 115–141.
- Wright, D.J.; Leach, I.B.; Wilding, P. Differential Scanning Calorimetric Studies of Muscle and Its Constituent Proteins. Journal of the Science of Food and Agriculture 1977, 28(6), 557–564.
- Murray, E.; Arntfield, S.; Ismond, M. The Influence of Processing Parameters on Food Protein Functionality II. Factors Affecting Thermal Properties as Analyzed by Differential Scanning Calorimetry. Canadian Institute of Food Science and Technology Journal 1985, 18(2), 158–162.
- Chanarat, S.; Benjakul, S. Impact of Microbial Transglutaminase on Gelling Properties of Indian Mackerel Fish Protein Isolates. Food Chemistry 2013, 136(2), 929–937.
- Funami, T.; Nakao, Y. Effects of Curdlan on the Rheological Properties and Gelling Processes of Meat Gels under a Model System Using Minced Pork. 1. Application of Curdlan to Meat Products. Journal of the Japanese Society for Food Science and Technology-Nippon Shokuhin Kagaku Kogaku Kaishi 1996, 43(1), 21–28.
- Funami, T.; Unami, M.; Yada, H.; Nakao, Y. Gelation Mechanism of Curdlan by Dynamic Viscoelasticity Measurements. Journal of Food Science 1999, 64(1), 129–132.
- Zhang, R.; Edgar, K.J. Properties, Chemistry, and Applications of the Bioactive Polysaccharide Curdlan. Biomacromolecules 2014, 15(4), 1079–1096.
- Hu, Y.; Liu, W.; Yuan, C.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Enhancement of the Gelation Properties of Hairtail (Trichiurus haumela) Muscle Protein with Curdlan and Transglutaminase. Food Chemistry 2015, 176, 115–122.
- Choi, S.-G.; Kerr, W.L. 1 H NMR Studies of Molecular Mobility in Wheat Starch. Food Research International 2003, 36(4), 341–348.
- Hazlewood, C.; Nichols, B. Evidence for the Existence of a Minimum of Two Phases of Ordered Water in Skeletal Muscle. Nature 1969, 222, 747–750.
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water Distribution and Mobility in Meat during the Conversion of Muscle to Meat and Ageing and the Impacts on Fresh Meat Quality Attributes—A Review. Meat Science 2011, 89(2), 111–124.
- Møller, S.M.; Grossi, A.; Christensen, M.; Orlien, V.; Søltoft-Jensen, J.; Straadt, I.K.; Thybo, A.K.; Bertram, H.C. Water Properties and Structure of Pork Sausages as Affected by High-Pressure Processing and Addition of Carrot Fibre. Meat Science 2011, 87(4), 387–393.