ABSTRACT
A gravimetric method (GM) that can evaluate oil exudation rate in yolk of salted eggs was studied. The samples of 1.5–2.5 g yolk were sandwiched between filter papers and pressed by 1000 g weights for 1–2 h at 25–35°C. Free oil exuded from yolk was absorbed by the filter papers, and the increased mass of the filter papers was designated as the amount of exuded oil. The GM showed satisfactory coefficient of variation at 5.4%, with recoveries ranging from 98.2 to 101.9%. Highly linear correlation of free oil amount detected by GM with levels added soybean oil in samples was demonstrated by R2 = 0.998. Cooked salted eggs from six companies and eggs brined different time were determined by the GM, which produced high consistency of the oil exudation ratio with the apparent state of different sample eggs. All the results indicated that the GM can be a practicable method to quantitatively evaluate oil exudation.
Introduction
Salted egg is a processed duck egg and is very popular in China and in other Asian countries.[Citation1,Citation2] Whole eggs are consumed as part of a regular diet, whereas yolks of salted eggs (YSE) are also used as stuffing in some food products, such as moon cake and other desserts and glutinous rice dumpling.[Citation1] Salted eggs are produced by brining eggs in saturated saline (immersing method) or by coating eggs with soil paste mixed with salt (coating method) for 20–45 days.[Citation2,Citation3] During salting, the yolk gradually solidifies and hardens, whereas the albumen loses its viscosity and becomes watery.[Citation1,Citation4]
Consumers love salted egg mainly for its yolk. Salted eggs are rich in protein. However, the egg whites possess neither attractive taste nor special aroma, and they are considerably salty because they contain at least 7–10% sodium chloride (NaCl). Salted egg whites are thus difficult to eat directly; for this reason, large amounts of salted egg whites are discarded yearly.[Citation5] Studies have focused on desalting salty albumin and on utilization of salted egg whites.[Citation5,Citation6] Compared with the salty egg white, the YSE is more delicious and nutritive part for its soft and gritty characteristics, aroma, beautiful colour, and richness in nutrients, including fat, protein, lecithin, carotenoids, vitamins, and minerals.[Citation6,Citation7] A desirable YSE possesses gritty texture, beautiful colour (orange to red), and apparent oil.
During salting, moisture leaves the egg while salt permeates into the egg white and yolk.[Citation1] Oil exudation in yolk increases with time resulting from dehydration and protein denaturation.[Citation3,Citation4] The oil exuded from YSE exists in the form of free lipids, which are released from lipoprotein. The free lipids on the interface of granulated yolk spheres provide the gritty texture of cooked salted egg yolk.[Citation4]
The degree of oil exudation (OE) is one of the most important indexes used to evaluate the quality of salted eggs because this parameter directly affects the consumer acceptability of this product. However, so far, there is no effective and simple procedure for evaluating the OE. Fletcher et al.[Citation8] comparatively evaluated four methods used to determine total lipid content of yolk; these methods are neither specialised for determination of lipid content of salted egg nor for quantification of exuded oil. Lai et al.[Citation4] and Kaewmanee et al.[Citation2] used a method to determine oil-off ratio, in which a yolk sample is mixed with distilled water, homogenised, and then centrifuged. The oil in liquid layer is extracted by an organic solvent, separated, evapourated, dried, and weighed. Exuded oil amount is the weight of oil extracted first by water and then by organic solvent.[Citation4] All the above methods involved extraction by organic solvent. The present study reported the effect of a gravimetric method without solvent on evaluation OE.
Materials and methods
Preparation of salted egg and yolk samples
Fresh duck eggs were collected from a suburb duckery in Hefei within 24 h after oviposition. The eggs were washed with tap water, left to dry, and then brined for 15 days in saline (25% NaCl) at 35°C. The eggs-to-brining solution ratio was approximately 1:1.5 (wt/wt). Both salted and fresh eggs (unsalted) were steamed for 10 min. The shell and egg white of the cooked eggs were removed, and the yolks were collected. Salted yolks (SY) and unsalted yolks (USY) were separately agitated and homogenised using a spoon and then used as sample for each test.
Determination of total lipids
According to methods described by Wang,[Citation9] approximately 2 g of homogenised yolk sample was weighed accurately and placed in 100 ml beaker. Approximately 15 g of anhydrous sodium sulfate powder was thoroughly mixed with the yolk sample by using a stainless spoon. The sample was subsequently transferred into a lipid extraction tube. The residual sample that adhered onto the beaker and spoon was scrubbed by using modicum cotton wool, which was added into the extraction tube. Neutral chloroform containing 1% (vol/vol) absolute ethyl alcohol was added into the tube to immerse and rinse the yolk sample for 10 times to extract all lipids; 10 ml of the solvents was used each time. The chloroform containing lipids was filtered into a fat bottle of known mass, and the solvent was recovered by using a rotary evapourator. The lipid contained in the fat bottle was dried at 105°C to constant weight. Total lipids (TL%) were calculated by Formula (1):
where M0 is the weight of the fat bottle; M1 is the weight of the sample; and M2 is the combined weight of the fat bottle and sample.
OE determination through solvent method
According to the method described by Lai et al.,[Citation4] 5 g of sample was mixed with 25 ml of distilled water and then homogenised at 5,000 rpm for 30 s. The homogenate was centrifuged (Hitachi Himac SCR 20B, Tokyo, Japan) at 9,500 × g for 30 min at 25°C, and then 25 mL of n-hexane:2-propanol (3:2, vol/vol) was added into the supernatant to dissolve the float. The solvent-lipid layer thus obtained was then separated using a separation funnel. The solvent in the solvent-lipid layer was evapourated in a water bath and subsequently heated at 105°C to constant weight. The residue was weighed, and OE% was counted; OE% is the mass percentage of the extract based on the weight of the sample.
OE determination by GM
For each test, four or six pieces of qualitative filter paper (Double Circle Brand, 7 cm in diameter, Hangzhou Special Paper Co., Ltd.) as a unit for one test were dried at 105°C to a constant weight. Approximately 1.5–2.5 g of yolk sample was evenly spread on the centre position of dried filter papers and precisely weighed with the papers. The filter papers unit was weighed before the sample is on it. After weighing, half the numbers of the filter papers below sample were transferred on top of the sample. The yolk sample, which was sandwiched between the papers, was laid on a glass board. Another glass board was placed over the top paper. A 1000-g weight (Tianjin Lilang Weighing Equipment Co., Ltd.) was used to press the upper board. The system contained yolk sample, filter papers, and glass boards, and weight was maintained at 25–35°C for 1–2 h. Subsequently, the entire yolk sample was removed, and the filter papers were heated at 105°C to constant weight. OE% was expressed on a wet basis of yolk and computed using Formula (2):
where M0 is the weight of papers prior to oil absorption; M1 is the combined weight of the yolk sample and papers; and M2 is the weight of the papers after absorbing oil.
Recovery and linearity experiment for validation
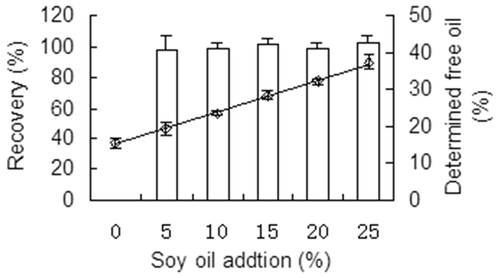
According to the method described by Odriozola-Serrano et al.,[Citation10] recovery and linearity of the GM were determined by adding five different amounts of refined soybean oil (SO) into the SY samples. The refined SO (0, 1, 2, 3, 4, and 5 g) were separately added into 20, 19, 18, 17, 16, and 15 g wet mass of SY to obtain 20 g of each sample; the samples were then properly agitated to obtain homogeneously mixed oil and yolk. The six samples, which were tested separately, contained 0%, 5%, 10%, 15%, 20%, and 25% SO. The percentages of free oil (FO) were determined both by GM. Five replicates were performed for each level. Linearity was evaluated based on the relationship between SO% (independent variable) and FO% (dependent variable). The recovery (RC%) was computed by Formula (3).
where M0 is the initial weight (grams) of FO in yolk; M1 is the weight (grams) of added SO; and M2 is the weight (grams) of FO in yolk after SO addition.
Sensory evaluation
According to the methods described in papers,[Citation11,Citation12] the cooked eggs were cut along their longitudinal axis to produce two symmetrical parts, and the sections were placed in a dish with their yolks shown. The sensory panel consisted of undergraduate students who had taken sensory evaluation course; they were asked to describe the amount of exuded oil in the samples by assigning a score based on a 10-point scale, where 1 indicates the least amount of oil and 10 indicates the greatest amount of oil. The scores should be given soon after the eggs were cut. Each member of the panel evaluated five eggs in a sample set, and mean scores were obtained from the score given by 10 members of the panel.
Statistical analysis
The statistical analyses for ANOVA were executed using the statistical software PASW Statistics v18.0 (SPSS, USA). Significant differences between samples were measured by T-test and LSD multiple comparison at p < 0.05. Linear correlation was determined, and figures were made by Excel (Microsoft, USA).
Results
Effect of some factors in GM on determination
Selected factors, including sample mass, temperature, and time, in GM procedure were studied through this experiment. Sample mass of SY from 1.5 g to 2.5 g and incubation time from 1 h to 2 h did not reveal significant difference on determination result of OE means, while temperature at 45°C made the OE significant higher than that at 25°C and 35°C (). In USY sample, OE determined by GM at 45°C was 0.68% which was significant higher than 0.50% at 35°C. The results suggested temperature affected the determination more than sample mass and incubation time did within the selected range. It was because melting point of oil increased with temperature, and oil exudation was promoted by high temperature. To avoid the interference of temperature, the determination temperature of GM should not be higher than 35°C.
Table 1. Oil exudation rate in different test conditions.
Determination results of OE by GM and SM
shows the OE%, standard deviation, and coefficient of variations (CV) of data obtained from GM and SM. The OE from GM is about 2–3 times more than that from SM for either SY or USY samples. For GM, Except for lipids, some other soluble solids and tiny particles were possibly absorbed by the filter paper, which would possibly increase the mass absorbed on the paper. But for SM, lipids are not soluble in water, and thus water can not completely extract the free lipids from the yolk; as a result, this would possibly lower the OE value. Although the CVs were higher in GM than in SM, all the CVs, including in , were within accepted range. As for the increased values by the possible non fat solids in GM, through limiting the spreading area of each SY sample on the filter paper to the same as USY spreading by a circle model, and subtracting OE% of USY from OE% of SY, the accuracy could be adjusted.
Table 2. Comparison effects of two methods on salted and unsalted egg.
Recovery and linearity of added soy oil with detected amount of free oil by GM
The added SO and detected FO showed high linear correlation (R2 = 0.9983), and the RC of the five levels of SO ranged from 98.21% to 101.92% as shown in . The recovery of SO did not change with the added amounts, and no significant differences (p > 0.05) existed among levels. Moreover, recovery was similar to the theoretical value (100%) for each assay, and all recovery values were satisfactory according to Student’s t-test.
The correlation of OE by GM with total lipids and sensory evaluation for SY of six brands
As shown in , total lipids percentages based on yolk weight of cooked salted duck egg ranged from 39.29% to 50.69% for the tested six brands. The OE% measured by GM and based on TL ranged from 24.64% to 44.96%. There were significant differences (p ≤ 0.05) among some samples for the TL%, sensory score, and OE%. However, the OE% by GM was significantly correlated with TL% and sensory score for the six samples, and the correlation coefficients were accordingly 0.785 and 0.820 of OE with TL and sensory score, which suggested consistence of GM and sensory evaluation.
Table 3. Oil exudations from six brands of salted yolk as evaluated by gravimetric method.
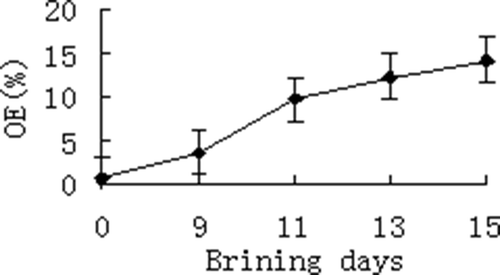
OE determined by GM during brining of duck eggs
The OE of duck eggs during brining period were measured by GM at regular intervals and the results showed a significant increasing trend () which was consistence with the development state of egg yolks at each stage. This result suggested that the GM can be used to evaluate oil evaluation development for salted egg processing and research work.
Discussion
All the above results demonstrated that the GM could be used as a practicable and effective method to quantitatively evaluate oil exudation in YSE. In addition, the GM offers several advantages, such as its is safer, simpler, and cheaper than other methods. The safety of the GM is attributed to its being solvent-free, wherein no organic solvent, which is possibly poisonous to human body, is used. Moreover, the GM is cost effective because the materials used, except for the filter papers and samples, are not consumable; the simplicity and practicability of the GM are obvious in the procedure described above. Knowing the absolute OE amounts in YSE is unnecessary, because only the state with apparent oil is consumers’ preference and concern. Companies processing salted egg just need to evaluate the oil exudation state by an effective method and expressed by a value which can be compared with each other or with a standard. Therefore, even if wide differences were between GM and SM, the GM can still be used as an alternative method to be applied in production and study works.
Despite the above advantages, some problems must be addressed. First, a dried filter paper rapidly absorbs moisture; for this reason, the filter papers and sample with filter papers must be weighed immediately, and the environment must be as dry as possible. Otherwise, the precision and accuracy of determination is affected. Second, slight agitation is necessary before sampling to prepare homogeneous samples; however, over agitation can result in re-emulsification of free lipids by water and proteins, reducing the detected values. Overall, skilled operation of the GM is important to obtain representative results.
Funding
The authors acknowledge the financial support provided by Science and Technology Department of Anhui Province in China (Grant number 1604a0702030).
Additional information
Funding
References
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Changes in Chemical Composition, Physical Properties and Microstructure of Duck Egg as Influenced by Salting. Food Chemistry 2009, 112, 560–569.
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effects of Salting Processes and Time on the Chemical Composition, Textural Properties, and Microstructure of Cooked Duck Egg. Journal of Food Science 2011, 76, S139–S147.
- Lai, K.M.; Chi, S.P.; Ko, W.C. Changes in Yolk States of Duck Egg during Long-Term Brining. Journal of Agriculture and Food Chemistry 1999, 47, 733–736.
- Lai, K.M.; Chung, W.H.; Jao, C.L.; Hsu, K.C. Oil Exudation and Histological Structures of Duck Egg Yolks during Brining. Poultry Science 2010, 89, 738–744.
- Zhao, N.N.; Hu, J.; Hou, T.; Ma, Z.; Wang, C.; He, H. Effects of Desalted Duck Egg White Peptides and Their Products on Calcium Absorption in Rats. Journal of Functional Foods 2014, 8, 234–242.
- Wang, Y.T.; Zheng, H.; Li, Y.; Li, B.; Chen, Y. One Step Procedure for Desalting Salty Egg White and Preparing Fat Analogue and Its Application in Mayonnaise. Food Hydrocolloids 2015, 45, 317–326.
- Blount, J.D.; Houston, D.C.; Møller, A.P. Why Egg Yolk Is Yellow. Trends in Ecology & Evolution 2000, 15, 47–49.
- Fletcher, D.L.; Britton, W.M.; Cason, J.A. A Comparison of Various Procedures for Determining Total Yolk Lipid Content. Poultry Science 1984, 63, 1759–1763.
- Wang, S.C. Handbook of Food Hygiene Inspection Technology (in Chinese, 2th ed); Press, Chemical Industry: Beijing, China, 1996; 138 pp.
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative Evaluation of UV-HPLC Methods and Reducing Agents to Determine Vitamin C in Fruits. Food Chemistry 2007, 105, 1151–1158.
- Chi, S.P.; Tseng, K.H. Physicochemical Properties of Salted Pickled Yolks from Duck and Chicken Eggs. Journal of Food Science 1998, 63, 27–30.
- Chong, L.C.; Aziz, N.A.A. Effects of Banana Flour and Β-Glucan on the Nutritional and Sensory Evaluation of Noodles. Food Chemistry 2010, 119, 34–40.