ABSTRACT
Cashew nut protein powder was hydrolysed by alkaline protease to prepare ACE inhibitory peptides. Results showed that the optimum conditions were: substrate concentration, 7 g/100 mL; amount of enzyme, 3%; pH, 10.5; hydrolysis time, 6 h; and temperature, 45°C. Under these conditions, ACE inhibitory rate was 57.45%. ACE inhibitory peptides were filtered using an ultrafiltration membrane of different molecular weights and Sephadex G-15 gel column. We found that the molecular weight was <3000 Da, and the ACE inhibitory rate concentration was 100 mg/mL. The M2 component, which was the most active component in ACE inhibitory peptides, yielded an ACE inhibitory rate of 77.58%. The M2 component contained 18 types of amino acids, including seven types of essential amino acids with the highest proline content reaching 23.56% (P < 0.01). The molecular weight of the M2 constituent was observed as 389 Da, with an amino acid sequence of Glu- Ser - Arg.
Introduction
Angiotensin-converting enzyme (ACE) is a dipeptide carboxylase that is an important regulator for blood pressure in humans.[Citation1] ACE can cause strong vascular smooth muscle contractions and increase blood pressure in the human renin–angiotensin system and kallikrein–excitation peptide system.[Citation2,Citation3] Moreover, ACE can soothe the activity of cream peptide, which means diastolic blood vessel function of the human endogenous step-down system could be regulated.[Citation4,Citation5] Therefore, inhibiting the cream peptide activity of regulating blood pressure has an important influence. The blood pressure peptide preparation methods mainly include direct extraction, fermentation, and enzymatic hydrolysis. Because the content of ACE inhibitory peptides is low in plants and animals, in order to prepare high activity and good purity peptide, it must be repetitively extracted, which greatly increased the economic cost. In addition, extraction method can cause environmental pollution and solvent residue;[Citation6] Defection of fermentation method: strains are easily varied and aged, contaminated by the bacterial, product preparation need a long time, and fermentation conditions are not easy to control;[Citation7] Enzymatic hydrolysis method has obvious advantages, which include having mild enzymatic conditions and specificity for protein, nutrients in the substrate are largely retained, and the solution with less impurity after enzymatic and operation is easy to control. Therefore, enzymatic hydrolysis method can better meet the needs of the preparation of ACE inhibitory peptides.[Citation8] Some researchers have reported different food protease solutions to prepare ACE inhibitory peptides.[Citation9,Citation10] The antihypertensive peptides after foodborne protein hydrolysis, which has high safety features, have been studied extensively,[Citation11,Citation12] so ACE inhibitory peptides isolated from foodborne materials have become a trend in the future development of health food[Citation13].
Table 1. Optimisation parameters used in response surface analysis.
Table 2. Experimental design and results for response surface analysis.
Table 3. Amino acid composition of the M2 component (%) (g/100 g protein).
Most reported ACE inhibitory peptides contain 2 to 15 peptides, hydrophobic amino acids, and aromatic amino acids, with a molecular weight between 200 and 1500 Da.[Citation14,Citation15] Cashew nut is rich in protein (17.7–21.29%) and amino acids (contains eight kinds of essential amino acids which cannot be synthesised from our body and have been reported in the preparation of antioxidant peptides and healthy oral liquid amino acids).[Citation16,Citation17] Research on Cashew nut protein-derived ACE inhibitory peptides mostly focused on enzyme screening and optimisation of the extraction process.[Citation18,Citation19] By contrast, few studies have reported on purification.[Citation13] Our research can explore potential biological utilisation value of cashew nut, abundant raw materials for the preparation of ACE inhibitory peptides, which is of positive significance for enriching cashew nut related product development.
Materials and methods
Materials and reagents
Skim cashew nut protein powder (protein content = 63.17%) was obtained from Biological Technology Co., Ltd. (Chongqing, China). Alkaline protease (enzyme activity = 1.89 × 10Citation4 µ/g), angiotensin I-converting enzyme (ACE), N-[3-(2-furylacryloyl)]-L-phenyalanyl-glycyl-glycine (FAPGG), and Sephadex G-15 glucan gel were purchased from Sigma-Aldrich Co. (USA). Tris-HCl buffer, NaCl, NaOH, formic acid, ethanol, and phenol reagents were of analytical grade and purchased from Beijing Chemical Reagent Co. (Beijing, China). Deionised water was used throughout the experiment. 50 mmol/l Tris-HCl: 6.055 g Tris was accurately weighed in a 1 L beaker and added with 800 ml of deionised water to dissolve thoroughly, concentrated hydrochloric acid was used to adjust the required pH, the solution volume was kept to 1 L, sterilised, and preserved it at room temperature.
Preparation of ACE inhibitory peptides
Cashew nut protein powder was dissolved in distilled water and then mixed with alkaline protease. The mixture was hydrolysed at a constant temperature, and the enzyme was inactivated via a boiling water bath for 10 min. After centrifugation for 10 min at 4°C and 10,000 r/min, the supernatant fluid was subjected to evaporation and freeze drying. Finally, ACE inhibitory peptides were obtained.[Citation18,Citation19]
Response surface design experiment under enzymatic hydrolysis conditions
According to the results of single-factor experiment, the experimental design was optimised using response surface methodology. The Box–Behnken centre combination experimental principle was also applied, in which we selected four factors affecting the ACE inhibitory rate.[Citation20,Citation21] These factors were substrate concentration, pH value, reaction temperature, and reaction time. Three levels per factor were used to optimise the test (), and the ACE inhibitory rate was used as the response variable.
Determination of ACE inhibitory rate
Distilled water (1 mL) was slowly injected into glass bottles containing 0.1 U ACE. After mixing, the bottles were set aside. Meanwhile, 10 µL of ACE aqueous solution and 10 µL of ACE inhibitory peptides (concentration of 1 mg/mL) were added to an enzyme label plate. Subsequently, the substrate (1.0 mmol/L FAPGG dissolved in 50 mmol/L Tris-HCl containing 0.3 mol/L NaCl, pH 7.5), which was preheated for 37°C for 5 min, was added to the plate and allowed to react for 2 min. The absorbance at 340 nm was recorded and labelled A1. After a reaction time of 30 min, the absorbance at 340 nm was determined and labelled A2. The control group comprised 10 µL of substrates instead of ACE inhibitory peptides. The initial absorbance of the blank solution was recorded as A01, and that after the reaction was recorded as A02. Changes in the absorbance value (ΔA inhibitor = A1 − A2, Δblank = A01 − A02) were calculated to obtain the ACE inhibitory rate.[Citation20,Citation21]
Ultrafiltration of ACE inhibitory peptides
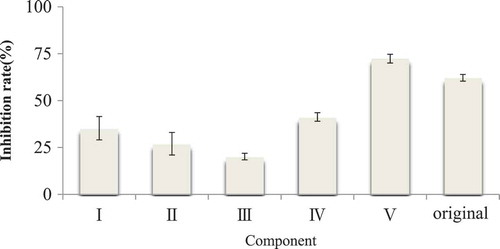
The hydrolytic liquid was filtered using four (30, 10, 5, and 3 kDa) ultrafiltration membranes and separated by a Vivaflow ultrafiltration system. After ultrafiltration, the hydrolytic liquid was divided into five components, namely, component I (M > 30 kDa), component II (10 kDa < M < 30 kDa), component III (5 kDa < M < 10 kDa), component IV (3 kDa < M < 5 kDa), and component V (M < 3 kDa). All components were collected and vacuum freeze-dried. The ACE inhibitory rates of these components were then determined.
Gel chromatography of ACE inhibitory peptides
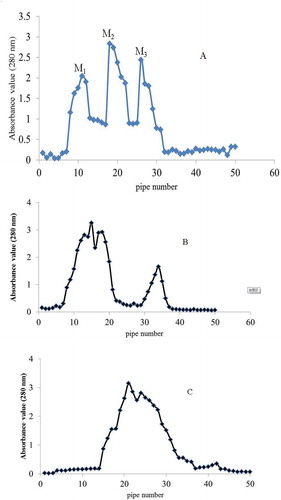
The processed Sephadex G-15 resin was loaded in a 1.6 × 60 cm glass chromatographic column. Samples of 100, 200, and 300 mg/mL solution were prepared, and 3 mL was eluted each time at a flow rate of 0.3 mL/min distilled water. Samples were collected by a tube every 10 min, and a total of 50 tubes were obtained. The absorbance of these 50 tubes was detected at 280 nm. The elution peak was collected at optimal state. After the components were freeze-dried, their ACE inhibitory rates were determined.
Determination of molecular weight distribution of ACE inhibitory peptides
The active components after SephadexG-15 filtration were identified by MALDI-TOF-MS to obtain the molecular weight of ACE inhibitory peptide. Myoglobin from horse heart trypsin digestion peptide was used as a standard peptide to calibrate the instruments. The instruments were calibrated to an error of ≤0.1 µ, and the relative standard deviation was ≤10 × 10Citation6. MS was performed under the following settings: positive 2 kv reflection mode, MALDI laser source, scanning range of m/z 120–760, laser energy of 5000, and positive ion mode. The samples were resolved in ultrapure water to a concentration of 5 µg/µL. Approximately 0.5 µL of samples were placed in MALDI stainless steel plates for natural drying, and 0.5 µL of 0.5 μg/μL alpha-cyano-4-hydroxycinnamic acid was used as the matrix[20,21].
Amino acid analysis of ACE inhibitory peptides
Samples (100 mg) in the hydrolysis tubes were initially added with 4.2 mol/L NaOH to hydrolyse tryptophan. Then 1 mL of performic acid reagent was added at room temperature, and the reagent was allowed to mix with the samples for 3 h. The oxidation reaction was stopped using 1 mL of ethanol. The tubes were placed in a pressure reducer, dried, rinsed with deionised water, and dried again. This process was repeated three times to remove performic acid. Subsequently, 10 mL of 6 mol/L hydrochloride was added to the aqueous solution, followed by three drops of phenol. The tubes were placed in a coolant, frozen for 3–5 min, vacuumed, and filled with nitrogen. This process was repeated three times. The tubes were then sealed with nitrogen, placed in a drying oven maintained at a constant temperature of 110°C, hydrolysed for 22 h, and cooled at room temperature. After hydrolysis of the tubes containing the samples, the hydrolytic solution was filtered and transferred to a 50 mL volumetric flask. Deionised water was used to reach a constant volume. 1 mL of the filtrate was taken into a 5 mL volumetric flask and dried in a vacuum drier. The residue was dissolved in deionised water, then dried, repeated for two times, and dissolved it in 1 mL of pH 2.2 in Tris-HCl buffer. Accurately absorbing 0.2mL mixed standard amino acids, diluting to 5mL with pH2.2 Tris-HCl buffer, which as the standard amino acid, the amino acid automatic analyzer with external standard method for determination the type and content of amino acids in ACE inhibitory peptides from cashew nut.[Citation20,Citation21]
Statistical analysis
Final data were expressed as the mean ± standard deviation () . ANOVA was employed to assess the differences among groups. Statistical analysis was performed using SPSS 21.0. Significant differences were denoted by P < 0.05.
Results and discussion
Response surface of optimisation test results
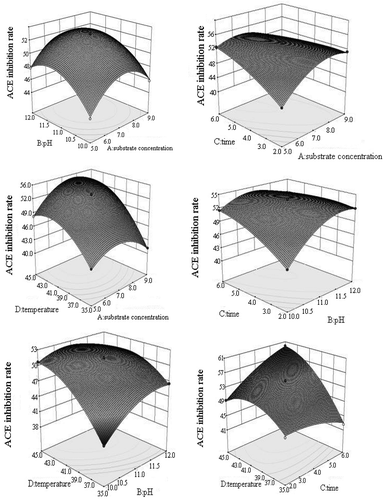
According to and the principle of response surface optimisation experiment, the P value of the model was significant. The P value of loss of quasi = 0.0639 > 0.05 was not significant, thereby suggesting that the unknown factors had a minimal influence on the test results (_ENSaikia et al. 2015, Jung et al. 2015)R2 = 0.9984, which indicated that the model exhibited a good fit, and the test error was small. The ACE inhibitory rate
Response surface analysis results
shows the response surface analysis of the interaction of various factors. The changes in response values exhibited a complex quadratic relationship, and interactions between any two factors were evident. The optimised hydrolytic conditions for the model were as follows: substrate concentration, 6.82 g/100 mL; pH, 10.5; time, 6 h; and temperature, 45°C. Under these conditions, the ACE inhibitory rate from the cashew nut peptide hydrolysed by papain reached 59.482%. For the validation experiments, the following conditions were set: substrate concentration, 7 g/100 mL; enzyme amount, 3%; pH, 10.5; time, 6 h; and temperature, 45°C. Under these conditions, the ACE inhibitory rate was 58.86%. The model had a relative error of 1.1%. Thus, the optimal cashew nut peptide hydrolysis conditions from the response surface method were accurate and reliable, and our proposed model was feasible in practical applications.
Ultrafiltration separation of different components of cashew nut peptide
The ACE inhibitory rates of components of different molecular weights were not the same after ultrafiltration separation. Based on the ACE inhibitory rate of components with a molecular weight >3000, such components exerted weak effects compared with the initial hydrolytic solution (P < 0.05). Among these components, only components V (molecular weight <3000 Da) had higher ACE inhibitory rates than the initial hydrolytic solution (P > 0.05). This finding illustrated that the most effective components of cashew nut ACE inhibitory peptides were from this component. This result also verified the conclusion that ACE inhibitory peptides are mainly composed of small molecular peptides. Therefore, in our subsequent analyses, we selected components V for further separation and purification ().
Sephadex G-15 chromatography separation of different components of cashew nut peptide
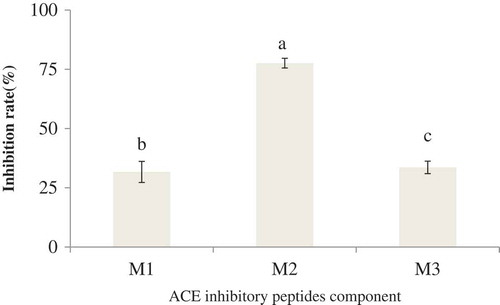
As shown in , the V components of cashew nut ACE inhibitory peptides were obtained after ultrafiltration. We selected samples of different concentrations, and three spectra were obtained after Sephadex-G15 resin separation. The elution peak of the sample at 200 mg/mL was poor (), and the components did not separate in the sample at 300 mg/mL (Fig. 3C). Based on the effects of the elution peak, we chose the sample concentration of 100 mg/mL. Three satisfactory elution peaks were obtained in this concentration, which were marked as M1, M2, and M3 (). The liquid attributed to the elution peak was collected, concentrated, and freeze-dried. Finally, the ACE inhibitory rate was determined. shows that the ACE inhibitory rate of the M2 component was the highest at 77.58% (P < 0. 05), with the IC50 of 0.5825 mg/mL.
Identification of amino acids in the ACE inhibitory peptides
As shown in , the amino acid content was rich in cashew nut ACE inhibitory peptides, which contained 18 types of amino acids, including seven essential amino acids for humans. Glutamate (Glu), Arginine (Arg), and Valine (Val) had the highest amino acid levels of 23.56% (P < 0.01), 9.01% (P < 0.05), and 6.78% (P < 0.05), respectively, which accounted for about 42% of the total amino acids. The n-terminal of the peptides containing Ala, Val, Leu, and Gly resulted in low blood pressure (); Moreover, the N-terminal containing Arg, Tyr, Phe, and Pro residues exerted blood pressure-lowering effects.[Citation20,Citation21] The contents of the aforementioned amino acids were relatively high in cashew nut ACE inhibitory peptides, which indicated good ACE inhibitory effects.
Determination of molecular weight distribution in ACE inhibitory peptides
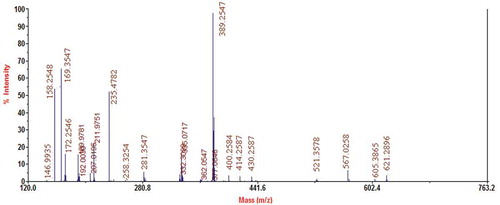
According to , the M2 component in the spectrum demonstrated three strong signal peaks. We observed a peak at 389.2 for molecular ion peaks (M+H)+, which indicated a molecular weight of 389 Da. Therefore, we presumed that the ACE inhibitory peptides comprised three peptides from the dehydration of three amino acids. The molecular weights of Glutamate (Glu), Arginine (Arg), and Serine (Ser) were 147, 174, and 105 Da, respectively, but their dehydration resulted in a molecular weight of only 389 Da. Therefore, the possible combinations of amino acids were Glu- Arg -Ser, Glu- Ser-Arg, Arg - Glu- Ser, Arg - Ser -Glu, Ser - Arg -Glu and Ser -Glu- Arg. According to the rules of sequence peptides, the characteristic ions at 169.3547 of the mass spectrum demonstrated that Ser was a c-terminal amino acid (in the formation of three peptides, Ser provided H+ for –NH2). The characteristic ions at 158.2548 showed that Arg was the middle peptide (in the formation of three peptides, Arg provided H+ for –NH2 and OH− for –COOH). Therefore, Glu should also provide OH−, which resulted in a characteristic ion at 58[20,21]. However, considering the data collected at m/z 120, the characteristic ions at 58 were not observed in the mass spectrum. The characteristics ions at 235.4782 could be attributed to the Glu- Ser-Arg lose-Arg residue, so we deduced that the amino acid sequence of the three peptides was Glu- Ser-Arg.[Citation20,Citation21]
Conclusion
The optimal parameters of ACE inhibitory peptides extracted from cashew nut protein powder after papain hydrolysis were as follows: substrate concentration, 7 g/100 mL; pH, 10.5; hydrolysis time, 6 h; and hydrolysis temperature, 45°C. Verification results showed that this model had good prediction ability. Ultrafiltration and Sephadex-G15 separation of the enzymatic hydrolysis product significantly increased the ACE inhibitory rate at a sample concentration of 100 mg/mL from 58.86% to 77.58% (P < 0.05). The cashew nut protein powder enzymatic hydrolysis product exhibited an ACE inhibitory effect of 389 Da. Its amino acid composition was rich, and the amino acid sequence may be Glu- Ser- Arg.
Declaration of interest
The author declares no conflict of interest.
LJFP_A_1325902_Supplemental_File.docx
Download MS Word (68.2 KB)Funding
This research was supported by ‘Hainan province natural science fund project (20163053)’, ‘Hainan university research funded projects (kyqd1553)‘, and ‘The Specialized Fund for the Modernization of Traditional Chinese Medicine of Hainan Province (2015ZY15)’.
Additional information
Funding
References
- White, B.L.; Sanders, T.H.; Davis, J.P. Potential ACE-Inhibitory Activity and NanoLC-MS/MS Sequencing of Peptides Derived from Aflatoxin Contaminated Peanut Meal. LWT-Food Science and Technology 2014, 56, 537–542.
- Wei, X.; Li, T.J.; Zhao, X.H. Coupled Neutrase-Catalyzed Plastein Reaction Mediated the ACE-inhibitory Activity In vitro of Casein Hydrolysates Prepared by Alcalase. International Journal of Food Properties 2013, 16, 429–443.
- Wang, S.; Lin, L.M.; Wu, Y.N.; Fang, M.; Yu, Y.Q.; Zhou, J.; Gong, Z.Y., Angiotensin I Converting Enzyme (ACE) Inhibitory Activity and Antihypertensive Effects of Grass Carp Peptides. Food Science and Biotechnology 2014, 23, 1661–1666.
- Wang, H.; Li, Y.; Cheng, Y.; Yin, L.; Li, L., Effect of the Maillard Reaction on Angiotensin I-converting Enzyme (ACE)-Inhibitory Activity of Douchi during Fermentation. Food and Bioprocess Technology 2013, 6, 297–301.
- Samarakoon, K.W.; O-Nam, K.; Ko, J.Y.; Lee, J.H.; Kang, M.C.; Kim, D.; Lee, J. B.; Lee, J.S.; Jeon, Y.J. Purification and Identification of Novel Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Cultured Marine Microalgae (Nannochloropsis oculata) Protein Hydrolysate. Journal of Applied Phycology 2013, 25, 1595–1606.
- Fu, Y.; Young, J.F.; Dalsgaard, T.K.; Therkildsen, M. Separation of Angiotensin I-converting Enzyme Inhibitory Peptides from Bovine Connective Tissue and Their Stability Towards Temperature, pH and Digestive Enzymes. International Journal of Food Science and Technology 2015, 50(5), 1234–1243.
- Elavarasan, K.; Shamasundar, B.A. Angiotensin I-converting Enzyme Inhibitory Activity of Protein Hydrolysates Prepared from Three Freshwater Carps (Catla catla, Labeo rohita and Cirrhinus mrigala) Using Flavorzyme. International Journal of Food Science and Technology 2014, 49(5), 1344–1350.
- Wang, L.; Mao, X.Y.; Cheng, X.; Xiong, X.X.; Ren, F.Z., Effect of Enzyme Type and Hydrolysis Conditions on the in Vitro Angiotensin I-converting Enzyme Inhibitory Activity and Ash Content of Hydrolysed Whey Protein Isolate. International Journal of Food Science and Technology 2010, 45(4), 807–812.
- Javier Espejo-Carpio, F.; De Gobba, C.; Guadix, A.; Guadix, E. M.; Otte, J. Angiotensin I-Converting Enzyme Inhibitory Activity of Enzymatic Hydrolysates of Goat Milk Protein Fractions. International Dairy Journal 2013, 32, 175–183.
- Intarasirisawat, R.; Benjakul, S.; Wu, J.; Visessanguan, W., Isolation of Antioxidative and ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack (Katsuwana pelamis) Roe. Journal of Functional Foods 2013, 5, 1854–1862.
- Gu, X.; Hou, Y.K.; Li, D.; Wang, J.Z.; Wang, F.J., Separation, Purification, and Identification of Angiotensin I-converting Dnzyme Inhibitory Peptides from Walnut (Juglans regia L.) Hydrolyzate. International Journal of Food Properties 2015, 18, 266–276.
- Gao, B.; Zhao, X.H., Modification of Soybean Protein Hydrolysates by Alcalase-Catalyzed Plastein Reaction and the Ace-inhibitory Activity of the Modified Product In vitro. International Journal of Food Properties 2012, 15, 982–996.
- Espejo-Carpio, F.J.; Perez-Galvez, R.; Almecija, M.d.C.;A. Guadix, E. M.; Guadix, Production of Goat Milk Protein Hydrolysate Enriched in ACE-inhibitory Peptides by Ultrafiltration. Journal of Dairy Research 2014, 81, 385–393.
- Dadzie, R.G.; Ma, H.; Abano, E.E.; Qu, W.; Mao, S. Optimization of Process Conditions for Production of Angiotensin I-converting Enzyme (ACE) Inhibitory Peptides from Vital Wheat Gluten Using Response Surface Methodology. Food Science and Biotechnology 2013, 22, 1531–1537.
- Su, R.; Xu, X.; Wang, X.; Li, D.; Li, X.; ZhangH, .;Yu, A. Determination of Organophosphorus Pesticides in Peanut Oil by Dispersive Solid Phase Extraction Gas Chromatography-Mass Spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 2011, 879(30), 3423–3428.
- Zuniga-Navarrete, F.; Gomez, I.; Pena, G.; Bravo, A.; Soberon, M. A Tenebrio Molitor GPI-anchored Alkaline Phosphatase is Involved in Binding of Bacillus Thuringiensis Cry3Aa to Brush Border Membrane Vesicles. Peptides 2013, 41, 81–86.
- Oppert, B.; Dowd, S.E.; Bouffard, P.; Li, L.; Conesa, A.; Lorenzen, M. D.; et al. Transcriptome profiling of the intoxication response of Tenebrio molitor Larvae to Bacillus thuringiensis Cry3Aa Protoxin. PLoS ONE 2012, 7(4), e34624 .
- Silva, M.R.; Silvestre, M.P.C.; Silva, V.D.M.; Souza, M.W.S.; Lopes Junior, C.O.; Afonso, W.O.; Lana, F.C.; Rodrigues, D.F. Production of Ace-inhibitory Whey Protein Concentrate Hydrolysates: Use of Pancreatin and Papain. International Journal of Food Properties 2014, 17, 1002–1012.
- Li, F.J.; Yin, L.J.; Lu, X.; Li, L.T. Changes in Angiotensin i-Converting Enzyme Inhibitory Activities during the Ripening of Douchi (a Chinese Traditional Soybean Product) Fermented by Various Starter Cultures. International Journal of Food Properties 2010, 13, 512–524.
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of Phenolic Extraction from Averrhoa Carambola Pomace by Response Surface Methodology and Its Microencapsulation by Spray and Freeze Drying. Food Chemistry 2015, 171, 144–152.
- Jung, K.W.; Hwang, M.J.; Cha, M.J.; Ahn, K.H. Application and Optimization of Electric Field-Assisted Ultrasonication for Disintegration of Waste Activated Sludge Using Response Surface Methodology with a Box-Behnken Design. Ultrasonics Sonochemistry 2015, 22, 437–445.