ABSTRACT
The combined effect of superchilling and tea polyphenols on the preservation quality of hairtails was assessed in terms of pH, electrical conductivity, K value, TVB-N, TBARS, total plate count, and sensory assessment. Hairtail blocks were subjected to three treatments: frozen at −18°C, superchilling at −3°C, and superchilling at −3°C after immersed in a 6.0 g/L tea polyphenols solution, respectively. For storage of 30 days, the TBARS value of hairtails increased steadily from 0.41 mg/100 g to 1.83, 1.38, 1.14 mg/100 g (p < 0.05) in the samples that were superchilled at −3°C, frozen at −18°C, and superchilled at −3°C with tea polyphenols, respectively. The TVB-N, microbial growth, electrical conductivity, and K values also exhibited an increasing trend (p < 0.05), similar to that obtained with the TBARS values, whereas the stability of sensory scores decreased (p < 0.05). Compared with superchilling samples, all these quality indicators have been improved in combined treated samples. Indicators such as pH, TVBN, and TBARS in combined treated samples even showed better results than those in frozen samples. These results suggested that superchilling with tea polyphenols provided an effective approach for inhibiting lipid oxidation and microbial growth, retarding the deterioration, and maintaining the inherent and fresh quality of hairtails.
Introduction
Hairtail (Trichiurus Haumela), also called cutlassfish or ribbonfish, is one of the most commercially important fish species in the Yellow Sea and East China Sea.[Citation1] In recent years, the production and consumption of hairtail is growing due to its delicious taste and high nutritional value. However, the vulnerability of hairtails to perish is even more severe than other marine fish species. It is well known that the refrigerated fish have limited shelf life, primarily associated with microbial activities and enzymatic activities.[Citation2,Citation3,Citation4] The initial quality deterioration in fish is primarily induced by autolytic enzymes.[Citation5] Lipid oxidation (both enzymatic and chemical) resulting in rancidity can also take place because of the presence of highly unsaturated fatty acids, where colour, flavour, texture, and nutritional value can be negatively affected.[Citation6,Citation7]
The most significant factor influencing the fresh quality and shelf life of fish that can be controlled is temperature.[Citation8] Therefore, to prolong the shelf life and minimize microbial and biochemical degradation, different preservation methods, mainly based on low temperatures, have been applied for fish preservation.[Citation9] Superchilling (−1 to −3°C), also known as partial freezing or deep chilling, has emerged as a promising process by which the temperature of food is reduced to 1–2°C below its freezing point. As the shelf life of superchilled products can be extended by 1.5–4 times that of conventional chilled food, superchilling got increasing emphasis in the frozen field. During superchilling, a cold reservoir is formed in the product, and additional chilling by addition of external ice might not be required during distribution, thus lowering the overall transport costs.[Citation10] Compared with freezing, less water is frozen (5–30%) in superchilled food, leading to a lower degree of freeze denaturation of the proteins and less mechanical damages to the muscle structure.[Citation11,Citation12] However, some negative quality effects on superchilled foods have also been found. Duun, A. S.[Citation10] revealed that both cod fillets and salmon exhibited higher degree of myofibrillar proteins denaturation during superchilling than those during chilled storage, and free amino acids formed rapidly as a result of exoproteolytic activity. What is more, the amount of ice crystals in superchilling food is highly dependent on the temperature, which has a great impact on the quality changes.[Citation13,Citation14]
To inhibit or mitigate these negative changes, various biopreservatives are normally added to the fish products to obtain optimum quality during ice, superchilling, or frozen storage.[Citation15,Citation16,Citation17] Tea polyphenol, a prominent physiological active component in tea, has many beneficial functions for human health, including anti-cancer[Citation18] and anti-obesity activities[Citation19], and could be used as antioxidant and potential preservative in food industry. Xu, Y.[Citation15] evaluated the inhibitory effect of tea polyphenols on the collagen degradation, collagenase activity, and texture change of grass carp fillets. They found tea polyphenols were able to inhibit the softening of fillets both under superchilling and ice storage.
Some studies have investigated the effect of superchilling on microbiology, sensory evaluations, and spoilage indicators, such as total volatile basic nitrogen (TVB-N).[Citation20,Citation21,Citation22] However, the effect of superchilling combined with tea polyphenols on the quality of hairtail has not yet been reported.The overall objective of this research was to evaluate the effects of superchilling combined with tea polyphenols on the quality of hairtails. The pH value, electrical conductivity (EC), TVBN, lipid oxidation, microbial growth, the freshness K value, and sensory evaluation were used as the quality indicators.
Materials and methods
Materials
Fresh hairtails (length 70.0 ± 2.0 cm) were purchased from a local aquatic market (Zhoushan, Zhejiang, China) and were immediately transported to the laboratory in an ice box. The proximate mean composition of the fish meat was determined as 78.12% moisture, 4.76% crude fat, 14.98% crude protein, and 1.21% ash. Tea polyphenols were provided by Nanning Zhongnuo bioengineering Co., LTD (Nanning, Guangxi, China). All other chemicals were ordered from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) at analytical reagent grade.
Sample preparation
The hairtail fishes were de-headed and gutted, tails cut followed by washing with sterile iced water. Then the fish were drained and cut into blocks of approximately 6.0 cm in length. All the fish blocks were randomly divided into three portions. The first two portions were vacuum-packed and stored at an automated temperature controlled refrigerator at −18°C and −3°C, respectively. The last portion was mixed with a 6.0 g/L tea polyphenols solution [6.0 g/L was determined by our pre-experiments (data not shown)] for 30 s, drained, vacuum-packed, and immediately transferred to a refrigerator at −3°C. Samples from each treatment were randomly selected at 0, 3, 6, 11, 16, 23, and 30 days for analysis.
Freezing curve
For hairtails stored at −18°C, the temperature change as a function of time was logged by an automatic thermocouple (L93-2L, Hangzhou LuGe technology co., LTD, Hangzhou, China) inserted deep into the sample. The temperature data was collected every 1 min during testing. The initial freezing point was determined from the freezing curve according to the previous method.[Citation23].
pH value
The pH of hairtail fish meat was measured according to the method of Song, Y.[Citation24] with some modifications. A 10 g sample of meat was homogenised with 100 mL of cold distilled water using a homogenizer at a speed of 1000 rpm for 1 min, and the pH of the homogenate was tested by a pH meter (EF20K, Shanghai Mettler Toledo instrument co., LTD, Shanghai, China). All measurements were done in triplicate.
Electrical conductivity
The EC of hairtail fish meat was measured as described by the literature.[Citation25] A sample (5 g) was homogenised with 45 mL distilled water and then stirred for 30 min. The mixture was filtered, and the EC of filtrate was determined by a digital EC meter (DDS-307A, Shanghai leici scientific instrument co., LTD, Shanghai, China).
Total volatile base nitrogen
TVB-N was estimated in accordance with the previous method.[Citation21] A 10 g sample was homogenised with 90 mL 0.6 mol/L perchloric acid solution, and the resulting suspension was centrifuged at 3000 r/min for 10 min at 4°C using a high-speed bench centrifuge (TGL-16G, Shanghai Anheng scientific instrument factory, Shanghai, China). A 10 mL supernatant was distilled and titrated in an automatic Kjeldahl Apparatus (Kjeltec2300, Foss, Sweden) with the blank experiment of 10 mL perchloric acid solution. The TVB-N value was estimated by the following equation:
Thiobarbituric acid-reactive substances
The determination of TBARS was performed according to methods previously described by Siu, G. M.[Citation26] with slight modifications. A 10 g sample was stirred with 50 mL 7.5% trichloroacetic acid (containing 0.1% EDTA) for 30 min. 5 mL of the resulting supernatant was reacted with 5 mL 0.02 mol/L TBA in boiling water for 40 min. After cooled, the sample solution was added 5 mL chloroform (1:1 ratio, v/v), vortexed, and subsequently stratified. The absorbance of the supernatant was determined at the wavelength of 532 nm and 600 nm. The TBARS value, which is expressed as mg of malonaldehyde/100 g muscle sample, was estimated using the following equation:
Total plate count
Total plate count determination was performed following the method of Li, X.[Citation27] with some modifications. A 25 g sample of haitails was obtained aseptically and homogenised with 225 mL of 0.9% physiological saline. The homogenates and serial 10-fold dilution in sterile 0.9% physiological saline were mixed with agar medium. The total plate count, which is expressed as lgCFU/g, was determined after incubation at 37°C for 48 h.
K-value
The hairtail fish samples were pre-treated by the presented way.[Citation28] Measurement of ATP-related compounds was performed by HPLC (Waters 2695, USA) with a Capcellpak C18 column (4.6 mm × 250 mm). Phosphate buffer (0.05 M, pH 6.3) was used as the mobile phase. The sample of 20 µL was injected (1mL/min), and the peaks were detected at 254 nm. The K-value was calculated as follows:
Sensory assessment
Hairtail samples were analysed according to the methodology presented by the author[Citation29] with some modifications. Sensory assessment of hairtails was performed by ten trained panelists from the laboratory staff. Each assessor scored the fish for colour (10, no discolouration; 1, extreme discolouration), odour (10, extremely desirable; 1, extremely unacceptable), morphology (10, normal; 1, sunken or swollen), and elasticity (10, elastic; 1, inelastic) of muscles. Scores of separate characteristics (the weight values: 0.2, 0.3, 0.2, 0.3, respectively) of fish were summed to give an overall sensory score (total score: 10).
Statistical analyses
All measurements were carried out in triplicate, and data were expressed as mean ± standard deviation. The least significant difference procedure was used to test for difference between means by the Compare Means Procedure of SPSS 19 with a value of P < 0.05 being regarded as statistically significant.
Results and discussion
Freezing point
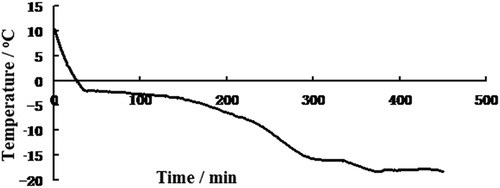
The freezing curve (time-temperature curve) for haitails stored at −18°C was shown in . In the early part of the freezing curve, the temperature of fish decreased rapidly from the initial temperature about 10°C. When the temperature was below zero, the freezing rate became slow. There is a temperature plateau around 0 to −5°C, which is regarded as a critical zone where most ice crystals form.[Citation30] At the frozen time of 30 min, an inflection point appeared in the freezing curve whose temperature was −1.9°C. Therefore, −1.9°C is the freezing point of hairtails attributed to liberation of the heat of fusion. According to the definition of superchilling,[Citation31] the superchilling temperature of hairtail is set to −3°C in this study.
Changes of pH
The changes of pH values of the hairtail fish meat during storage are shown in ). As can be seen from the figure, the initial pH of hairtails was 7.2. At the end of storage, pH value reached 7.29, 7.19, 7.1 in the samples that were superchilled at −3°C, frozen at −18°C, and superchilled at −3°C with tea polyphenols, respectively. Changes in pH value of different treatments showed similar trend in which the values decreased within the initial 3 days and then increased in different degrees. This result was in accordance with the findings that for grass carp subjected to superchilling and freezing.[Citation21] The initial decrease may be explained by the accumulation of lactic acid from anaerobic glycolysis and the production of inorganic phosphate due to ATP degradation,[Citation32] whereas rising pH value during the later period of storage may be associated with decomposition of amino compounds resulting from microbial and autolytic reactions.[Citation33]
Figure 2. Effect of frozen at −18°C (×), superchilled at −3°C (▲), and superchilled at −3°C with tea polyphenols (●) on the (A) pH, (B) EC, (C) TVB-N, and (D) TBARS of the hartails during storage. The error bars indicate the standard deviation obtained from a total of three analyses.
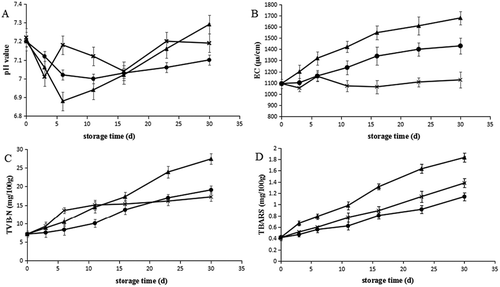
In the initial 3 days, the pH of samples superchilled at −3°C with tea polyphenols decreased slower than that frozen at −18°C and superchilling at −3°C, which indicated that tea polyphenols may interact with oxidase proteins and reduce their activity, inhibiting the rate of pH reduction due to glycolysis and ATP degradation. During the following storage, samples of superchilling at −3°C with tea polyphenols showed no significant increase in pH value (p > 0.05); however, the pH of samples frozen at −18°C and superchilled at −3°C increased significantly (p < 0.05), which was similar to the findings by He, L.[Citation34] This result may be attributed to that tea polyphenols can retard the autolysis and protein degradation caused by microorganism during superchilling storage. Therefore, superchilling with tea polyphenols may achieve a better prevention of microbial spoilage which may give rise to the accumulation of basic compounds, such as trimethylamine and ammonia.
Changes in EC
Electrical conductivity (EC), as an important indicator of the concentration of electrolytes in the fish tissues, has an impact on body fluid balance and survival and therefore was widely used to predict the quality of meat.[Citation35] As shown in ), the initial EC value of hairtail fish was 1094μs/cm. The samples storage at −18°C did not show significant increase in EC value (p > 0.05). The EC value of samples superchilled at −3°C without or with tea polyphenols increased significantly, and the latter batch was significantly lower (p < 0.05) during the storage time. The significantly increase of EC value was induced by the fact that the fish tissues are decomposed and fluids flow out. Similar trends were found.[Citation36] This result also suggested that tea polyphenols may retard the leakage membrane structures that allow fluids to flow out,[Citation28] and inhibit the decomposing of fish proteins, thus inhibiting the increase of EC during superchilling storage.
Changes in TVB-N
The changes in TVB-N values of hairtails with different treatments are shown in ). The TVB-N value in hairtails steadily increased from 7.13 mg/100 g (at day 0) to 27.36, 19.08, 17.21 mg/100 g in the samples that were superchilled at −3°C, superchilled at −3°C with tea polyphenols, and frozen at −18°C, respectively, over 30 days of storage (Fig. 2C). The TVB-N values for hairtails stored at −3°C either with or without tea polyphenols increased rapidly (p < 0.05), accounting for the increases in pH during the later period of storage, whereas, for samples stored at −18°C, the increase of TVB-N is not prominent, especially during the later storage period, which reflected that lower storage temperatures could inhibit the microbial and enzymatic reaction and thus inhibit TVB-N formation. Besides, the addition of tea polyphenols to the superchilled samples significantly lower TVB-N values relative to the superchilled samples without tea polyphenols, suggesting that tea polyphenols can alleviate the formation of TVB-N during superching storage.
Changes in TBARS
He, L.[Citation34] also revealed that the increase in TVB-N values was significantly lower in the experiment group treated with several biopreservatives containing tea polyphenols than in the control group (p < 0.01). This difference may be owing to that the tea polyphenols may have inhibitory effect on bacterial population or on oxidative deamination of nonprotein nitrogen compounds.[Citation34]
According to the current hygienic standards, a level of 20 mg TVB-N/100 g in fish sample is recognised as a spoilage limit.[Citation37] Thus, our results illustrated that the TVB-N values in samples in superchilled with tea polyphenols and frozen storage were below the test limitation throughout the storage stages.
The lipid oxidation of hairtail fishes stored under superchilling or frozen conditions was evaluated by measuring the TBARS value. As is presented in ), the TBARS values steadily increased from 0.41 mg/100 g (initial value) to 1.83, 1.38, 1.14 mg/100 g in the samples that were superchilled at −3°C, frozen at −18°C, and superchilled at −3°C with tea polyphenols, respectively, over 30 days of storage. All these treatments exhibited a significant increasing trend in TBARS value during the storage (p < 0.05). Compared with the samples superchilled at −3°C, the TBARS in the −18°C frozen samples was significantly reduced with decreased storage temperature, which indicates that a low temperature can significantly decrease the formation of malonaldehyde and other secondary products. In addition, the TBARS production in the superchilling samples with tea polyphenols was the lowest among all these treatments after 30 days of storage. It demonstrated that the addition of tea polyphenols to the samples in the superchilling storage can significantly decrease the TBARS and inhibit lipid oxidation.
Oxidation is regarded as a major cause of deterioration of the quality of fish during processing and storage, generally characterised by a loss of nutrients, flavour deterioration, discolouration, and the possible production of toxic compounds.[Citation38] Hydroperoxides produced during lipid oxidation can subsequently decompose to some secondary products, such as ketones and aldehydes, which are closely associated with fish rancidity. The hairtails meat in this study is rich in proteins and fat, especially unsaturated fatty acids, which is susceptible to the pro-oxidants and oxygen attack and form hydroperoxides that easily decompose to secondary products, including malonaldehyde (MDA). Tea polyphenol, acted as a natural antioxidant, can effectively clear free radicals and thus prevent lipid oxidation during superchilling storage. Cheng, A.[Citation39] also discovered the same results that the incorporation of tea polyphenol was effective in diminishing the lipid oxidation, measured as TBARS values, of chilled pork during cold storage.
Changes in total plate count
Microbiological analysis results are shown in ). Changes in total plate count show similar trends as changes in EC during superchilling and frozen storage. At the beginning of the storage, total plate values for fresh hairtail samples were 2.50 lgCFU/g samples. For samples frozen at −18°C, total plate count increased to 3.2 lgCFU/g and exhibited the lowest bacterial count as the storage time increased, which indicated that low temperature storage can significantly inhibit the growth of microorganisms. The total plate value of samples superchilled at −3°C with or without tea polyphenols increased to 6.85, 5.06 lgCFU/g, respectively, for 30 days of storage, and the latter batch was significantly lower (p < 0.05) during the storage time. This results reflected that superchilling combined with tea polyphenols can inhibit the microbial growth during superchilling storage to some extent though they cannot attain the level of frozen storage. According to the proposed limits (7 lgCFU/g) for fresh fish by ICMSF (1986), all treatments maintained relatively good quality throughout the storage.
Figure 3. Effect of frozen at −18°C (×), superchilled at −3°C (▲), and superchilled at −3°C with tea polyphenols (●) on the (A) total plate count, (B) K value, and (C) sensory scores of the hartails during storage. The error bars indicate the standard deviation obtained from a total of three analyses.
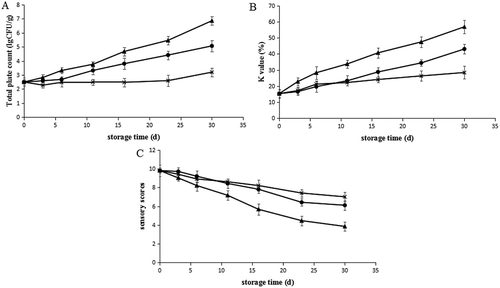
Cheng, A.[Citation32] also found a similar increase of total plate count in golden gray mullet during ice storage. This can be induced by possible leakage of internal substances including various nutrients from muscle cells which offered a proper medium for microbial growth during superchilled and frozen storage. Tea polyphenols have a good antimicrobial activity so that they are widely used in many areas in food industry. Antimicrobial activities of tea polyphenols were also reported before,[Citation34] who reported that tea polyphenols exhibited inhibitory effects against both Gram-negative bacteria (GNB) and Gram-positive bacteria (GPB) and extended the shelf life of chilled mutton.
Changes in K value
The changes in K value can reflect the loss of freshness in fish objectively. As is shown in ), the changes in K value in superchilled and frozen storage are similar to the changes of EC and total plate count. The K values increased from 15.35% to 56.85%, 43.06%, and 28.45% in the samples that were superchilled at −3°C, superchilled at −3°C with tea polyphenols, and frozen at −18°C, respectively, over 30 days of storage. According to the description,[Citation28] fish with K values <20% are considered as very fresh, with <50% as moderately fresh, and >70% as not fresh. In this study, both the two groups superchilled at −3°C with tea polyphenols and frozen at −18°C were still recognised as moderately fresh at day 30 storage. Shen, S. demonstrated similar findings that the K values increased in rainbow trout both during storage at 3°C and −3°C.[Citation22]
With the storage time increasing, many biochemical reactions take place in fish that impact on its freshness quality. Among them, the concentration of ATP and its breakdown products are mostly widely used as index of fish freshness. Tea polyphenols may lower the rate of ATP degradation by the way of inhibiting the activity of endogenous autolytic enzymes and thus contribute to the freshness of the hairtails in the superchilled storage.
Sensory assessment
) shows the sensor assessment of hairtails. Sensory scores decreased with storage time in all the three cases, and the group of superchilling at −3°C showed higher reducing rate than the other two groups (p < 0.05). On day 16, the fish maintained superchilled at −3°C (score is lower than 6) had bad colour, odour, texture, and unacceptable appearance, while those stored in −18°C and −3°C with tea polyphenols still had high degrees of quality. On day 30, the scores of samples stored in −18°C and −3°C with tea polyphenols are 7, and 6.1, respectively, still had acceptable quality. This result is in agreement with the TBARS values that tea polyphenols can retard lipid oxidation during superchilling, retain the inherent odour and quality, and therefore extend the shelf life of hairtails.
Conclusion
Superchilling of hairtail fish at −3°C with tea polyphenols retarded the increasing of pH, EC, and K values; lowered total plate count; reduced the production of TVB-N; and inhibited lipid oxidation compared with storage at −3°C and −18°C though some quality indices were not better than storage at −18°C in the longer period of storage. The sensory assessment also indicated that the addition of tea polyphenols in the superchilling method extended the spoilage time of hairtails, while the superchilling samples without tea polyphenols showed bad quality on day 16. The results showed that tea polyphenols were very effective in preventing hairtail quality deterioration by lowering the activity of endogenous autolytic enzymes, inhibiting the lipid oxidation and microbial growth. Therefore, superchilling with tea polyphenols was proved to be a promising preservation method of hairtails and might contribute to the reservation of inherent and fresh quality of other fish species in aquatic industry.
Funding
This study was supported by project NSFC31671918, project 2017YFD0400400, project 2016YFD0400102 and project SQ2017YFNC010025.
Additional information
Funding
References
- Zhao,X. In situ Target-Strength Measurement of Young Hairtail (Trichiurus haumela) in the Yellow Sea. ICES Journal of Marine Science: Journal du Conseil 2006, 63, 46–51.
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-life Extension on Fillets of Atlantic Salmon (Salmo salar) Using Natural Additives, Superchilling and Modified Atmosphere Packaging. Food Control 2009, 20, 1036–1042.
- Fernández, K.; Aspé, E.; Roeckel, M. Scaling Up Parameters for Shelf-life Extension of Atlantic Salmon (Salmo salar) Fillets Using Superchilling and Modified Atmosphere Packaging. Food Control 2010, 21, 857–862.
- Yesudhason, P.; Lalitha, K.V.; Gopal, T.K.S.; Ravishankar, C.N. Retention of Shelf Life and Microbial Quality of Seer Fish Stored in Modified Atmosphere Packaging and Sodium Acetate Pretreatment. Food Packaging and Shelf Life 2014, 1, 123–130.
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of Food: A Review. Journal of Food Engineering 2011, 107, 141–146.
- Maqsood, S.; Benjakul, S.; Kamal-Eldin, A. Haemoglobin-mediated Lipid Oxidation in the Fish Muscle: A Review. Trends in Food Science & Technology 2012, 28, 33–43.
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G. Lipid Oxidation and Fishy Odour in Protein Hydrolysate Derived from Nile Tilapia (Oreochromis niloticus) Protein Isolate as Influenced by Haemoglobin. Journal of the Science of Food and Agriculture 2014, 94, 219–226.
- Song, Y.; Luo, Y.; You, J.; Shen, H.; Hu, S. Biochemical, Sensory and Microbiological Attributes of Bream (Megalobrama amblycephala) during Partial Freezing and Chilled Storage. Journal of the Science of Food and Agriculture 2012, 92, 197–202.
- Gallart-Jornet, L.; Rustad, T.; Barat, J.M.; Fito, P.; Escriche, I. Effect of Superchilled Storage on the Freshness and Salting Behaviour of Atlantic Salmon (Salmo salar) Fillets. Food Chemistry 2007, 103, 1268–1281.
- Duun, A.S.; Rustad, T. Quality of Superchilled Vacuum Packed Atlantic Salmon (Salmo salar) Fillets Stored at −1.4 and −3.6°C. Food Chemistry 2008, 106, 122–131.
- Kaale, L.D.; Eikevik, T.M. The Development of Ice Crystals in Food Products during the Superchilling Process and Following Storage, a Review. Trends in Food Science & Technology 2014, 39, 91–103.
- Gaarder, M.Ø.; Bahuaud, D.; Veiseth-Kent, E.; Mørkøre, T.; Thomassen, M.S. Relevance of Calpain and Calpastatin Activity for Texture in Super-Chilled and Ice-Stored Atlantic Salmon (Salmo salar L.) Fillets. Food Chemistry 2012, 132, 9–17.
- Hartel, R.W. Advances in Food Crystallization. Annual review of food science and technology 2013, 4, 277–292.
- Duun, A.S.; Rustad, T. Quality Changes during Superchilled Storage of Cod (Gadus morhua) Fillets. Food Chemistry 2007, 105, 1067–1075.
- Xu, Y.; Jiang, X.; Ge, L.; Zang, J.; Xia, W.; Jiang, Q. Inhibitory Effect of Edible Additives on Collagenase Activity and Softening of Chilled Grass Carp Fillets. Journal of Food Processing and Preservation 2016, 41, n/a–n/a.
- Kong, B.; Guo, Y.; Xia, X.; Liu, Q.; Li, Y.; Chen, H. Cryoprotectants Reduce Protein Oxidation and Structure Deterioration Induced by Freeze-Thaw Cycles in Common Carp (Cyprinus carpio) Surimi. Food Biophysics 2013, 8, 104–111.
- Liu, Q.; Chen, Q.; Kong, B.; Han, J.; He, X. The Influence of Superchilling and Cryoprotectants on Protein Oxidation and Structural Changes in the Myofibrillar Proteins of Common Carp (Cyprinus carpio) Surimi. LWT - Food Science and Technology 2014, 57, 603–611.
- Yang, C.S.; Li, G.; Yang, Z.; Guan, F.; Chen, A.; Ju, J. Cancer Prevention by Tocopherols and Tea Polyphenols. Cancer Letters 2013, 334, 79–85.
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y., Mechanisms of Body Weight Reduction and Metabolic Syndrome Alleviation by Tea. Molecular Nutrition & Food Research 2016, 60, 160–174.
- Erikson, U.; Misimi, E.; Gallart-Jornet, L. Superchilling of Rested Atlantic Salmon: Different Chilling Strategies and Effects on Fish and Fillet Quality. Food Chemistry 2011, 127, 1427–1437.
- Mi, H.; Qian, C.; Zhao, Y.; Liu, C.; Mao, L. Comparison of Superchilling and Freezing on the Microstructure, Muscle Quality and Protein Denaturation of Grass Carp (Ctenopharyngodon idellus). Journal of Food Processing and Preservation 2013, 37, 546–554.
- Shen, S.; Jiang, Y.; Liu, X.; Luo, Y.; Gao, L. Quality Assessment of Rainbow Trout (Oncorhynchus mykiss) Fillets during Super Chilling and Chilled Storage. Journal of Food Science and Technology 2015, 52, 5204–5211.
- Chen, Y.-L.; Pan, B.S. Freezing Tilapia by Airblast and Liquid Nitrogen – Freezing Point and Freezing Rate. International Journal of Food Science & Technology 1995, 30, 167–173.
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of Sodium Alginate-Based Edible Coating Containing Different Anti-oxidants on Quality and Shelf Life of Refrigerated Bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615.
- Yao, L.; Luo, Y.; Sun, Y.; Shen, H. Establishment of Kinetic Models Based on Electrical Conductivity and Freshness Indictors for the Forecasting of Crucian Carp (Carassius carassius) Freshness. Journal of Food Engineering 2011, 107, 147–151.
- Siu, G.M.; Draper, H.H. A Study of the Malonaldehyde Content of Retail Meats and Fish. Journal of Food Science 1978, 43, 1147–1149.
- Li, X.; Li, J.; Zhu, J.; Wang, Y.; Fu, L.; Xuan, W. Postmortem Changes in Yellow Grouper (Epinephelus awoara) Fillets Stored Under Vacuum Packaging at 0°C. Food Chemistry 2011, 126, 896–901.
- Hong, H.; Luo, Y.; Zhou, Z.; Bao, Y.; Lu, H.; Shen, H. Effects of Different Freezing Treatments on the Biogenic Amine and Quality Changes of Bighead Carp (Aristichthys nobilis) Heads during Ice Storage. Food Chemistry 2013, 138, 1476–1482.
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of Chitosan Coatings Enriched with Cinnamon Oil on the Quality of Refrigerated Rainbow Trout. Food Chemistry 2010, 120, 193–198.
- Chen, Y.L.; Pan, B.S. Morphological Changes in Tilapia Muscle Following Freezing by Airblast and Liquid Nitrogen Methods. International Journal of Food Science and Technology 1997, 32, 159–168.
- Kaale, L.D.; Eikevik, T.M.; Bardal, T.; Kjorsvik, E.; Nordtvedt, T.S. The Effect of Cooling Rates on the Ice Crystal Growth in Air-packed Salmon Fillets during Superchilling and Superchilled Storage. International Journal of Refrigeration 2013, 36, 110–119.
- Bahmani, Z.A.; Rezai, M.; Hosseini, S.V.; Regenstein, J.M.; Böhme, K.; Alishahi, A.; Yadollahi, F. Chilled Storage of Golden Gray Mullet (Liza aurata). LWT - Food Science and Technology 2011, 44, 1894–1900.
- Hernández, M.D.; López, M.B.; Álvarez, A.; Ferrandini, E.; García García, B.; Garrido, M.D. Sensory, Physical, Chemical and Microbiological Changes in Aquacultured Meagre (Argyrosomus regius) Fillets during Ice Storage. Food Chemistry 2009, 114, 237–245.
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y.; Han, X.; Pu, B.; Hu, B.; Deng, W.; Liu, S. Antimicrobial Activities of Nisin, Tea Polyphenols, and Chitosan and their Combinations in Chilled Mutton. Journal of Food Science 2016, 81, M1466–M1471.
- Ekanem, E.O.; Achinewhu, S.C. Mortality and Quality Indices of Live West African Hard-Shell Clams (Galatea paradoxa born) during Wet and Dry Postharvest Storage. Journal of Food Processing and Preservation 2006, 30, 247–257.
- Lu, H.; Luo, Y.; Zhou, Z.; Bao, Y.; Feng, L. The Quality Changes of Songpu Mirror Carp (Cyprinus carpio) during Partial Freezing and Chilled Storage. Journal of Food Processing and Preservation 2014, 38, 948–954.
- Liu, Q.; Kong, B.; Han, J.; Chen, Q.; He, X. Effects of Superchilling and Cryoprotectants on the Quality of Common Carp (Cyprinus carpio) surimi: Microbial Growth, Oxidation, and Physiochemical Properties. LWT - Food Science and Technology 2014, 57, 165–171.
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chemical Society Reviews 2010, 39, 4067–4079.
- Cheng, A.; Wan, F.; Xu, T.; Du, F.; Wang, W.; Zhu, Q. Effect of Irradiation and Storage Time on Lipid Oxidation of Chilled Pork. Radiation Physics and Chemistry 2011, 80, 475–480.