ABSTRACT
The objective of this study was to determine the functional and structural properties of Maillard reaction products of mung bean [Vigna radiate (L.)] protein isolate with dextran obtained under the temperature of 80°C or 90°C with different times (0, 1, 2, 3, 4, 5, and 6 h). The mung bean protein isolate–dextran conjugate had better solubility, emulsifying activity, and emulsifying stability than mung bean protein isolate; however, the protein aggregation decreased the solubility, surface hydrophobicity, emulsifying activity, and emulsifying stability of mung bean protein isolate–dextran conjugate. In general, these functional properties of mung bean protein isolate–dextran conjugate obtained at 80°C with lower degree of glycosylation and browning degree were better than mung bean protein isolate–dextran conjugate obtained at 90°C. Both vicilin (8S) and legumin type (11S) globulins both participated in the graft reaction, the subunit with a molecular weight of 21 kDa that contributed to 11S globulin might be the most vulnerable to dextran followed by the 60, 32, and 26 kDa subunits. Maillard reaction between mung bean protein isolate and dextran led to a decreased fluorescence intensity and a bathochromic shift. The fluorescence intensity of mung bean protein isolate–dextran conjugate generally decreased, and λmax of mung bean protein isolate–dextran conjugate first increased and then decreased. The grafted mung bean protein isolates had lower α-helix content but higher β-sheet content. As the Maillard reaction between mung bean protein isolate and dextran proceeded, the α-helix contents of the mung bean protein isolate–dextran conjugates were significantly increased and then decreased, and the β-sheet content generally decreased and then increased. Excessive grafted dextran and more protein aggregation was observed in mung bean protein isolate–dextran conjugate obtained at 90°C, which induced structural changes that might have impaired the functions. Our findings suggest that conjugation of mung bean protein isolate with dextran through the Maillard reaction is an effective way of improving the functional properties of mung bean protein isolate for its application in the food industry.
Introduction
Mungbean [Vigna radiata (L.)] has been traditionally grown in most parts of China and has been accepted as part of the local diet, such as soups and desserts. Mungbean contains balanced nutrients including protein, dietary fiber, and significant amounts of bioactive phytochemicals, and is a popular food in China due to its detoxifying, anti-inflammatory, antitumorigenic, cholesterol-lowering, and diuretic properties.[Citation1] The high levels of proteins, amino acids, oligosaccharides, and polyphenols in mung beans are thought to be the main contributors to the antioxidant, antimicrobial, anti-inflammatory, and antitumor activities of this food and are involved in the regulation of lipid metabolism.[Citation2] Mung bean protein isolate (MBPI) has many desirable functions in processed foods, such as foaming, emulsifying, gelling, and water absorption.[Citation3] The biofunctional properties of mung bean protein hydrolysate and peptides, such as antioxidant capacity of andangiotensin-converting enzyme (ACE) inhibitory activity, have also been previously reported.[Citation4]
The techno-functional characteristics of proteins can be intensified using physical, chemical, or enzymatic treatments to obtain food ingredients for different applications.[Citation5] One modification recognized as being suitable for producing speciality ingredients for food applications is protein–polysaccharide conjugation via a Maillard-type reaction. In order to obtain better functions, we previously conjugated MBPI with glucose to increase its solubility, emulsification activity, and emulsification stability.[Citation6] Conjugation of proteins with polysaccharides leads to better emulsifying properties and heat stability compared with that achieved with mono- or disaccharides.[Citation7] Different proteins such as whey protein isolate, lactoglobulin, and β-conglycinin have therefore been conjugated with dextran to improve their solubility, emulsifying and heat stability properties.[Citation8] In this study, we modified MBPI using a Maillard-type conjugation with dextran for its future application in the food industry.
Two approaches have been used to carry out the Maillard reaction: the dry heating method and the wet heating method. The former generally takes a long time (several weeks) to form the protein–saccharide grafts and does not conform to the requirements of industrial production; the latter takes less time, usually tens of hours (especially between a protein and a polysaccharide), but its disadvantages include the need for the removal of the water and buffer when recovering the products.[Citation9] Therefore, from the industrial point of view, we used the wet-heating method for the synthesis of the MBPI–dextran (MBPI-D) conjugate in our study.
We also then studied the physicochemical properties of the MBPI-D conjugates prepared using the wet-heating method. This study extends our knowledge of the functional changes caused due to Maillard reactions of MBPIs and provides an effective way to improve the functional properties of MBPIs for their better application in the food industry.
Materials and methods
Materials
Mung bean [Vigna radiata (L.)] seeds were purchased from a local market. MBPIs were prepared according to Kudre’s method,[Citation10] with a slight modification. The hulls were removed manually after soaking the mung bean seeds for 12 h in distilled water (1:10, w/v) at 4°C. The dehulled seeds were then broken in a beater. The pH of suspension was adjusted to 9 using 1 M NaOH. The mixture then was stirred continuously for 20 min at room temperature, followed by centrifugation at 10,000g for 30 min at 4°C. Next, the supernatant was collected and the pH was adjusted to 4.0 using 1 M HCl, the supernatant was then again centrifuged at 10,000g for 30 min, followed by collection of the precipitated proteins and dissolving them in distilled water. The pH was then again adjusted to 7.0 using 2 M NaOH followed by dialysis with distilled water at 4°C for 24 h and freeze-drying. The dried powders obtained were referred to as MBPIs. The MBPIs were placed in polyethylene bags, sealed, and stored at 4°C until further analysis. The final MBPI product had a protein content of 90.76 ± 1.12%, as determined using the Kjeldahl method. All the other chemicals were of analytical grade.
Preparation of MBPI-D conjugates
For preparing the MBPI-D conjugates, the MBPIs (10 mg/mL) prepared as described in the previous section were first dispersed with dextran (10 mg/mL) in phosphate buffer solution (0.2 mol/L, pH 7.8). The solution was divided into multiple samples, which were then placed in a water bath at a temperature of 80°C or 90°C for different times (0, 1, 2, 3, 4, 5, and 6 h). Thereafter, the slurry was cooled in ice-water to stop the reaction, followed by dialysis of the slurry at 4°C for 24 h. Finally, the sample was freeze-dried and stored at −20°C. The MBPI-D conjugates obtained by heat treatment at 80°C and 90°C were labeled as MBPI-D-80 and MBPI-D-90, respectively; the MBPI-D conjugates obtained by heat treatment at 80°C and 90°C with 0–6 h were labeled as MBPI-D-80/90-0–6.
Measurement of the degree of glycosylation
The degree of glycosylation (DG) was calculated based on the loss of amino groups. The amount of free amino groups was determined using the o-phthaldialdehyde (OPA) method[Citation11] with some modifications. The OPA reagent was prepared by dissolving OPA (40 mg) in 1 mL of methanol, 2.5 mL of 200 g/L sodium dodecyl sulfate (SDS), and 25 mL of 0.1 mol/L borax, and then adding 100 μL of 2-mercaptoethanol (2-ME). Finally, distilled water was added to make the volume up to 50 mL. The OPA reagent (4 mL) was then placed in a tube and injected with 200 μL of sample solution. After blending, the solution was incubated at 35°C for 2 min and the absorbance at 340 nm was measured. The DG was calculated as follows:
where A0 and A1 are the absorbance values before and after glycosylation with glucose, respectively.
Browning
The browning of Maillard reaction products (MRPs) was measured according to the method described by Diftis and Kiosseoglou[Citation12] with a slight modification. The samples were diluted with 1 mg/mL SDS to a concentration of 2 mg/mL of protein and the blank samples were diluted with deionized water. The extent of browning was determined by measuring the absorbance at 420 nm using a UV-2550 spectrophotometer (Jinghua, Shanghai, China).
Solubility
Protein solubility was determined by dispersing the samples in distilled water to obtain a final solution of 2 mg/mL in protein. The pH values of the protein solution were adjusted in the range of 3–9 using 0.5 M HCl or 0.5 M NaOH and the solutions were then centrifuged at 12,000 g for 30 min (4°C). The protein content of the resulting solution was analyzed using the Lowry’s method.[Citation13]
Emulsifying properties
The samples were dissolved in phosphate buffer solution (10 mmol/L, pH 7.0) to obtain a final protein content of 2 mg/mL. For emulsion, soybean oil and protein solution (1:3, v/v) were homogenized using a homogenizer (AE300L-H, Shanghai Angni Instruments Co., Shanghai, China) at 24,000 rpm for 1 min. After homogenization for 0 and 10 min, 50 μL of the emulsion was immediately taken from the bottom of the beaker, and diluted to 1:100 using 1 mg/mL SDS solution. The absorbance of the diluted emulsion was then recorded at 500 nm. The emulsification activity index (EAI) and emulsification stability index (ESI) were calculated as follows:
where DF is the dilution factor (100), C is the protein concentration (g/mL), φ is the optical path (1 cm), θ is the oil volume fraction (0.25), and A0 and A10 are the absorbance values of the emulsion at 0 and 10 min, respectively.
Surface hydrophobicity
Surface hydrophobicity (H0) values of the proteins in the samples were determined by using 1,8-anilinonaphthalenesulfonate (ANS) as the fluorescence probe. The ANS solution (20 μL, 8.0 mmol/L) was added to 4 mL of 0.05, 0.1, 0.2, 0.5, and 1 mg/mL protein solution, prepared using phosphate buffer solution (10 mmol/L, pH 7.0). Fluorescence intensity (FI) was measured at 390 nm (excitation) and 470 nm (emission) using a Hitachi F-7000 fluorescence spectrophotometer (Hitachi Ltd., Tokyo, Japan) with a slit width of 5 nm for both. The initial slope of the FI versus protein concentration plot was used as the index of H0.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Gel electrophoresis was conducted according to Laemmli’s method[Citation14] using 12% and 5% acrylamide separating and stacking gels, respectively. The samples were prepared in 0.125 mol/L Tris-HCl buffer, containing 10 mg/mL SDS, 2% (v/v) 2-ME, 20% (v/v) glycerol, and 0.25 mg/mL bromophenol blue, and heated for 5 min in boiling water before electrophoresis. After the electrophoresis, the gel sheets were stained for protein with Coomassie brilliant blue R-250. The protein stain was destained using 10% acetic acid (v/v) containing 10% methanol (v/v).
Intrinsic fluorescence emission spectroscopy
The intrinsic emission fluorescence spectra of the protein samples were obtained using a Fluor photometer (Hitachi Ltd.) as described previously.[Citation15] Protein dispersions (0.15 mg/mL) were prepared in 0.01 mol/L phosphate buffer (pH 7.0). The excitation wavelength was 290 nm, and the emission spectra were recorded from 300 to 400 nm at a 10 nm/s scanning speed by using a constant 5 nm slit for both excitation and emission. Each scan was performed five times.
Measurement of circular dichroism spectra
Circular dichroism (CD) spectroscopy in the far-ultraviolet region was used to examine the change in protein secondary structure as described by Jiang et al.[Citation15] The samples were weighed and dissolved in a 0.01 mol/L phosphate buffer (pH 7.0) to a concentration of 0.4 mg protein/mL and then centrifuged at 12,000g for 20 min at 20°C to remove any insoluble residues. The final concentration of the proteins used for CD analysis was 0.2 mg/mL. A Jasco-815 CD spectrometer (Jasco Co., Tokyo, Japan) was used for scanning between 200 and 250 nm, and the experiments were conducted at 20°C. The optical length of the sample cell was 1 mm, the sensitivity was 100 mdeg/cm, the scan speed was 100 nm/min, and the resolution was 0.1 nm. Each scan was performed five times, and the mean was calculated from the experimental data. The software package CDPro (Jasco Co.) was used to model the secondary structure of the proteins, and CONTIN/LL was used as the algorithm. The mean residue weight of the proteins was 115 g/mol. The wavelength range used for calculations was 200–240 nm, and each sample was measured three times.
Statistical analysis
All the tests were performed in triplicate, and the results are given as means ± standard deviations. Duncan’s multiple range test was used to evaluate significant differences (P < 0.05) between results.
Results and discussion
Browning and degree of glycosylation
The MBPI-D glycosylation reaction was mainly based on the reaction between the free amino group of the protein amino acid side-chain and the carbonyl group at the end of polysaccharide molecules. Hence, changes in the free amino group on the MBPI molecular chain can reflect the level of glycosylation grafting reaction, which can be quantified by the DG.[Citation16]
The browning and the DG of MBPI-D conjugates obtained under different treatment conditions are shown in and . The DG values of the MBPI-D conjugates were higher than those of MBPI. The DG of the MBPI-D-80 conjugates increased with time. To initiate grafting reaction between MBPI and dextran, the reducing aldehyde group needs to come in contact with the ε-amino group on the protein chain. Not all the ε-amino groups on MBPI chains are exposed at the beginning of reaction; however, this exposure can be promoted by incubation.[Citation17,Citation18] Therefore, more dextran could have been grafted on the MBPI chain, which increased the DG. The DG of the MBPI-D-90 conjugates generally increased with time and was higher than that of MBPI-D-80, which suggested that more dextran had been grafted with MBPI at 90°C.
Figure 1. Effects of reaction time on DG and brown degree of MBPI–Dextran conjugates obtained at 80°C (A) and 90°C (B).
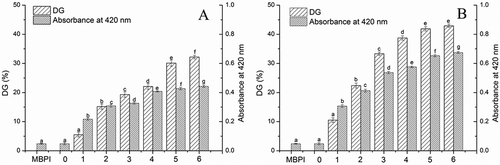
The browning of MBPI-D was generally higher than that of native MBPIs, which could have been because the reaction between the free amino groups of proteins, and the reducing end carbonyl group of the sugars could increase the color intensity.[Citation6] The increasing absorbance values resulting from browning can be used as an indicator of the browning degree caused by the Maillard reaction at more advanced stages.[Citation19] The higher browning degree of MBPI-D-90 confirmed more advanced glycosylation end products or even melanoidins.
Solubility
The Maillard reaction can be divided into three stages: initial, intermediate, and advanced stages. The initial stage involves the condensation of the carbonyl group of the reducing sugar with the available ε-amino groups (lysine being the primary reactive amino group) of the protein, which results in the formation of an Amadori product via the formation of a Schiff base with the release of water and the Amadori rearrangement.[Citation20] The intermediate stage involves the degradation of Amadori products, which results in the formation of a wide range of compounds. The final stage of the production of extensively colored, water-insoluble, nitrogen-containing polymeric compounds is referred to as “melanoidins.”[Citation20]
As shown in and , the MBPI-D exhibited substantially improved solubility properties compared with MBPI. Enhanced solubility was also been observed in other proteins, such as whey protein isolate,[Citation21] peanut protein isolate,[Citation22] lysozyme and casein,[Citation23] β-lactoglobulin, and bovine serum albumin[Citation24] after conjugation with dextran.
Figure 2. Effects of reaction time on solubility of MBPI–Dextran conjugates obtained at 80°C (A) and 90°C (B).
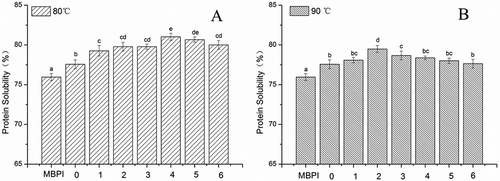
The solubility of MBPI-D-80 and MBPI-D-90 generally first increased and then slightly decreased with graft time. The solubility of MBPI-D-80 was higher than that of MBPI-D-90. This can be explained by the fact that the increased number of hydrophilic hydroxyl brought by the grafted dextran formed a hydration layer on the surface of MBPI, which increased the hydrophilicity of MBPI.[Citation25] However, protein oxidation and protein aggregation increased with the degradation of Amadori products and formation of “melanoidins,” which decreased the solubility of MBPI-D. Insoluble protein aggregation could also be an important factor in the decrease of the solubility of MBPI-D. Oliver et al.[Citation26] also suggested that the occurrence of disulfide bridges, as well as cross-linking among peptides, reduces protein solubility.
Emulsifying properties
Pea proteins are known to be able to decrease the oil–water interfacial tension and contribute to emulsion stabilization by the formation of a rigid membrane.[Citation27] As depicted in and , adding dextran slightly increased the EAI and ESI of MBPI, which is consistent with a previous report indicating that the properties of protein emulsions can be enhanced by using high-molecular-weight polysaccharides that keep emulsion droplets apart after their formation and thus protect them against aggregation and coalescence.[Citation8]
Figure 3. Effects of reaction time on emulsifying properties of MBPI–Dextran conjugates obtained at 80°C (A) and 90°C (B).
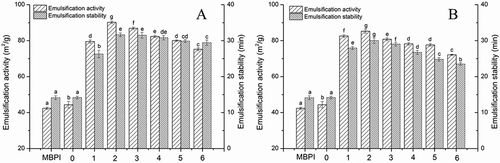
Furthermore, the EAIs of MBPI-D-80 and MBPI-D-90 were higher than that of MBPI. It is now well-recognized that the formation of protein–polysaccharide conjugates combines the characteristic property of proteins to facilitate their strong adsorption to the oil–water interface with the characteristic property of the polysaccharides for solvation in the aqueous phase medium.[Citation28] Therefore, the most striking characteristic of the protein–polysaccharide conjugates is the excellent emulsifying properties.[Citation29,Citation30]
also shows that the EAIs and ESIs of MBPI-D-80 and MBPI-D-90 both first increased and then decreased, while the EAI and ESI of MBPI-D-80 were both higher than those of MBPI-D-90. This result suggested that the hydrophobic protein moiety firmly attached to the oil droplet surface and the bulky hydrophilic part of the covalently linked polysaccharide highly oriented to the water phase, which has been supposed to provide a steric stabilizing layer to inhibit the coalescence of the oil droplets.[Citation21] Improvements in emulsifying properties by heating can be mainly attributed to increased hydrophobic surface and flexibility as a consequence of thermal denaturation.[Citation31] However, the heat aggregation of proteins would impair their emulsifying properties.[Citation32] Besides, Dickinson and Semenova[Citation33] showed that when more dextran attaches to proteins, it reduced the surface activity, presumably due to a lower rate of protein adsorption at the oil–water interface. In our study, we also found that the ESI of MBPI-D-90 decreased from 2 to 6 h more significantly than that of MBPI-D-80 did, which might be because polymeric aggregation at the later stage of the Maillard reaction did not benefit the emulsifying capacity of the hydrophobic protein.[Citation34]
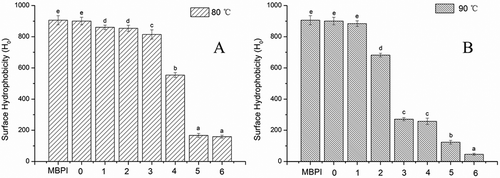
Surface hydrophobicity
urface hydrophobicity is a structure-related function, which is dependent on the size and shape of the protein molecule, amino acid composition, and sequence, as well as any intramolecular or intermolecular cross-links. As shown in and , no significant change was observed between MBPI and MBPI-D; however, a slight decrease was found in the MBPI-D conjugate compared with the MBPI. Glycation has been suggested to reduce the exposure of hydrophobic groups buried intramolecularly; additionally, the bonding of hydrophilic straight-chain dextran increases the hydrophilicity on the molecule surface.[Citation35] In our study, we also found a sharp decrease in the surface hydrophobicity of MBPI-D-80 with 4 h of graft treatment, which might be related to protein aggregation. We previously showed that protein denaturation increases the surface hydrophobicity, and that surface hydrophobicity decreases as aggregates are formed.[Citation36] In general, the surface hydrophobicity of MBPI-D-90 was lower than that of MBPI-D-80, which suggested that more dextran grafting and aggregation formation decreased protein hydrophobicity to a large extent.
SDS–PAGE profile
SDS–PAGE has been used as a reliable method for identifying covalent coupling between proteins and polysaccharides.[Citation29] Mendoza et al.[Citation37] reported that mung bean consists of 89% 8S vicilin, 3.4% 7S globulin, and 7.6% 11S globulin, and detected that the native molecular weights of the different globulin types were 360,000 for 11S legumin, 200,000 for 8S vicilin, and 135,000 for basic 7S. Vicilin consists of four types of peptides with molecular weights of 60, 48, 32, and 26 kDa. 7S globulin has a molecular weight of 28 and 16 kDa, while 11S globulin has a molecular weight of 40 and 24 kDa. Ericson and Chrispeels[Citation38] reported the subunit molecular weights obtained for 8S and 11S mungbean globulins were 56, 44, and 165 kDa for 11S and 63, 50, 34, and 24 kDa for 8S.
The SDS–PAGE profiles of MBPI and MBPI-D obtained in our study are shown in and . Six bands of 60, 56, 43–48, 32, 26, and 21 kDa were obtained for MBPI. The bands of 60, 32, and 26 kDa were attributed to vicilin, those of 56 and 21 kDa were attributed to 11S globulin. The wide bands of 43–48 kDa might be related with both vicilin and 11S globulin. No distinct bands of 7S were obtained; however, the 26 kDa band might correspond to basic 7S. Vicilin (8S) was found to be the main component in MBPI, and legumin type (11S) globulins were the other main proteins. A general decrease in the intensities of subunits with molecular weights of 60, 43–48, 32, and 26 kDa attributed to vicilin was observed in MBPI-D; the intensities of all these subunits decreased with conjugation time, which suggested that these subunits participated in the graft reaction between MBPI and dextran. The bands in MBPI-D-80 corresponding to 32 and 26 kDa even disappeared after 5 h of treatment, and the 60 kDa band faded away after 4 h of conjugation; the 21 kDa bands could hardly be found after 2 h of treatment. This result suggested that the 21 kDa bands in MBPI-D-80 corresponding to 11S globulin were the most vulnerable to dextran followed by the 60, 32, and 26 kDa. The intensities of these bands in MBPI-D-90 decreased more significantly than those in MBPI-D-80.
Figure 5. SDS-PAGE patterns of MBPI and MBPI–Dextran conjugates obtained at 80°C (A) and 90°C (B). (Lane M: Protein standards; Lane 1: native mung bean protein isolates; Lane 2: mixture of mung bean protein isolates and dextran; Lane 3–8: MBPI-D conjugates incubated under 1–6 h).
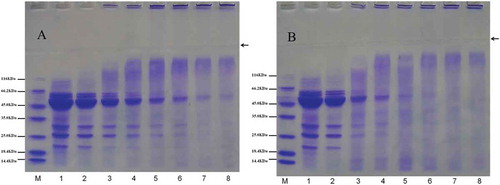
Some new bands were obtained in MBPI-D-80 and MBPI-D-90 with high molecular weight, which may have been due to conjugate formation or protein aggregation. Previously, Diftis and Kiosseoglou[Citation12] showed that SBPI–NaCMC results in the appearance of two broad bands near the tops of the stacking and separation gels, which indicates a wide distribution of molecular weights of the products of the reaction, which, apart from protein–polysaccharide hybrids, might consist of polymerized protein molecules due to isopeptide or disulfide bond formation.
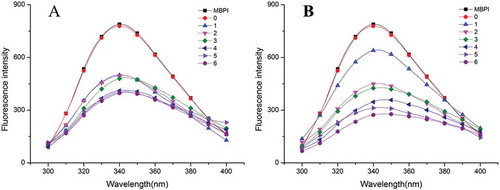
Fluorescence spectrum
Average fluorescence spectra ( and ) of the MBPI-D samples were obtained to evaluate conformational changes around the Trp residues. Because Trp residues of a protein are excited at 280 nm, the changes in the tertiary structure of the protein can be determined from the fluctuations of the fluorescence.[Citation39,Citation40] In plant protein, two aromatic amino acid residues, tryptophan (Trp) and tyrosine (Tyr), make the major contribution to the ultraviolet fluorescence of the protein. As the energy transfer from the tyrosine residues to the tryptophan residues leads to quenching of the fluorescence of the tyrosine residues and enhancement of the fluorescence of the tryptophan residues, it is generally accepted that the protein fluorescence results mainly from the fluorescence of Trp residues, whose peak position is between 325 and 350 nm.[Citation15,Citation41]
We found that the fluorescence intensity of MBPI-D was lower than that of MBPI and it decreased with graft time. Consistent with this, previously, Hattori et al.[Citation42] indicated that the polysaccharide chains shield the area around the Trp residues and thus decrease the fluorescence intensity of Trp. Li et al.[Citation43] showed that high degree of graft during glycation leads to a higher decrease in fluorescence intensity. These reports also explain why fluorescence intensity of MBPI-D-90 with same graft time was markedly decreased.
Generally, when using Trp fluorescence λmax information, a Trp is assigned as being buried and in a “nonpolar” environment if λmax is <330 nm; if λmax is >330 nm, the Trp is assigned to be in a “polar” environment.[Citation44] In this study, the λmax of MBPI and MBPI-D samples was >330 nm in all cases, which suggested that the tryptophans of MBPI and MBPI-D were in a “polar” environment. The Maillard reaction between MBPI and dextran led to a general shift of λmax to longer wavelengths (bathochromic shift). The λmax of MBPI-D-80 increased from 340.4 up to 344.2 nm with an increase in treatment time to 4 h, which suggested that the conjugated dextran partially unfolded the structure of MBPI to be less compact, which was favorable for emulsifying property; MBPI denaturation is also a possible reason for this phenomenon. A decrease in λmax of MBPI-D-80 was observed for 5 and 6 h of treatment time, which might be related to protein aggregation; this result was also consistent with the results of CD analysis. A similar change was observed in the λmax of MBPI-D-90; the highest λmax was observed for MBPI-D-90 with 3 h of graft treatment. In comparison, the λmax of MBPI-D-90 was higher than that of MBPI-D-80 for same graft time, which suggested that more grafted dextran or higher degree of denaturation led to a less compact structure.
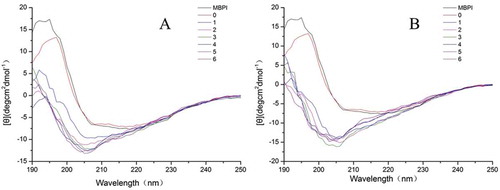
CD analysis
The far-ultraviolet CD spectra were used to determine changes in the secondary structures of the native MBPI and MBPI-D conjugates prepared using the Maillard reaction ( and ). The negative shoulder peak at 215 nm indicated the existence of a β-sheet structure in MBPI, whereas the negative groove at 205 nm might have arisen from the negative Cotton effect caused by the α-helix structure. The secondary structure was estimated using the CONTIN/LL algorithm, which was provided by Jasco Corp. and originated from the method described by Sreerama et al.[Citation45] The estimated secondary structure content is shown in and .
Table 1. Secondary structural content of native MBPI and MBPI–Dextran conjugates treated at 80°C estimated from circular dichroism spectra.
Table 2. Secondary structural content of native and MBPI–Dextran conjugates treated at 90°C estimated from circular dichroism spectra.
We found that MBPI contained 19.7% α-helix, 26.7% β-sheet, 21.3% β-turns, and 32.3% unordered structure. The addition of dextran did not significantly change the secondary structure of MBPI. In general, the α-helix content of MBPI-D-80 was lower than that of MBPI, while the β-sheet content of MBPI-D-80 was higher than that of MBPI. It has been reported that the decrease in α-helix content is attributed to polysaccharides being bound to the ε-amino group in the α-helix region.[Citation46] A loss of α-helix structure has also been observed in whey protein isolate conjugated with dextran, where the loss of secondary structure was considered a result of heating unfolding or from the attachment of dextran.[Citation47] In conclusion, heating unfolding and grafted dextran might have decreased the α-helix content of MBPI-D-80 and transformed to β-sheet.
With the proceeding of the Maillard reaction between MBPI and dextran, the content of α-helices and unordered structures of the MBPI-D-80 conjugates were significantly increased and then decreased, and the content of β-sheets and β-turns generally decreased and then increased. Previously, Chen et al.[Citation48] explained that the introduction of a Dex chain to the protein chain may be attributed to hydrogen bonds, Van der Waals interactions, and molecular entanglement between Dex and the charged phosphatidylserine groups in the core of phosvitin. However, in our study, the decreased α-helix content and unordered structure and increased β-sheet content of MBPI-D-80 under 4–6 h of heat treatment could be attributed to glycoprotein aggregation. Previously, Lee et al.[Citation49] reported that the involvement of β-sheets in the secondary structure of protein aggregates might be attributed to the relatively large surface areas for ordered hydrogen bonding. Consistent with the results of our study, our previous research also showed that protein aggregate have higher β-sheet and β-turn content.[Citation36]
The α-helix content of MBPI-D-90 gradually increased and then decreased with treatment time, while the β-sheet content first decreased and then increased. The change in these structures of MBPI-D-90 was similar to that observed in MBPI-D-80. Further, the β-turn content first increased and then decreased, but the content of unordered structure generally increased with time. The increase in the content of unordered structures might be related with protein denaturation.[Citation47,Citation50] Our results suggested that 4–6 h of treatment might have led to a greater degree of unfolding than protein aggregation, which is consistent with the results of our fluorescence analysis.
Conclusion
Maillard reaction between MBPI and dextran led to structural modifications and partial unfolding and endowed MBPI-D conjugate better solubility, EAI, and ESI than MBPI, however, the formation of protein aggregation decreased the solubility, surface hydrophobicity, EAI, and ESI of MBPI-D. In general, these functional properties of MBPI-D obtained at 80°C with lower DG and browning degree were better than those of MBPI-D obtained at 90°C, which could have been because the excessive grafted dextran and more protein aggregation formed in MBPI-D-90, which induced structural changes and impaired these functions. All the results suggested that wet-heating Maillard reaction could potentially be applied as a means to improve the functional properties of MBPI. Further investigations need to be conducted to achieve more improvements in the protein physicochemical properties of proteins and to elucidate the mechanism by which the Maillard reaction facilitates such improvements.
Funding
The authors acknowledge the financial support from the NNSF of China (number 31301501, number 31430067, and number 31571876), Natural Science Foundation of Heilongjiang Province (number ZD201302), and Young Innovative Talent Training Plan of Heilongjiang Province (number UNPYSCT-2015011), Fok Ying Tung Education Foundation (number 151032), Postdoctoral Start-funded Research of Heilongjiang Province (number LBH-Q13018), as well as the Key Laboratory of Soybean Biology of Chinese Education Ministry, “Young Talents Project” of Northeast Agricultural University (17QC16), and National Natural Science Foundation of China (31701568).
Additional information
Funding
References
- Li, G.H.; Shi, Y.H.; Liu, H. Le, G.W. Antihypertensive Effect of Alcalase Generated Mung Bean Protein Hydrolysates in Spontaneously Hypertensive Rats. European Food Research and Technology 2006, 222, 733–736.
- Kanatt, S.R.; Arjun, K.; Sharma, A. Antioxidant and Antimicrobial Activity of Legume Hulls. Food Research International 2011, 44, 3182–3187.
- El-Adawy, T.A. Functional Properties and Nutritional Quality of Acetylated and Succinylated Mung Bean Protein Isolate. Food Chemistry 2000, 70, 83–91.
- Wongekalak, L.O.; Sakulsom, P.; Jirasripongpun, K.; Hongsprabhas, P. Potential Use of Antioxidative Mungbean Protein Hydrolysate as an Anticancer Asiatic Acid Carrier. Food Research International 2011, 44, 812–817.
- Kato, A. Industrial Applications of Maillard-Type Protein-polysaccharide Conjugates. Food Science and Technology Research. 2002, 8, 193–199.
- Wang, Z.J.; Han, F.F.; Sui, X.N.; Qi, B.K.; Yang, Y.; Zhang, H.; Wang, R.; Li, Y.; Jiang, L.Z. Effect of Ultrasound Treatment on the Wet Heating Maillard Reaction between Mung Bean [Vigna radiate (L.)] Protein Isolates and Glucose and on Structural and Physico-chemical Properties of Conjugates. Journal of the Science of Food and Agriculture 2016, 96, 1532–1540.
- Dunlap, C.A.; Côté, G.L. β-Lactoglobulin-dextran Conjugates: Effect of Polysaccharide Size on Emulsion Stability. Journal of Agricultural and Food Chemistry 2005, 53, 419–423.
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and Emulsifying Properties of Whey Protein Isolate (WPI)− Dextran Conjugates Produced in Aqueous Solution. Journal of Agricultural and Food Chemistry 2010, 58, 2988–2994.
- Guan, J.J.; Qiu, A.Y.; Liu, X.Y.; Hua, Y.F.; Ma, Y.H. Microwave Improvement of Soy Protein Isolate–Saccharide Graft Reactions. Food chemistry 2006, 97, 577–585.
- Kudre, T.G.; Benjakul, S.; Kishimura, H. Comparative Study on Chemical Compositions and Properties of Protein Isolates from Mung Bean, Black Bean and Bambara Groundnut. Journal of the Science of Food and Agriculture 2013, 93, 2429–2436.
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using o-phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. Journal of Dairy Science 1983, 66, 1219–1227.
- Diftis, N.; Kiosseoglou, V. Improvement of Emulsifying Properties of Soybean Protein Isolate by Conjugation with Carboxymethyl Cellulose. Food Chemistry 2003, 81, 1–6.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. Journal of biological Chemistry 1951, 193, 265–275.
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Jiang, L.Z.; Wang, Z.J.; Li, Y.; Meng, X.H.; Sui, X.N.; Qi, B.K.; Zhou, L.Y. Relationship Between Surface Hydrophobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. International Journal of Food Properties 2015, 18, 1059–1074.
- Echavarría, A.; Pagán, J.; Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Engineering Reviews 2012, 4, 203–223.
- Geng, X.; Cui, B.; Li, Y.; Jin, W.; An, Y.; Zhou, B.; Ye, T.; He, L.; Liang, H.; Wang, L. Preparation and Characterization of Ovalbumin and Carboxymethyl Cellulose Conjugates Via Glycosylation. Food Hydrocolloids 2014, 37, 86–92.
- Zhuo, X.Y.; Qi, J.R.; Yin, S.W.; Yang, X.Q.; Zhu, J.H.; Huang, L.X. Formation of Soy Protein Isolate–Dextran Conjugates by Moderate Maillard Reaction in Macromolecular Crowding Conditions. Journal of the Science of Food and Agriculture 2013, 93, 316–323.
- Sun, W.W.; Yu, S.J.; Yang, X.Q.; Wang, J.M.; Zhang, J.B.; Zhang, Y.; Zheng, E.L. Study on the Rheological Properties of Heat-induced Whey Protein Isolate–Dextran Conjugate gel. Food Research International 2011, 44, 3259–3263.
- Friedman, M. Food Browning and Its Prevention: an Overview. Journal of Agricultural and Food Chemistry 1996, 44, 631–653.
- Sun, W.W.; Yu, S.J.; Zeng, X.A.; Yang, X.Q.; Jia, X. Properties of Whey Protein Isolate–Dextran Conjugate Prepared Using Pulsed Electric Field. Food Research International 2011, 44, 1052–1058.
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of Functional Properties of Peanut Protein Isolate by Conjugation with Dextran Through Maillard Reaction. Food Chemistry 2012, 131, 901–906.
- Aminlari, M.; Ramezani, R.; Jadidi, F. Effect of Maillard-based Conjugation with Dextran on the Functional Properties of Lysozyme and Casein. Journal of the Science of Food and Agriculture 2005, 85, 2617–2624.
- Jimenez-Castano, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of Individual Whey Proteins by Maillard Reaction Using Dextran of Different Molecular Mass. Food Hydrocolloids 2007, 21, 433–443.
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F. Proteins, Polysaccharides, and Their Complexes Used as Stabilizers for Emulsions: Alternatives to Synthetic Surfactants in the Pharmaceutical Field? International Journal of Pharmaceutics 2012, 436, 359–378.
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating Proteins with Novel Functionality Via the Maillard Reaction: A Review. Critical Reviews in Food Science and Nutrition 2006, 46, 337–350.
- Ducel, V.; Richard, J.; Popineau, Y.; Boury, F. Adsorption Kinetics and Rheological Interfacial Properties of Plant Proteins at the Oil-Water Interface. Biomacromolecules 2004, 5, 2088–2093.
- Dickinson, E.; Galazka, V. Emulsion Stabilization by Protein–Polysaccharide Complexes. Gums and Stabilisers for the Food Industry 1992, 6, 351–362.
- Akhtar, M.; Dickinson, E. Whey Protein–Maltodextrin Conjugates as Emulsifying Agents: An Alternative to Gum Arabic. Food Hydrocolloids 2007, 21, 607–616.
- Diftis, N.; Kiosseoglou, V. Physicochemical Properties of Dry-Heated Soy Protein Isolate–Dextran Mixtures. Food Chemistry 2006, 96, 228–233.
- Raymundo, A.; Franco, J.; Gallegos, C.; Empis, J.; Sousa, L. Effect of Thermal Denaturation of Lupin Protein on Its Emulsifying Properties. Nahrung-Food 1998, 42, 220–224.
- Traynor, M.; Burke, R.; Frias, J.M.; Gaston, E.; Barry-Ryan, C. Formation and Stability of an Oil in Water Emulsion Containing Lecithin, Xanthan Gum and Sunflower Oil. International Food Research Journal 2013, 20, 2173–2181.
- Dickinson, E.; Semenova, M.G. Emulsifying Properties of Covalent Protein—Dextran Hybrids. Colloids and Surfaces 1992, 64, 299–310.
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional Properties of Maillard Reaction Products of Rice Protein Hydrolysates With Mono-, Oligo-and Polysaccharides. Food Hydrocolloids 2013, 30, 53–60.
- Zhang, X.; Qi, J.R.; Li, K.K.; Yin, S.W.; Wang, J.M.; Zhu, J.H.; Yang, X.Q. Characterization of Soy β-conglycinin–dextran Conjugate Prepared by Maillard Reaction in Crowded Liquid System. Food Research International 2012, 49, 648–654.
- Wang, Z.J.; Li, Y.; Jiang, L.J.; Qi, B.K.; Zhou, L.Y. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. Journal of Chemistry 2014, 2014
- Mendoza, E.M.T.; Adachi, M.; Bernardo, A.E.N.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] Globulins: Purification and Characterization. Journal of Agricultural and Food Chemistry 2001, 49, 1552–1558.
- Ericson, M.C.; Chrispeels, M.J. Isolation and Characterization of Glucosamine-Containing Storage Glycoproteins from the Cotyledons of Phaseolus Aureus. Plant physiology 1973, 52, 98–104.
- Liu, W.; Zhang, Z.Q.; Liu, C.M.; Xie, M.Y.; Tu, Z.C.; Liu, J.H.; Liang, R.H. The Effect of Dynamic High-Pressure Microfluidization on the Activity, Stability and Conformation of Trypsin. Food Chemistry 2010, 123, 616–621.
- Shen, L.; Tang, C.-H. Microfluidization as a Potential Technique to Modify Surface Properties of Soy Protein Isolate. Food Research International 2012, 48, 108–118.
- Jiang, L.J.; Wang, J.; Li, Y.; Wang, Z.J.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.J.; Qi, B.K.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Research International 2014, 62, 595–601.
- Hattori, M.; Ogino, A.; Nakai, H.; Takahashi, K. Functional improvement of β-lactoglobulin by Conjugating with Alginate Lyase-lysate. Journal of Agricultural and Food Chemistry 1997, 45, 703–708.
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative Studies on the Physicochemical Properties of Peanut Protein Isolate–Polysaccharide Conjugates Prepared by Ultrasonic Treatment or Classical Heating. Food Research International 2014, 57, 1–7.
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophysical Journal 2001, 80, 2093–2109.
- Sreerama, N.; Venyaminov, S.Y.; Woody, R.W. Estimation of the Number of α-helical and β-strand Segments in Proteins Using Circular Dichroism Spectroscopy. Protein Science 1999, 8, 370–380.
- Hattori, M.; Nagasawa, K.; Ametani, A.; Kaminogawa, S.; Takahashi, K. Functional Changes in. Beta.-lactoglobulin by Conjugation with Carboxymethyl Dextran. Journal of Agricultural and Food Chemistry 1994, 42, 2120–2125.
- Wooster, T.J.; Augustin, M.A. Rheology of Whey Protein–dextran Conjugate Films at the Air/Water Interface. Food Hydrocolloids 2007, 21, 1072–1080.
- Chen, H.; Jin, Y.; Ding, X.; Wu, F.; Bashari, M.; Chen, F.; Cui, Z.; Xu, X. Improved the Emulsion Stability of Phosvitin from Hen Egg Yolk Against Different pH by the Covalent Attachment with Dextran. Food Hydrocolloids 2014, 39, 104–112.
- Lee, H.J.; Choi, C.; Lee, S.J. Membrane-Bound α-Synuclein Has a High Aggregation Propensity and the Ability to Seed the Aggregation of the Cytosolic form. Journal of Biological Chemistry 2002, 277, 671–678.
- Sun, Y.; Hayakawa, S.; Izumori, K. Modification of Ovalbumin with a Rare Ketohexose Through the Maillard Reaction: Effect on Protein Structure and Gel Properties. Journal of Agricultural and Food Chemistry 2004, 52, 1293–1299.