ABSTRACT
In this study, stability of Lactobacillus acidophilus and L. paracasei in synbiotic sugared and sugar-free milk chocolates within 90-day storage was investigated, considering inline as prebiotic. Probiotic bacteria inoculation at 9.0 log cfu/25 g and usage of inulin with Degree of Polymerisation (DP)<10 and DP > 23 at a concentration of 9.0 g/100 g were investigated. At the end of 90 days, at least 6.5 log cfu/25 g probiotic bacteria was determined in the samples containing L. acidophilus, whereas this value was determined as 5.9 log cfu/25 g for L. paracasei. Therefore, it was concluded that L. acidophilus was more stable in terms of viability level in the milk chocolates prepared by sugar, maltitol, and inulin DP was effective on viability levels of probiotics (p < 0.05). Moreover, inulin DP was also found effective on water activity, melting, rheological and textural properties and colour of milk chocolate (p < 0.05). Quality parameters were affected by inulin DP, except rheological properties.
Introduction
The chocolate is a foodstuff which has been consumed by wide social layers and age groups in the world. The biggest advantage of chocolate is its sensorial properties and consumer perception. However, attitudes and behaviours of consumers have changed significantly especially for the last 25 years. Existence of saturated fat and sugar in the ingredients are negative aspects for chocolate products. Alternatively, bioactive compounds (e.g., plant extracts, fibres, vitamins, minerals and phytochemicals) and/or sugar substitutes can be added to chocolate products to eliminate these disadvantages. Especially, the presence of beneficial components and fortifications has at least positive impact on purchasing decisions.[Citation1]
Chocolate consumers have functional product expectations at a significant level.[Citation2] Also, in developed economies, a key current trend is confectionery products that deliver functional benefits for health and well-being, such as sugarless sweets and functional chocolate.[Citation3] Among the main bioactive compounds, probiotics may be used with this aim. Probiotics modify the composition of the gut microflora, and as a consequence, they have been shown to influence both intestinal and body functions.[Citation4] Various foodstuffs, especially the dairy products, may be used as probiotic sources. Results of previous studies have shown that introduction of encapsulated probiotic strains into chocolate can be an excellent media to protect them from environmental stress. In chocolate, cocoa butter was shown to be protector for bifidobacteria.[Citation5,Citation6] Also, the chocolates with probiotics have the potential to offer double benefits: (i) a longer shelf life than traditional probiotic products, (ii) better protection of probiotics during passage through the stomach and more effective colonisation of the intestinal tract due to high fat content.[Citation7–Citation9]
Previous studies concerning development of probiotic dark,[Citation5,Citation7,Citation9–Citation12] milk[Citation7,Citation9,Citation11,Citation13] and white[Citation7] chocolates were conducted by using different probiotic bacteria. However, in these studies, prebiotic and sugar-free ingredient usage was not investigated. When the trends in food technology area are taken into consideration, it is a significant requirement to develop and study quality parameters of functional sugared and sugar-free synbiotic chocolates. One of the main bulk sweeteners used in sugar-free chocolate development studies was maltitol.[Citation3,Citation14–Citation18]
Roberfroid[Citation4] suggested that synbiotic products could improve the survival of bacteria when they pass into the upper part of the gastrointestinal tract and produce greater effects in the large bowel. However, the studies devoted to developing synbiotic chocolate are very limited,[Citation5,Citation19] and any study in which inulin was used as a prebiotic material in chocolate product[Citation3,Citation15–Citation17,Citation20–Citation23] did not exist.
Milk chocolates are the most preferred chocolates among children as well as most of adults.[Citation3] This study aimed to investigate viability levels of different probiotic bacteria (Lactobacillus paracasei and L. acidophilus) and main quality parameters (melting, textural, rheology and colour properties) of developed sugared and sugar-free milk chocolates prepared by inulin having low (DP < 10) and high (DP > 23) degree of polymerisation.
Materials and methods
Materials
For the preparation of synbiotic chocolate samples, cocoa butter, cocoa mass (Altinmarka, Istanbul Turkey), fine sugar (SMS Kopuz, Istanbul, Turkey), whole and skim milk powder (Besel, Konya, Turkey), soy lecithin (Brenntag Chemistry, Istanbul, Turkey), polyglycerol polyricinolate (PGPR) (Palsgaard, Zierikzee, Netherlands), vanillin (Ekin Chemistry, Istanbul, Turkey), DP<10 and DP > 23 inulin (Beneo Orafti, Oreye, Belgium), lyophilised L. paracasei (LOT No 4112648741) and L. acidophilus (LOT No 41127003932) (Danisco, Niebüll, Germany), and maltitol (Roquette Frenes, Lestres, France) were used.
Sample preparation and probiotic addition
Each sample group was prepared in lots of 10 kg batch using the formulations presented in . For this purpose, the melted fat components (comprising 20% of the total cocoa butter) and the dry powders (fine sugar or maltitol, cocoa mass and inulin) were mixed until homogeneous mixture was formed while being heated to 40°C. At the end of the mixing and warming, the chocolate mass was first pre-refined on a pilot-scale 3-roll refiner (Lehmann, Aaelen, Germany) and then mixed again and warmed to 50°C. To achieve a mean particle size of 20 µm, the gap size/pressure between the rollers of the 3-roll refiner was adjusted, and d90 values were measured using a micrometer (Mitutoyo, Manufacturing Co. Ltd., Japan, 0.001 mm accuracy) (). After measuring the particle size, dry conching was performed for 45 min, and the remaining cocoa butter (80% of the total), soy lecithin and PGPR were added (resulting a fat content of 18.5%). The total conching time was 270 min at 55°C.
Table 1. The formulations used for the production of sugar-free and sugared synbiotic milk chocolate samples (g/100g).
Table 2. D90 values of synbiotic milk chocolate samples (μm).
After conching, L. paracasei or L. acidophilus (9.0 log cfu/25 g) were added to chocolate mass at 35°C. Then the mass was mixed for nearly 5 min. Afterwards, a three-stage tempering process (33–35, 24–25 and 25–26°C) was implemented. Temper index was in the range of 5.50–6.00 which was measured by a temper meter (Chocometer, Aasted Farum, Denmark). Subsequently, the moulding and vibration process were conducted at 27–30°C. After 20 min of cooling at 5°C, the process was completed. Samples were stored at temperatures between 13°C and 15°C and kept away from light and heat prior to analysis.
Viability of probiotic bacteria
Approximately 20 g of chocolate was taken under aseptic conditions and mixed with 180 mL peptone water solution (0.1%). To melt the chocolate, the mixture was hold at 40°C. After preparation of appropriate dilutions, 200 μL of each dilution was plated on specific media for viable cell counts. LAB was counted on De Man, Rogosa, and Sharpe Agar (Oxoid CM 361) after the plates were incubated at 37.5°C for 48 h.[Citation24] The analyses were carried out on the 0th, 30th, 60th and 90th days of the storage. All of the analyses replicated three times.
Instrumental texture analysis
Hardness values were measured using texture analyser (TA-TX plus Stable Micro Systems, UK) equipped with 5 kg load cell. Three-point bend ring was used to determine force required to break chocolate samples. Pre-test, test and post-test speeds were set to be 1 mm/sec, 1 mm/sec and 10 mm/sec, respectively. Results for the hardness (N) were expressed as the mean value of five replicates conducted on different samples of the same lot of each chocolate.
Melting properties
Melting properties of chocolate samples were determined according to the method described by Glicerina et al.[Citation25] and using a differential scanning calorimeter (DSC) (TA Q20, USA). Samples (approximately 5 mg) were loaded into 40 ml capacity pans and sealed with hermetic lid using a sample press. Pans were heated from 0 to 60°C at 10°C/min in N2 stream. Onset temperature (Tonset), peak temperature (Tpeak), end temperature (Tend) and energy required for complete melting of the samples (ΔH) were calculated using thermograms obtained from the measurements.[Citation26]
Rheological analyses
Rheological properties of chocolate samples were determined at 40°C using stress or strain controlled rheometer (Anton Paar, Austria) equipped with a Peltier system and water bath for temperature control and CC27 probe. Steady shear rheological properties of chocolate samples melted at 40°C prior to the analysis were determined according to the method of International Confectionery Association (ICA). This method is consisted of the following four steps:
1st Step: Shear rate of 5 s−1 was performed for 500 s to homogenise the sample and balance the temperature.
2nd Step: Shear rate, which started from 2 s−1 to 50 s−1, was applied, and this step proceeded for 180 s.
3rd Step: The samples were sheared at 50 s−1 for 60 s.
4th Step: The descending shear rates that ranged from 50 s−1 to 2 s−1 were applied for 180 s.
By using the obtained data, τ0 (yield stress) and ηpl (plastic viscosity values) were determined.
Moisture analyses and water activity
Moisture contents of chocolate samples were performed using the method previously reported by Lonchampt and Hartel.[Citation27] Water activity of the chocolates was measured using a Lab-Master aw (Novasina, Switzerland) according to the method used by Konar.[Citation15] aw values of each sample were measured in triplicate 24 h after sample preparation.
Colour measurement
Colour parameters (L: brightness, a: ±red-green and b: ±yellow-blue) of the compounds produced were measured by colourimeter (Chroma Meter CR-400, Konica Minolta, Japan), and chroma (C*), hue (h°) and whiteness index (WI) values were calculated depending of those measured parameters by the following equations[Citation28]:
CIE Lab system was used in order to examine these colour parameters.
Statistical analyses
The quantitative data were expressed as the mean. The results were analysed using a factorial design with analysis of variance (ANOVA). Tukey’s test was applied to determine if the differences were significant. The statistical analyses were performed using the MINITAB-Express (Minitab Inc., State College, PA, USA) and MSTAT statistical packages (Michigan State University, East Lansing, MI, USA). Differences with p values less than 0.05 were considered significant.
Results and discussion
Viability of probiotic bacteria
L. paracasei or L. acidophilus at the level of 9.0 log cfu/25 g was inoculated to sugared and sugar-free chocolate samples after conching. showed viability of Lactobacillus paracasei (LP) and L. acidophilus (LA) in the samples during 90 days of storage. It was determined that probiotic viability losses remained generally under 1 log cfu/25 g in the all sample groups after the production (day 0), and no loss for L. paracasei was determined in the milk chocolate samples prepared with maltitol and inulin DP < 10 (). However, decrease in viability was observed during storage period for all samples. At the end of 90 days, at least 5.9 log cfu/25 g probiotic bacteria was determined in the samples containing L. paracasei, and at least 6.5 log cfu/25 g probiotic bacteria was determined in the samples containing L. acidophilus. Maragkoudakis et al.[Citation29] considered probiotic load between 5 and 8 log cfu/g acceptable for dairy products. However, Canada and Italy authorities accepted the use of term ‘probiotic’ on food labels when there was a minimum 9 lof cfu/serving size or day,[Citation30] and for chocolate, the accepted serving size is 25 g.[Citation31]
Table 3. Viability of Lactobacillus paracasei (LP) and L. acidophilus (LA) in sugar-free and sugared synbiotic milk chocolate samples (log cfu/25 g).
In the previous studies, different results were obtained relevant to the change in the number of probiotic bacteria during storage. Lalicic-Petronijevic et al.[Citation9] determined that the number of probiotic bacteria decreased gradually within the storage period. But Zaric et al.[Citation32] determined that the number of probiotic bacteria showed an increase even at a low level in the first 90 days and then started to decrease in milk chocolate samples developed by using L. acidophilus and L. rhamnosus. They determined a correlation between the change in the viable cell numbers within the storage period and inoculation temperature to chocolate. They found that viability of the probiotics which were inoculated at 35°C was higher compared to the ones inoculated at 40°C. Lalicic-Petronicevic et al.[Citation9] determined that storage temperature of probiotic chocolates was an influential factor. In our study, the samples were stored at ambient temperature same as in market conditions. According to results, L. acidophilus had higher level of viability in the milk chocolates (containing maltitol or sugar), and the use of DP < 10 inulin helped increase viability level (p < 0.05).
In a recent study, Mueller et al.[Citation33] investigated the prebiotic effects of fructans from different sources having different structures and DP when used with L. casei, L. paracasei, L. acidophilus, L. reuteri, L. rhamnosus and Bifidobacterium animalis and cultures in agar environment. As a result of the study, it was determined that lower DP fructans improved probiotic stability more than higher DP inulin. These findings were in agreement with this study. The results verified that the type of probiotic bacteria and prebiotic substance, its structure and polymerisation degree were required to be taken into consideration to have positive effects on the bioavailability of products.
Textural properties
The hardness was used as textural quality parameter of chocolate. The hardness values of the samples ranged between 8.28–9.74 N and 8.48–10.01 N in the maltitol included samples and sugar included samples, repectively (). It was seen that the use of inulin affected the hardness parameter differently regarding the type of bulk sweetener used. While the use of inulin and DP did not affect the hardness value of the samples containing sugar, inulin addition caused higher hardness value in the samples containing maltitol compared to the control sample (p < 0.05).
Table 4. Moisture contents, water activities, hardness values of sugar-free and sugared synbiotic milk chocolate samples.
Belcak-Cvitanovic et al.[Citation3] and Farzanmehr and Abbasi[Citation22] determined that inulin addition increased the hardness of chocolates. This effect might be due to water absorbing property of prebiotic compound. Also it was observed that probiotic bacteria inoculation and type did not change the hardness values of the samples significantly (p > 0.05).
Water activities (aw) and moisture content
The factors such as ingredients, temperatures applied during refining and conching procedures and surface areas of materials may affect the water activity and humidity level of chocolate.[Citation34,Citation35] Humidity content and water activity should be below 1.50 g/100 g and 0.4, respectively, in chocolates. Regarding the maltitol-containing samples, water activity was determined within the range of 0.327–0.406, while it was found in the range of 0.295–0.460 for sugar-containing samples, and moisture contents were determined in the range of 1.28–1.79g and 1.10–1.56g/100g, respectively (). Determination of high moisture content and water activity values in some samples might be resulted from the conching and refining procedures applied during sample preparation. There was no clear trend observed between factors studied within the scope of this study (sweetener type, DP value of inulin, existence and type of probiotic bacteria) and water activity values of the samples.
Previously, the effect of inulin on the water activity of milk chocolates was studied. Konar et al.[Citation16] did not find a significant difference on the water activity values of maltitol-included milk chocolates which were obtained with the use of inulin at 9.00% concentration (w/w). Farzanmehr and Abbasi[Citation22] determined that the humidity content of milk chocolates including inulin, maltodextrin and polydextrose was higher than the control samples. When the water absorbing property of inulin is taken into consideration, this result can be expected. Nevertheless, desired water activity and moisture content values of chocolate samples could be reached by optimisation of particle size, conching period and temperature during production.
Melting properties
Tonset, Tpeak and Tend values belonging to milk chocolate samples were determined within the range of 15.71–16.81, 19.17–20.36 and 29.62–32.94°C, respectively (). The enthalpy required for melting the samples (ΔH) was determined within the range of 7.88 and 12.72 J/g. Melting temperatures, one of the main quality criteria for chocolate, is basically based on the fat used in the formulation. Use of same amount and types of fat in the product formulations led the melting properties to vary within a narrow range.
Table 5. Melting characteristics of sugar-free and sugared synbiotic milk chocolate samples.
The knowledge about melting properties provides information about oral melting behaviour with an impact on temporal components of flavour release and also oral epithelial sensation.[Citation36] For maltitol including samples containing DP > 23 inulin, Tonset and Tpeak were determined to be closer to the control sample, whereas any significant difference was not determined among the sugar-included samples (p > 0.05). Although there were significant differences for Tend and ΔH of two groups at a low level, it was tolerable in terms of quality properties of a product. This could be caused by crystal structure, particle size and interaction, and especially physicochemical properties of compounds used such as bulk sweetener, use of inulin and its DP. In the previous studies, it was determined that the fat ratio,[Citation36,Citation37] lecithin amount and particle size[Citation36] in the chocolate compound affected the melting properties.
It might be concluded that the use of inulin and its DP affected the melting properties. Interestingly, this effect varied depending on the existence of probiotic bacteria. Bacteria included samples with higher DP inulin showed higher Tend values. However, without using bacteria, samples with lower inulin value had higher Tend values. Regarding these effects, type of sweetener did not affect this trend.
Rheological properties
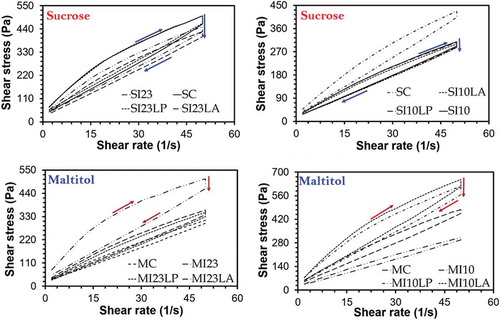
Rheograms are given in . As seen in the figure, the samples had a shear thinning flow behaviour. σ0 and ηpl values of the samples are summarised in . σ0 and ηpl values were determined in the range of 52.1 and 134.0 Pa and 6350 and 14400 mPa.s, respectively. According to results, it could be seen that inulin and probiotic strains added to the formulation significantly affected the rheological properties of the products (p < 0.05).
Table 6. σ0 and ηpl values of sugar-free and sugared synbiotic milk chocolate samples.
Increase of surface contact between particles may lead to an increase on the textural parameters such as the density, consistency and stickiness in the chocolate. In this case, it may result in the increase of viscosity. Concerning σ0 and ηpl values, it was determined that the samples prepared with DP > 23 inulin and maltitol had rheological properties closer to the control samples in conformity with the results obtained for the hardness value. Even if a significant difference was not determined among the hardness values of sugar group, the rheological properties closer to the control sample were revealed with the use of DP < 10 inulin for both σ0 and ηpl values. The plastic viscosity and yield stress showed increase along with the particle surface area in contact with the cacao butter. In this case, it may be also required to select inulin DP in the synbiotic chocolates in accordance with the type of bulk sweetener used.
Another remarkable result was that the existence of probiotic bacteria was effective on the rheological properties. For maltitol and higher DP inulin including samples, addition of L. paracesei decreased yield stress and plastic viscosity while L. acidophilus increased these parameters. For sugar-including samples L. paracesei did not affect these parameters, whereas only L. acidophilus decreased these parameters in the samples which include higher DP inulin. Rheological properties of the samples can also be modified by particle size and the use of ratios of lecithin and PGPR.
Another factor which is required to be taken into consideration when interpreting rheological results should be the moisture contents of samples because high humidity values increase the viscosity. This factor should be considered to make rheological properties at desired quality during the product development.
Colour properties of samples
Colour and brightness of appearance are accepted as one of the main quality parameters of chocolate. With this aim, whiteness index (WI), the brightness (L*), chroma (C*) and hue angle (h°) of synbiotic milk chocolate samples were examined in our study ().
Table 7. Colour values of sugar-free and sugared synbiotic milk chocolate samples.
In conformity with the results obtained by Bolenz et al.[Citation21] and Shouridehet al.,[Citation23] DP < 10 inulin caused a decrease in L* value in the samples containing sugar, but the use of DP > 23 inulin decreased the level of this effect (p < 0.05). However, it was observed that the use of inulin in the samples containing maltitol led to brighter milk chocolate compared to the control samples, and the existence and type of probiotic bacteria did not affect the brightness for all sample groups (p < 0.05).
In the same way, it was determined that the existence of inulin increased C* in the maltitol group samples and decreased WI. DP > 23 inulin containing samples had chroma, h° and WI values closer to the control samples. It was observed that all colour parameters changed within a narrow range, and the existence and type of probiotic bacteria did not cause significant change on mainly L* value which was the main colour quality parameter for chocolate (p < 0.05). However, inulin DP affected the colour of the milk chocolate depending on the sweetener type.
Conclusion
The consumer interest to the functional foods, especially the probiotic foods, has been increasing every passing day. As a result of the study, it was revealed that the chocolate matrix had an advantage for carrying the probiotic bacteria, and this was also valid for the sugar-free chocolates. However, more studies are required to be conducted to decrease viability loss of probiotic bacteria during the chocolate production process. The inulin was a significant compound as a prebiotic substance. Viability of the probiotics did not decrease more than 3 log after 90-day storage, and most durable strain was found as L. acidophilus in sugar and lower DP inulin including samples. Although studied parameters did not affect hardness values of the samples, inulin DP and probiotic type affected the water activity of the samples. There was no clear trend found in melting properties of the samples. However, existence of probiotic bacteria influenced rheological parameters. It was also positively found that probiotic addition did not affect the colour of the milk chocolates. This study showed that it was important to consider sweetener type, inulin and probiotic strain type to produce optimum milk chocolate at the best quality conditions.
Funding
This work was funded by the Scientific and Technological Research Council of Turkey (TUBITAK), Project No. TEYDEB-3140541.
Additional information
Funding
References
- Harwood, M.L. A Novel Method to Assess Consumer Acceptance of Bitterness in Chocolate Products. Thesis in the Pennsylvania State University The Graduate School College of Agricultural Sciences 2013, US. 123 pp.
- Callebaut. Barry Callebaut International Consumer Survey Shows: Chocolate Lovers Want Functional Chocolate With Proven Health Benefits. 2008, http://www.barry-callebaut.com/51?release=4030.
- Belscak-Cvitanovic, A.; Komes, D.; Dujmovic, M.; Karlovic, S.; Biskic, M.; Brncic, M.; Jezek, D. Physical, Bioactive and Sensory Quality Parameters of Reduced Sugar Chocolates Formulated with Natural Sweeteners as Sucrose Alternatives. Food Chemistry 2015, 167, 61–70.
- Roberfroid, M.B. General Introduction: Prebiotics in Nutrition In Handbook of Prebiotics; Gibson, G.R.; Roberfroid, M.B.; Ed.; CRC Press Boca Raton, FL: US, 2008; 435.
- Erdem, O.; Gultekin-Ozguven, M.; Berktas, I.; Ersan, S.; Tuna, H.E.; Karadag, A.; Ozcelik, B.; Gunes, G.; Cutting, S.M. Development of a Novel Synbiotic Dark Chocolate Enriched with Bacillus indicus HU36, Maltodextrin and Lemon Fiber: Optimization by Response Surface Methodology. LWT – Food Science and Technology 2014, 56, 187–193.
- Pimentel, F.A.; Nitzke, J.A.; Klipel, C.B.; de Jong, E.V. Chocolate and Red Wine e a Comparison Between Flavonoid Content. Food Chemistry 2010, 120, 109–112.
- Maillard, M.; Landuyt, A. Chocolate: An Ideal Carrier for Probiotics. Agro Food Industry Hi-Tec 2008, 19(3 Suppl), 13–15.
- Possemiers, S.; Marzorati, M.; Verstraete, W.; Van de Wiele, T. (2010). Bacteria and Chocolate: A Successful Combination for Probiotic Delivery. International Journal of Food Microbiology 2010, 141, 97–103.
- Lalicic-Petronijevic, J.; Popov-Raljic, J.; Obradovic, D.; Radulovic, Z.; Paunovic, D.; Petrusic, M.; Pezo, L. Viability of Probiotic Strains Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 and Their Impact on Sensory and Rheological Properties of Milk and Dark Chocolates During Storage for 190 Days. Journal of Functional Foods 2015, 15, 541–550.
- Raymond, Y.; Champagne, C.P. (2015). The Use of Flow Cytometry to Accurately Ascertain Total and Viable Counts of Lactobacillus Rhamnosus in Chocolate. Food Microbiology 2015, 46, 176–183.
- Coman, M.M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Functional Foods as Carriers for SYNBIO, a Probiotic Bacteria Combination. International Journal of Food Microbiology 2012, 157, 346–352.
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Belanger, G. Effects of Storage Conditions, Microencapsulation and Inclusion in Chocolate Particles on the Stability of Probiotic Bacteria in Ice Cream. International Dairy Journal 2015, 47, 109–117.
- Yonejima, Y.; Hisa, K.; Kawaguchi, M.; Ashitani, H.; Koyama, T.; Usamikrank, Y.; Kishida, N.; Kishino, S.; Ogawa, J. Lactic Acid Bacteria-containing Chocolate as A Practical Probiotic Product with Increased Acid Tolerance. Biocatalysis and Agricultural Biotechnology 2015, 4(4), 773–777.
- Aidoo, R.P.; De Clerq, N.; Afoakwa, E.O.; Dewettinck, K. Industrial Manufacture of Sugar-free Chocolates- Applicability of Alternative Sweeteners and Carbohydrate Polymers as Raw Materials in Product Development. Trends in Food Science and Technology 2013, 32, 84–96.
- Konar, N. Influence of Conching Temperature and Some Bulk Sweeteners on Physical and Rheological Properties of Prebiotic Milk Chocolate Containing Inulin. European Food Research and Technology 2013, 23, 135–143.
- Konar, N.; Ozhan, B.; Artik, N.; Poyrazoglu, E.S. Using Polydextrose as a Prebiotic Substance in Milk Chocolate-Effects of Process Parameters on Physical and Rheological Properties. CyTA Journal of Food 2014, 12(2), 150–159.
- Konar, N.; Ozhan, B.; Artik, N.; Dalabasmaz, S.; Poyrazoglu, E.S. Rheological and Physical Properties of Inulin-Containing Milk Chocolate Prepared at Different Process Conditions. CyTA Journal of Food 2014, 12(1), 55–64.
- Sokmen, A.; Gunes, G. Influence of Some Bulk Sweeteners on Rheological Properties of Chocolate. LWT-Food Science & Technology 2006, 39, 1053–1058.
- Beards, E.; Tuohy, K.; Gibson, G. A Human Volunteer Study to Assess the Impact of Confectionery Sweeteners on the Gut Microbiota Composition. British Journal of Nutrition 2010, 104, 701–708.
- Aidoo, R.P.; Afaokwa, E.O.; Dewettinck, K. Rheological Properties, Melting Behaviours and Physical Quality Characteristics of Sugar-free Chocolates Processed Using Inulin/polydextrose Bulking Mixtures Sweetened with Stevia and Thaumatin Extracts. LWT - Food Science and Technology 2015, 62, 592–597.
- Bolenz, S.; Amtsberg, K.; Schape, R. The Broader Usage of Sugars and Fillers in Milk Chocolate Made Possible by the New EC Cocoa Directive. International Journal of Food Science and Technology 2006, 41, 45–55.
- Farzanmehr, H.; Abbasi, S. Effects of Inulin and Bulking Agents on Some Physicochemical, Textural and Sensory Properties of Milk Chocolate. Journal of Texture Studies 2009, 40, 536–553.
- Shourideh, M.; Taslimi, A.; Azizi, M.H.; Mohammadifar, M.A. Effects of D-tagatose and Inulin of Some Physicochemical, Rheological and Sensory Properties of Dark Chocolate. International Journal of Bioscience, Biochemistry and Bioinformatics 2012, 2(5), 314–319.
- Aragon-Alegro, L.C.; Alarcon Alegro, J.H.; Cardarelli, H.R.; Chiu, M.C.; Saad, S.M.I. Potentially Probiotic And Synbiotic Chocolate Mousse. LWT - Food Science and Technology 2007, 40, 669–675.
- Glicerina, V.; Balestra, F.; Rosa, M.D.; Romani, S. Rheological, Textural and Calorimetric Modifications of Dark Chocolate During Process. Journal of Food Engineering 2013, 119(1), 173–179.
- Gloria, H.; Sievert, D. Changes in the Physical State of Sucrose During Dark Chocolate Processing. Journal of Agriculture and Food Chemistry 2001, 49(5), 2433–2436.
- Lonchampt, P.; Hartel, R.W. Surface Bloom on Improperly Tempered Chocolate. European Journal of Lipid Science and Technology 2006, 108(2), 159–168.
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M.L. Potential Use of Isomaltulose to Produce Healthier Marshmallows. LWT - Food Science and Technology 2015, 62, 605–612.
- Maragkoudakis, P.A.; Miaris, C.; Rojez, P.; Manalis, N.; Magkanari, F.; Kalantzopoulos, G. Production of Traditional Greek Yoghurt using Lactobacillus Strains with Probiotic Potential as Starter Adjuncts. International Dairy Journal 2006, 16, 52–60.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; Calder P.C.; Sanders, M.E. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nature Reviews – Gastroenterology & Hepatology 2014, 11, 506–514.
- Watson, W.L.; Kury, A.; Wellard, L.; Hughes, C.; Dunford, E.; Chapman, K. Variations in Serving Sizes of Australian Snack Foods and Confectionery. Appetite 2016, 96, 32–37.
- Zaric, D.B.; Bulatovic, M.L.; Rakin, M.B.; Krunic, T.Z.; Loncarevic, I.S.; Pajin, B.S. Functional, Rheological and Sensory Properties of Probiotic Milk Chocolate Produced in a Ball Mill. RSC Advances 2016, 6, 13934–13941.
- Mueller, M.; Viernstein, H.; Loeppert, R.; Praznik, W. Prebiotic Effect of Fructans with Different Structure and Polymerization Degree. International Scientific Conference on Probiotics and Prebiotics ( IPC 2015), June 23–25, 2015. Budapest Hungary, pp. 22–23.
- Rossini, K.; Norena, C.P.Z.; Brandelli, A. Changes in the Colour of White Chocolate During Storage: Potential Roles of Lipid Oxidation and Non-Enzymatic Browning Reactions. Journal of Food Science and Technology 2011, 48(3), 305–311.
- Vercet, A. Browning of White Chocolate During Storage. Food Chemistry 2003, 81(3), 371–377.
- Afaokwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Oxford, UK; 2010; 275.
- Do, T.A.L.; Hargreveas, J.M.; Wolf, B.; Hort, J.; Mitchell, J.R. Impact of Particle Size Distribution on Rheological and Textural Properties of Chocolate Models with Reduced Fat Content. Journal of Food Science 2007, 72(9), E541–E552.