ABSTRACT
In this study, we obtained the liquid–solid phase diagrams of a ternary solution of sodium chloride, acetic acid, and water, and a diagram of the frozen ratio of this ternary mixture. Based on the data from differential scanning calorimetry (DSC) measurement, the phase equilibria of this ternary system were successfully visualized. The formation of the ternary eutectic phase occurred between −34°C and −35°C, and the formation of the binary eutectic phase was suggested around −22°C to −34°C. Higher concentrations of a component had a significant effect on the phase equilibrium. A diagram of the frozen ratio of the ternary solution of sodium chloride at −20°C was also obtained by a simple temperature measurement method. This diagram is useful for estimating the ice fraction at the phase equilibrium of a frozen food system containing sodium chloride and acetic acid.
Introduction
Food processing and preservation techniques are largely influenced by the phase equilibria that prevail in the food system. Food products are usually multi-component and multi-phase systems, where phase transitions occur in a complicated manner as a consequence of changes in state variables such as temperature and pressure. When a food product is subjected to freezing, the formation of ice crystals causes considerable stress to the food system. This is due to the mechanical and interfacial stress from the ice, and chemical and physical stress caused by the formation of the freeze-concentrated phase (cryoconcentrated phase). The crystallization of ice expels the solute components to form a concentrated liquid phase that coexists with the solid phase (ice). Ice crystals possibly incorporate a small amount of the solute components; however, in many cases, the distribution coefficients of solutes between ice and the freeze-concentrated solution are negligibly small.[Citation1–Citation3]
Most of the typical food systems contain sodium chloride. The eutectic point of the water–NaCl•2H2O system is −21.2°C at a concentration of 22.4% (wt), and that of the water–NaCl system is −22.4°C at a concentration of 23.1% (wt).[Citation4] Similarly, aqueous acetic acid has its eutectic point at −26.3°C at a concentration of 23.95% (wt).[Citation4,Citation5] The properties of a frozen salt/vinegar-containing food, such as mayonnaise, are under the influence of these phase equilibria.[Citation6] An equilibrium freezing point, also denoted as the depression in freezing point, is dependent on the solute compositions. Solute concentrations in the freeze-concentrated phase are governed by the liquid–solid equilibrium, whereas the magnitude of the ice fraction coexisting with the freeze-concentrate is dependent on the composition of the original solution. In order to realize complete solidification, the temperature of the system must be lowered to the eutectic temperature.
It could be considered that many factors associated with the loss of quality of frozen food are linked to phenomena in the freeze-concentrated phase, where even at sub-zero temperatures the mobility of the substrates is not completely restricted or could even be promoted by the concentration.[Citation7] Denaturation of proteins in freezing and/or frozen systems is of great interest to biologists.[Citation8] Gel formation due to freezing has been intensively studied for food applications.[Citation9–Citation13] Nakagawa et al. recently reported that aggregated casein nanoparticles changed their microstructure in the freeze-concentrated phase,[Citation14] highlighting the importance of understanding phenomena that occur in this phase.
The phase equilibrium is the most important property in order to assess the thermodynamic state of the system. This study provides liquid–solid phase diagrams of a ternary solution of sodium chloride, acetic acid, and water. The measurements were conducted using DSC. Based on the diagrams obtained and alternative experiments, the frozen ratio of this ternary system was determined and visualized.
Materials and method
Materials
Sodium chloride (JIS Special Grade, ≥99.5%) and acetic acid (JIS Special Grade, ≥99.7%) were purchased from Wako Pure Chemical Industries, Osaka, Japan. Sodium chloride and acetic acid solutions were prepared by dissolving in water purified by an Elix® 3 water purification system (Merck Millipore Corporation, Darmstadt, Germany).
DSC measurements for melting point and frozen ratio estimation
Ternary aqueous solutions of various concentrations of sodium chloride and acetic acid were prepared, and their melting temperatures were analysed by DSC (DSC7020, Hitachi High-Technologies Corporation, Tokyo, Japan). About 2 mg of the sample solution was placed in an Alodine-coated aluminium sample pan and tightly clamped with a lid. This was placed in the DSC, and first stabilized at 30°C for 10 min. The sample was subsequently cooled down to −120°C at 5°C/min, and then heated up to 30°C at 5°C/min. The obtained DSC curves were analysed to detect melting points and to estimate the fraction of crystallized ice (i.e. frozen ratio).
Frozen ratio estimation by temperature profile measurement
The temperature profiles of the sample solutions were separately measured to estimate the frozen ratio. A sample solution was filled in a plastic centrifuge tube (PP microtube, 2 mL), and a type-K thermocouple (with a wire diameter of 0.25 mm) was fixed in the centre of the solution. The solution in the tube was then immersed in a cold bath held at −20°C for 120 min, for complete solidification, and subsequently immersed in a water bath at 30°C for thawing. The transition from the solid–liquid coexistence state to the liquid state appeared in the patterns of the thawing temperature profiles. Assuming that the heat flux given to the system during thawing is equivalent for each experimental run, the time duration of the transition step corresponds to the amount of ice in the system. This enables us to estimate the frozen ratio of a solution by calculating the ratio of the duration for the solution and the pure solvent (water).
Results and discussion
Liquid–solid phase equilibrium
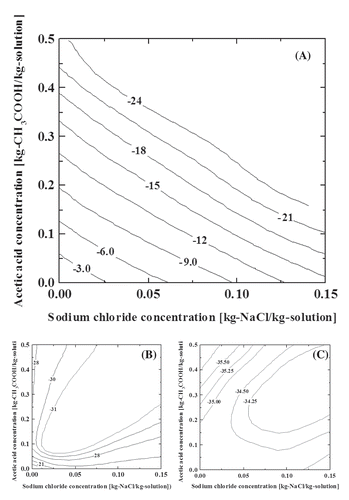
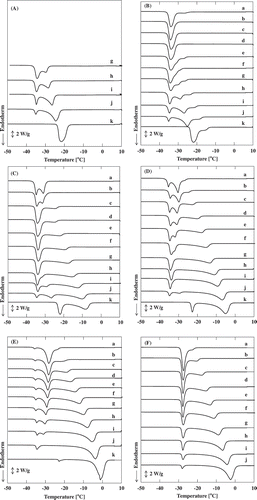
The DSC curves for different compositions of the ternary aqueous solutions of acetic acid and sodium chloride are shown in . In all the DSC curves, three major endothermic peaks were confirmed between −5°C and −22°C, −22°C and −34°C, and around −35°C, and their patterns were dependent on the compositions. The eutectic temperature of the sodium chloride and water system obtained from the present DSC measurement was around −21.9°C (–; line k). On the other hand, the eutectic temperature of the acetic acid and water system was confirmed around −27.6°C (; lines a–j). These eutectic point values were slightly lower than the reported values. This difference corresponds to the accuracy of measurement of the present device. A comparison of the DSC curves j and k in reveals that the peak around −35°C could be attributed to acetic acid. This peak appeared at almost the same position regardless of the amount of acetic acid and sodium chloride (–; –), suggesting that it corresponded to the eutectic point of the ternary mixture. The peaks that appeared in the range from −5°C to −22°C were strongly dependent on the solute compositions, and they were attributed to the equilibrium freezing points. The peaks between −22°C and −34°C were not so sensitive to the change in composition, and the component with higher concentration had a significant effect on these peak points. These peaks could probably be attributed to the formation of water–sodium chloride or water–acetic acid eutectics. However, the formation of the hydrate that may occur in this temperature range also complicated the assignment, which was based on the DSC results only. We did not carry out further detailed investigations in the present study. The analytical results on the phase equilibria could be summarized in contour diagrams as shown in . , , and correspond to the equilibrium freezing points, the eutectic points of the binary mixtures, and the eutectic points of ternary mixture, respectively.
Figure 1. The DSC curves for various solutions. The weight ratios of sodium chloride to water are (A) 3:10, (B) 2:10, (C) 1:10, (D) 0.5:10, (E) 0.1:10, and (F) 0:10. The weight ratios of acetic acid to the sodium chloride aqueous solutions are (a) 5:5, (b) 4.5:5.5, (c) 4:6, (d) 3.5:6.5, (e) 3:7, (f) 2.5:7.5, (g) 2:8, (h) 1.5:8.5, (i) 1:9, (j) 0.5:9.5, and (k) 0:10.
Frozen ratio estimation
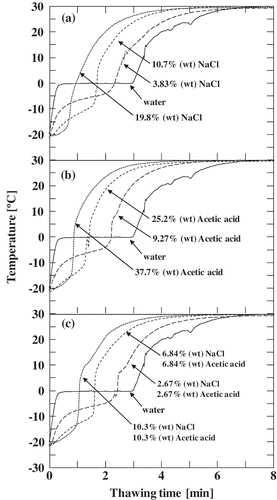
The frozen ratio was estimated from the temperature profile data obtained during the thawing of solutions. Examples of the temperature profiles are shown in . During the water-thawing process, a plateau can be observed in the temperature profile at the equilibrium frozen temperature, i.e. around 0°C. When thawing a solution, a slope (instead of plateau) appeared due to the dependence of the equilibrium frozen temperature on the solute concentration. These plateaus and slopes thus correspond to the transition of ice into water. The steep rise temperature after the plateaus and slopes are evidence of the disappearance of the ice fraction. Assuming that the heat flow (qi [J/s]) transferred from the bath medium to the solution in the test tube was all used for thawing, this heat flow can be related to the amount of ice fraction (mi [kg]) in the solution with the enthalpy of fusion (ΔHi [J/kg]) by following equation:
Figure 3. Temperature measurement during the thawing of (a) sodium chloride solution, (b) acetic acid solution, and (c) ternary mixture.
The time duration of the plateau or slope (ti [s]) can be given by integrating this equation, namely
The ratio of the time duration of the plateau of thawing water (t0) to the duration of a slope of thawing solution (t1) thus yields the ice fraction ratio (i.e. frozen ratio) of the frozen solution system by assuming equivalent qi and ΔHi values to the water and solution systems.
All the measurements were conducted in a setting where we can assume the equivalence of heat flux from the water bath to the sample solution. In order to validate the frozen ratio values obtained by this method, comparisons were made () with the ratio of the enthalpy of fusion of ice in a solution to that of pure ice. The enthalpy values were estimated by DSC analyses. From , it is seen that the frozen ratio values obtained by the two methods were in good accordance. This method directly yields the apparent ice fraction values, with the advantage of simplicity. Overlapping thermal peaks in DSC curves often lead to errors in peak separation that potentially allow mistakes in the estimation of enthalpy values. This drawback can be avoided in the temperature measurement method. However, care must be taken in this measurement that the separation of the melt from the thermocouple potentially gives data irregularity. And this method is not suitable for detecting sensible heat flow during thawing.
Figure 4. Correlation of the frozen ratio values obtained by DSC analysis and by temperature measurement.
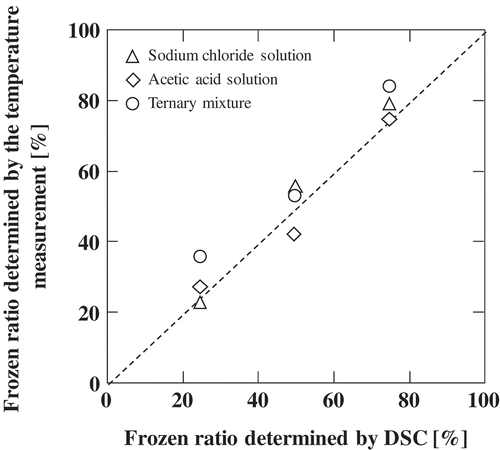
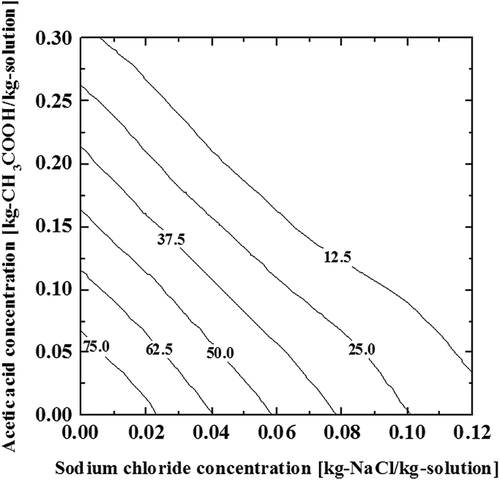
This measurement was carried out for about 60 solutions with different compositions of acetic acid and sodium chloride. The frozen ratio of the ternary aqueous solution at −20°C is shown in the contour lines in . Based on this figure, even in a common food that contains vinegar and salt with concentrations of 0.05 and 0.04 kg/kg, about 50% of the water remains unfrozen when subjected to a temperature of −20°C. The frozen ratio values increase at lower temperatures and decrease at higher temperatures. This figure is useful to estimate the mass fraction values of the liquid phase (or solid phase) that is important information for investigating possible phenomena that may occur in a frozen food system with sodium chloride and acetic acid.
Conclusion
Liquid–solid phase equilibria of a ternary solution of sodium chloride, acetic acid, and water, and the frozen ratios of the ternary mixtures were measured and successfully visualized in diagrams. Liquid–solid phase diagrams could be summarized in three contour diagrams that corresponded to the equilibrium freezing point, binary eutectic, and ternary eutectic. The eutectic temperature of the acetic acid and water system was confirmed around −27.6°C, and that of sodium chloride and water system was around −21.9°C. The formation of ternary eutectic phase mainly occurred between −34°C and −35°C, suggesting that this temperature is required for complete solidification. The peaks that appeared in the range from −5°C to −22°C were attributed to the equilibrium freezing points, and they were strongly dependent on the solute compositions. On the other hand, the peaks between −22°C and −34°C, probably be attributed to the formation of water–sodium chloride or water–acetic acid eutectics, were not so sensitive to the change in composition. As expected, a component with higher concentration had a significant effect on the phase equilibria. However, the formation of the hydrate cannot be ignored in this temperature range, and further investigations are required. A diagram of the frozen ratio of the ternary solution of sodium chloride at −20°C was obtained by a simple temperature measurement method. This would be useful for estimating the ice fraction at a state of the phase equilibrium of a frozen food system that contains sodium chloride and acetic acid. The results, for instance, suggested that 50% of the water remains unfrozen in a frozen food at –20°C that contain vinegar and salt with concentrations of 0.05 and 0.04 kg/kg.
Acknowledgement
This study was carried out during the course of a collaborative project by the Graduate School of Agriculture, Kyoto University, the Graduate School of Applied Biological Sciences, Gifu University, and the Nisshin Seifun Group, Inc.
References
- Fukui, K.; Maeda, K. Distribution of Solute at Solid–Liquid Interface during Solidification of Melt. J. Chem. Phy. 1998, 109, 7468–7473. DOI: 10.1063/1.477369.
- Fukui, K.; Maeda, K. Effects of Crystal Growth Rate and Heat and Mass Transfer on Solute Distribution. Chem. Eng. Sci. 2002, 57, 3133–3140. DOI: 10.1016/S0009-2509(02)00174-4.
- Fukui, K.; Kouuchi, S.; Asakuma, Y.; Maeda, K. Distribution of Cations in Ice Grown on a Rotating Cold Cylinder. J. Chem. Eng. Japan. 2008, 41, 344–349. DOI: 10.1252/jcej.08we009.
- Washburn, E. W.; West, C. J. International Critical Tables of Numerical Data, Physics, Chemistry and Technology. National Academies, 1928.
- Dahms, A.;. Nachträge Und Bemerkungen Zu Der Arbeit Über Gefrierpunkte Binärer Gemenge. Annalen. Der. Physik. 1896, 296, 119–123. DOI: 10.1002/(ISSN)1521-38889.
- Miyagawa, Y.; Ogawa, T.; Nakagawa, K.; Adachi, S. Destabilization of Mayonnaise Induced by Lipid Crystallization upon Freezing. Bio. Biotech. Biochem. 2016, 80, 786–790. DOI: 10.1080/09168451.2015.1123611.
- Champion, D.; Blond, G.; Le Meste, M.; Simatos, D. Reaction Rate Modeling in Cryoconcentrated Solutions: Alkaline Phosphatase Catalyzed DNPP Hydrolysis. J. Agri. Food Chem. 2000, 48, 4942–4947. DOI: 10.1021/jf000457s.
- Bhatnagar, B. S.; Bogner, R. H.; Pikal, M. J. Protein Stability during Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Tech. 2007, 12, 505–523.
- Lozinsky, V. I.;. Polymeric Cryogels as a New Family of Macroporous and Supermacroporous Materials for Biotechnological Purposes. Russian. Chem. Bulletin. 2008, 57, 1015–1032.
- Sowasod, N.; Nakagawa, K.; Charinpanitkul, T.; Tanthapanichakoon, W. Encapsulation of Curcumin Loaded Oil Droplets with Chitosan Based Cryogel: Influence of Freezing Condition on Nanocapsule Properties. Food. Sci. Tech. Res. 2013, 19, 633–640.
- Lozinsky, V. I.; Damshkaln, L. G.; Brown, R.; Norton, I. T. Study of Cryostructuration of Polymer Systems. XXI. Cryotropic Gel Formation of the Water-Maltodextrin Systems. J. Appl. Poly. Sci. 2002, 83, 1658–1667.
- Sowasod, N.; Nakagawa, K.; Tanthapanichakoon, W.; Charinpanitkul, T. Development of Encapsulation Technique for Curcumin Loaded O/W Emulsion Using Chitosan Based Cryotropic Gelation. Mater. Sci. Eng. C. 2012, 32, 790–798.
- Nakagawa, K.; Sowasod, N.; Tanthapanichakoon, W.; Charinpanitkul, T. Hydrogel Based Oil Encapsulation for Controlled Release of Curcumin by Using a Ternary System of Chitosan, Kappa-Carrageenan, and Carboxymethylcellulose Sodium Salt. LWT – Food. Sci. Tech. 2013, 54, 600–605.
- Nakagawa, K.; Jarunglumlert, T.; Adachi, S. Structural Changes in Casein Aggregates under Frozen Conditions Affect the Entrapment of Hydrophobic Materials and the Digestibility of Aggregates. Chem. Eng. Sci. 2016, 143, 287–296.