ABSTRACT
In this study, changes of protein and lipid in mud shrimp during frozen storage were investigated. Protein changes were evaluated through sulfhydryl content, disulfide bond, carbonyls, surface hydrophobicity, and intrinsic fluorescence intensity, whereas lipid oxidation was measured by free fatty acids, thiobarbituric acid reactive substances, peroxide value, and fluorescent compounds. In addition, radial basis function neural network (RBFNN) model was established to predict the quality of shrimp. Results indicated that the lipid changes of frozen mud shrimp were dominant. Mud shrimp stored at −20°C exhibited more lipid oxidative damage compared to those stored at −30 and −40°C. Protein denaturation occurred gradually with the prolongation of frozen storage time and protein oxidation mainly occurred after 12 weeks. RBFNN model can accurately predict and evaluate the protein and lipid qualities of shrimp stored at −20 to −40°C.
Introduction
The mud shrimp (Solenocera melantho) is one of the most abundant species in the East China Sea and the Yellow Sea. Mud shrimp have become one of the most popular seafood around the world and is the main target species for the beam trawls and bottoms trawls.[Citation1] Freezing is one of the most important preservation techniques for shrimp because of minimal loss of quality during long-term storage. Frozen storage can effectively inhibit the bacterial growth and enzyme activity, and prolong the shelf life of shrimp and other seafood.[Citation2] However, prolonged frozen storage can lead to denaturation, aggregation of myofibrillar proteins, and oxidation of lipids.[Citation3,Citation4] Previous studies have shown a close association between the protein degeneration and lipid oxidation during frozen storage and reported that proteins are susceptible to damage by oxidative reactions in the muscle.[Citation5,Citation6] The compounds generated from lipid oxidation can induce a number of changes in proteins, such as the formation of protein polymers, loss of sulphydryl groups, and an increase in carbonyl groups. In addition, several kinds of reactive oxygen species can also catalyze oxidative reactions and result in the detrimental effects on the quality of proteins and lipids.[Citation7] Several studies have investigated the protein and lipid changes in seafood during frozen storage, most of which have focused on different kinds of fishes.[Citation3,Citation8] But the influence of freezing temperature and duration on protein denaturation and lipid oxidation in mud shrimp has not been studied and needs to be investigated scientifically.
In recent years, different types of models have been widely used for predicting the quality changes in aquatic products.[Citation9–Citation11] Radial basis function neural network (RBFNN) is a feed-forward neural network and has been proved to have a nearly perfect predictive accuracy.[Citation12] RBFNN can develop a meaningful relationship between inputs and outputs through a learning process and obtain accurate predictive results by mimicking the real neural activity in the human brain.[Citation13] Several previous studies have reported the benefits of using RBFNN model for quality prediction of aquatic products. Xu et al.[Citation12] used the RBFNN model for predicting the quality of thawed shrimp stored at different temperatures (−3, 0, 3, and 6°C) and reported the accurate prediction. Wang et al.[Citation13] and Kong et al.[Citation14] applied RBFNN model to predict the quality changes of bream and common carp fillets during storage. However, the studies are scanty on the application of RBFNN to predict the quality changes of protein and lipid in shrimp during frozen storage.
Therefore, the objective of this study was to investigate the effects of prolonged frozen storage at different temperatures (−20, −30, and −40°C) on protein denaturation and lipid oxidation in mud shrimp. Additionally, a RBFNN predictive model was established based on several indicators to estimate the quality of protein and lipid in mud shrimp during frozen storage at different temperatures.
Materials and methods
Materials
Mud shrimp (Solenocera melantho) (average weight 11.55 ± 5.11 g) were obtained from Zhejiang Tianhe Aquatic Products Co., Ltd. (Zhejiang, China). The mud shrimp used in this study were captured in the East China Sea and transported on ice to the laboratory immediately. Upon arrival, the shrimp were washed with running tap water, drained for 5 min on stainless steel mesh, and packed with polyvinyl chloride bags (150 g/bag). All the shrimp were divided into three groups and stored in a refrigerator at −20, −30, and −40°C. Before all analyses, the shrimps were beheaded, peeled, and deveined. All determinations were performed in triplicates.
Preparation of myofibril protein
Myofibrillar protein (MFP) was prepared according to Kong et al.[Citation14] with some modification. Two grams of minced shrimp was mixed with 15 ml of cold distilled water and homogenized for 1 min. The homogenate was extracted at 4°C for 30 min and then centrifuged at 14,000g for 10 min to remove the supernatant. The abovementioned washing step was repeated using the 15 ml cold 0.3% NaCl (instead of water) to remove more water-soluble substances. Then, the precipitate was mixed with 30 ml Tris–maleate buffer (0.6 M NaCl–20 mM Tris–maleate, pH 7.0) and homogenized for 10 s. After staying at 4°C for 1 h to extract MFP, the homogenate was centrifuged at 14,000g and 4°C for 10 min. The supernatant was collected and washed with four volumes of cold distilled water. After centrifugation at 14,000g and 4°C for 5 min, the precipitate of MEP was collected and dissolved in chilled 0.6 M NaCl. The protein concentration of the MFP solution was determined by Biuret assay[Citation15] and adjusted to 4 mg/ml.
Lipid extraction
The lipids were extracted according to the method described by Bligh and Dyer[Citation16] with some modification. Briefly, 35 g minced shrimp meat was homogenized with 35 ml of chloroform and 70 ml of methanol for 2 min. After that, an additional 35 ml of chloroform and 35 ml of distilled water were added and the mixture was homogenized again for 30 s. Then, the homogenate was vacuum filtered and the residue was washed with 35 ml of chloroform. The filtrate was then transferred to a separating funnel and allowed to stand for several hours until divided completely. The organic phase was collected from the bottom of the funnel and dried under nitrogen. The obtained lipids were kept in a desiccator until (~12 h) further experiments.
Total sulfhydryl content
Total sulfhydryl (SH) content was determined using 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) and following the method of Kong et al.[Citation14] Briefly, 0.5 ml of MFP solution (4 mg/ml) was mixed with 4.5 ml 0.2 M Tris–HCl buffer (8 M urea, 2% SDS, 10 mM EDTA, pH 8.0). Then, 4 ml of the mixture was added to 0.5 ml of 0.1% DTNB in 0.2 M Tris–HCl buffer (pH 8.0) and incubated at 40°C for 25 min. The absorbance was measured at 412 nm using a UV-2600 spectrophotometer (Unico Instrument Co., Ltd., Shanghai, China). The SH content was calculated from the absorbance using the molar extinction of 13,600 (M cm)−1 and expressed as mol/105 g protein.
Disulfide bond content
The disulfide bond content was determined using 2-nitro-5-thiosulfobenzoate (NTSB) and according to the method of Sriket et al.[Citation17] with some modification. Five hundred microliters of MFP solution (4 mg/ml) was mixed with 4.5 ml 0.2 M Tris–HCl buffer (8 M urea, 1% SDS, 3 mM EDTA, pH 8.0). Then, 4 ml of the mixture was added to 0.5 ml 10 mM NTSB in 0.2 M Tris–HCl buffer (8 M urea, 3 mM EDTA, 1% SDS, 0.1 M Na2SO3, pH 9.5) and incubated at 40°C for 25 min. The absorbance was measured at 412 nm using a UV-2600 spectrophotometer. Disulfide bond content was calculated using the molar extinction of 13,600 (M cm)−1 and expressed as mol/105 g protein.
Protein carbonyls
Measurement of carbonyls is the most common method to determine protein oxidation. Protein carbonyls were measured using 2,4-dinitrophenylhydrazine (DNPH) and following the method of Srinivasan and Hultin[Citation18] with modification. An amount of 1 ml of MFP solution (2 mg/ml) was reacted with 1 ml of 10 mM DNPH in 2 M HCl for 1 h at room temperature in the dark. An amount of 1 ml of sample with 1 ml 2 M HCl served as control. The mixture was shaken every 15 min during the reaction period. Proteins were precipitated with 1 ml 20% TCA and washed three times with 1 ml of ethanol:ethyl acetate mixture (1:1) to remove unreacted DNPH. Then, the pellet was dissolved in 3 ml of 6 M guanidine in 2 M HCl and kept at 37°C for 15 min before centrifugation at 14,000g for 3 min. The absorbance of the supernatant was measured at 370 nm using a UV-2600 spectrophotometer. Protein carbonyls content was calculated using an absorption coefficient of 22,000 (M cm)−1 and expressed as nmol carbonyl per mg protein.
Surface hydrophobicity
Surface hydrophobicity was measured using the fluorescent probe 1-anilinonaphthalene-8-sulphonic acid (ANS) and according to the method of Wang et al.[Citation19] with modification. The MFP was diluted to 0.2, 0.3, 0.5, and 1.0 mg/ml in 0.6 M NaCl–10 mM phosphate buffer (pH 7.0). The diluted MFP solution (4 ml) was mixed with 20 μl 8 mM ANS in 0.1 M phosphate buffer (pH 7.0). The fluorescence intensity of ANS–protein conjugates was measured at an excitation wavelength of 390 nm and an emission wavelength of 470 nm using a F97 fluorescence spectrophotometer (Shanghai Lengguang Technology Co., Ltd., Shanghai, China). The excitation and emission slit widths were 10 nm. The slope of the fluorescence intensity versus protein concentration was calculated by linear regression analysis and referred as S0ANS.
Intrinsic fluorescence intensity
Intrinsic fluorescence was measured as described by Lina et al.[Citation20] with minor modification. Diluted MFP (0.05 mg/ml) in 0.6 M NaCl solution was scanned using a F97 fluorescence spectrophotometer. Intrinsic fluorescence spectroscopy was obtained between 300 and 400 nm at an excitation wavelength of 295 nm with a scanning speed of 1000 nm/min. The excitation and emission slit widths were 10 nm. All spectra were performed in triplicate and the corresponding protein-free sample (0.6 M NaCl solution) was used as blank for all samples.
Free fatty acid
Free fatty acid (FFA) content of lipid extract was determined according to the method of Sanchez-Alonso et al.[Citation21] Lipid sample (0.05–0.1 g) was dissolved in 5 ml of toluene and then treated with 1 ml of 5% (w/v) cupric acetate–pyridine reagent (pH 6.0). The mixture was vortex for 2 min and then centrifuged at 3000 rpm for 5 min at room temperature. The absorbance of the upper layer was measured at 715 nm, and the FFA content was expressed as g FFA/100 g lipid.
Thiobarbituric acid reactive substances
The thiobarbituric acid reactive substances (TBARS) were determined by following the method of Erkan and Özden.[Citation22] An amount of 2 g minced mud shrimp was added to 16 ml of 5% TCA and 100 μl of 2 g/l butylated hydroxytoluene (in ethanol). The mixture was homogenized for 2 min and then centrifuged at 5000 rpm for 3 min. Five milliliters of the supernatant was mixed with 1 ml of 0.01 M TBA and heated in a boiling water bath for 40 min. The mixture was allowed to cool down to room temperature, and the absorbance of the mixture was measured at 532 nm using a UV-2600 spectrophotometer. The results were expressed as mg/kg.
Peroxide value
Peroxide value (PV) was measured according to the method of Soyer et al.[Citation6] The lipid sample (0.01–0.3 g) was dissolved in 9.8 ml of chloroform–methanol (7:3, v/v) and mixed for 4 s. The mixture was added with 0.05 ml of 30% ammonium thiocyanate and mixed for 4 s. Then, 0.05 ml of 3.5 g/l ferrous chloride in 10 M HCl was added and mixed again for 4 s. After 5 min standing at room temperature, the absorbance was measured at 500 nm using a UV-2600 spectrophotometer. The PV was expressed as milliequivalents (meq) of peroxide/kg lipid, and a reference curve was plotted using ferric chloride standard.
Fluorescent compounds
Formation of fluorescent compounds (FCs) was determined in the aqueous phase obtained during the lipid extraction and following the method described by Rodríguez et al.[Citation23] Fluorescence formation was studied at an excitation/emission wavelength of 393/463 and 327/415 nm. The relative fluorescence (RF) was calculated as RF = F/Fst, where F is the fluorescence of sample measured at each excitation/emission maximum, and Fst is the fluorescence intensity of quinine in sulphate solution (1 μg/ml, 0.05 M H2SO4) at the corresponding wavelength. The FC was expressed as the fluorescence ratio (FR) and the FR was calculated as the ratio between the two RF values: FR = RF393/463 nm/RF327/415 nm.
Establishment of RBFNN model
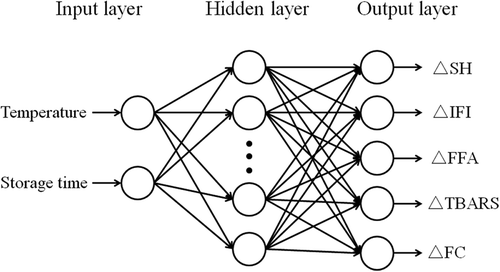
The RBFNN model is a feed-forward neural network with a flexible structure that can make a nonlinear mapping between input and output spaces.[Citation24] In this study, five indicators (SH, intrinsic fluorescence intensity [IFI], FFA, TBARS, and FC) were selected for the establishment of RBFNN model. The structure of RBFNN consists of three layers: an input layer, a hidden layer with sufficiently large number of neurons (hidden nodes), and an output layer. The RBFNN structure applied to predict quality changes for mud shrimp is shown in . The input layer links the neural network with the external environment. Neurons in the hidden layer provide a radial basis function to apply a nonlinear transformation from the input nodes to the hidden nodes. The output layer uses a linear activation function to supply the eventual response of the network. The hidden layer of RFBNN has radbas neurons and calculates its weighted inputs with the following Euclidean distance weight function:
Figure 1. Structure of radial basis function neural network (RBFNN) for predicting quality changes of mud shrimp during frozen storage.
where uj is the jth output vector of the hidden layer, xi is the ith input vector, wj is the center vector of the jth hidden neuron, bj is the input to hidden neurons weights, and C is the spread of radial basis functions. The output layer of RBF can be calculated using the following dot product weight function:
where Yk is the kth output vector of the output layer, n represents the number of neurons in hidden layer, wkj are weights of hidden neurons to output neurons (when j = 0, i.e., bias). In this study, the input layer consisted of two neurons that represented temperature (°C) and storage time (week). The output layer had five neurons, which are ΔSH, ΔIFI, ΔFFA, ΔTBARS, and ΔFC. ΔC was calculated using the following equation:
where C is the experimental value of quality indicator at time t, C0 is the corresponding initial value. The input and output variables were normalized to the range of −1–1 by the network and then used to train the RBFNN. The performance of trained network was evaluated by the mean square error (MSE) between the predicted and experimental values. The number of neurons in the hidden layer and the spread parameter was determined by calibration through several test runs.
Statistical analysis
Experimental data and linear regression were analyzed by using Microsoft Office Excel 2010 software. The RBFNN model was performed using Matlab R2013 (Mathworks). The SPSS 18.0 for Windows was used to perform analysis of variance, and the difference between means was compared by using the least significant difference procedure. Statistical significance was reported at a level of P < 0.05.
Results and discussion
Changes in total SH and disulfide bond contents
The total SH and disulfide bond contents of MFP from mud shrimp during frozen storage at different temperatures are shown in and , respectively. The total SH of all samples decreased sharply (P < 0.05) during the first 4 weeks of storage and then decreased slowly. The SH content of mud shrimp decreased by 18.71%, 15.04%, and 10.11% after 12 weeks of storage at −20, −30, and −40°C, respectively, when compared to the initial value. The decrease in SH content is generally associated with protein oxidation caused by the formation of disulfide bond or disulfide interchanges.[Citation8] During the frozen storage, a lower SH content was observed in mud shrimp stored at −20°C than the samples stored at −30 and −40°C. These results suggest that lower frozen temperatures (−30 and −40°C) can protect the protein from oxidation as compared to the higher frozen temperature (−20°C). The current study results are in agreement with Wu et al.[Citation15] who found a sharper and greater decrease in SH content of bighead carp stored at −20°C than the samples stored at −30 and −40°C. Soyer et al.[Citation6] also reported a similar result for chicken meat during frozen storage at 6, 12, and 18°C.
Figure 2. Changes in SH (a), disulfide bond (b), carbonyls (c), and surface hydrophobicity (d) in mud shrimp during frozen storage at different temperatures.
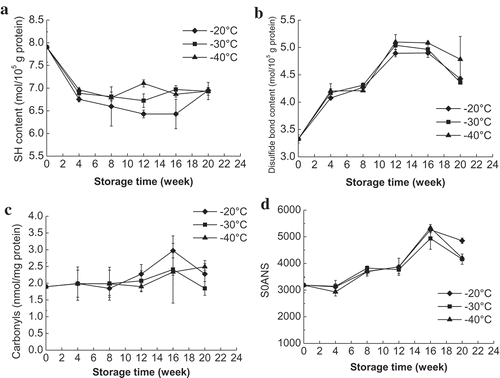
The disulfide bond content of MFP in mud shrimp increased significantly (P < 0.05) during the frozen storage for up to 12 weeks with a concomitant decrease in SH content (). The increased disulfide bond formation is generally due to the oxidation of SH groups and conformational changes. After 12 weeks of storage at −20, −30, and −40°C, the disulfide bond content of shrimp increased by 46.85%, 51.35%, and 53.15%, respectively. There was no significant difference in disulfide bond content of samples stored at different frozen temperatures, indicating that the formation of disulfide bond might be less susceptible to the influence of frozen temperatures. Benjakul et al.[Citation8] reported that the changes of the disulfide bond in tropical fish during frozen storage varied depending on the species. In that study, after 24 weeks of storage, disulfide bond in croaker, lizardfish, threadfin bream, and bigeye snapper increased by 129.5%, 325.4%, 32.9%, and 41.7%, respectively.
Changes in protein carbonyls
Protein carbonyls in all samples showed no significant changes before 12 weeks of storage and then increased significantly from 12th week to 16th week (). The increase of carbonyls was much higher (P < 0.05) in shrimp stored at −20°C than samples at other two temperatures (−30 and −40°C). After 16 weeks of storage, protein carbonyls in shrimp stored at −20, −30, and −40°C showed the highest value of 2.97, 2.43, and 2.27 nmol/mg protein (initial value 1.90 nmol/mg protein), respectively. Since carbonyls are the principal products of autoxidation, the significant increase of protein carbonyls in this study indicated the protein oxidative changes in mud shrimp after 12 weeks of frozen storage. The current study results are similar to the protein carbonyls reported for fish[Citation3] and chicken meat[Citation6] during frozen storage.
Changes in surface hydrophobicity
The changes in surface hydrophobicity of MFP from mud shrimp during frozen storage are shown in . The surface hydrophobicity increased sharply up to 16 weeks of frozen storage and followed by a slight decrease at the end of storage. The increased surface hydrophobicity in this study indicated the structural and conformational changes of MFPs, in mud shrimp during frozen storage, which can cause the unfolding of protein and exposure of more hydrophobic residues.[Citation19] The decrease in surface hydrophobicity at the end of storage might be attributed to the aggregation of unfolded protein molecules and masking of previously exposed hydrophobic portions.[Citation20] Lu et al.[Citation25] also reported that mild oxidation could change the conformational structure of MFP that was expressed by high surface hydrophobicity, but further oxidation induced a balance of protein structure. No statistically significant difference was found in surface hydrophobicity of the samples stored at the three temperatures, which is consistent with the changes of disulfide bond content. This indicated the structural changes of MFP in mud shrimp occurred gradually with the prolongation of frozen storage time and was less influenced by the frozen temperatures.
Intrinsic fluorescence intensity
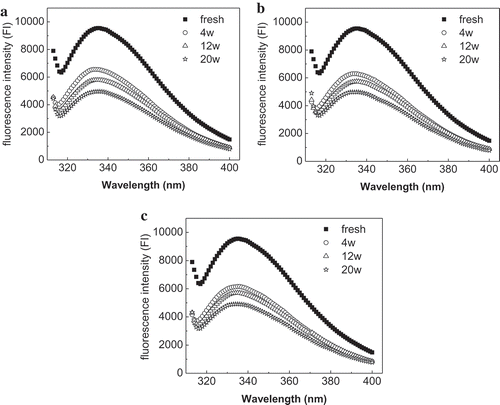
Aromatic amino acids are present in the inner core of the native proteins, and their emergence indicates the extent of protein unfolding. Intrinsic fluorescence measurements are widely used as an exposure magnitude index of aromatic amino acids, but the major contribution to the intrinsic fluorescence comes from tryptophan (Trp).[Citation26] Since myosin has Trp residues both in its rod and head regions, it is essential to monitor the conformational changes of MFPs by detecting the intrinsic fluorescence spectra of Trp residues. Moreover, the intrinsic fluorescence is sensitive to the polarity of microenvironment and the transition of tertiary structures of proteins.[Citation20]
The fluorescence spectra of MFP in mud shrimp during frozen storage at −20, −30, and −40°C are shown in . The data demonstrated that fresh myofibrils excited at 295 nm had a broad band with a maximum at 336 nm and exhibited high fluorescence intensity. The emission intensity of MFP in all samples decreased sharply (P < 0.05) during first 4 weeks and then reduced gradually with the prolonged frozen storage time. The decrease in intrinsic fluorescence is associated with the denaturation and exposure of indole side chain of Trp.[Citation27] This indicated that prolonged frozen storage resulted in the exposure of buried Trp residues and tertiary structures changes of MFP in mud shrimp. The decrease of IFI was greater when mud shrimp stored at higher temperatures and demonstrated that lower frozen temperatures could protect the protein from denaturation and structural changes. A similar result was reported by Lina et al.,[Citation20] who showed that MFPs in silver carp stored at −20°C suffer more pronounced structural alteration than the samples stored at −50°C.
Changes in FFA
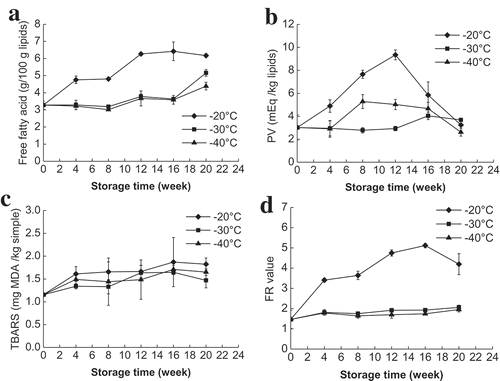
The formation of FFA is measured to evaluate the degree of lipid hydrolysis.[Citation28] Since FFA is more prone to oxidation than esterified fatty acids, lipid hydrolysis is a major factor that affects lipid oxidation.[Citation29] Changes in FAA of mud shrimp during frozen storage at −20, −30, and −40°C are shown in . The FFA content in mud shrimp stored at −20°C was significantly (P < 0.05) higher than those stored at −30 and −40°C throughout the whole frozen storage. At −20°C, the FFA content increased rapidly from 3.28 to 6.27 g/100 g lipids during the first 12 weeks of storage and indicated the extensive hydrolysis of lipids. However, at −30 and −40°C, a significant increase of FFA content was only observed at 12th and 20th weeks, respectively, and showed the highest value of 5.16 and 4.39 g/100 g lipids at the end of storage. These results suggested that frozen storage temperatures and duration had a marked impact on lipid hydrolysis. This is in agreement with Baron et al.,[Citation3] who reported that lower temperature could reduce the formation of FFA by inhibiting chemical and enzymatic reactions.
Lipid oxidation assessment
The process of lipid oxidation in frozen aquatic products is attributed to the action of endogenous enzymes.[Citation30] Frozen temperature and duration are the two most important factors that affect the rate of enzyme reactions and extent of lipid oxidation. The changes in PV in mud shrimp during frozen storage at −20, −30, and −40°C for 20 weeks are shown in . The PV at −20°C showed a marked increase (P < 0.05) from the initial value of 3.02 to 9.35 meq/kg lipids at 12th week and subsequently decreased to 3.25 meq/kg lipids at the end of the storage. The PV value of samples stored at −40°C exhibited similar varying tendency with the highest value of 5.28 meq/kg lipids at the eighth week of storage. In contrast, PV at −30°C maintained stable during the first 12 weeks of storage and increased to 4.04 meq/kg lipids at 16th week. The increase in PV was probably due to the faster rate of peroxides formation than degradation of peroxides into secondary oxidation products.[Citation6] The maximum PV of −20°C was much higher than that of −30 and −20°C and indicated that lower frozen temperatures could effectively slow down the primary oxidation process. A similar trend of PV was reported by Haghshenas et al.[Citation4] for shrimp nuggets during frozen storage.
In this study, TBARS increased slowly during frozen storage, reached a maximum value at 16th week, and then decreased slightly at 20th week (). The increased TBARS in this study indicated that secondary lipid oxidation in mud shrimp occurred with a slight degree during the frozen storage. The small decrease in TBARS at the end of the storage may be due to the formation of protein polymers. de Abreu et al.[Citation28] reported that TBARS could interact with other components such as proteins to form polymers when lipid or fatty acids are oxidized during the frozen storage. The TBARS content in mud shrimp stored at −20°C was little higher than that stored at −30 and −40°C during the entire storage. However, no significant difference was found among the three freezing temperatures (P > 0.05).
Formation of FCs
The formation of FCs is due to the interaction between primary and secondary lipids oxidation products and nucleophilic molecules such as proteins. The FC was measured using their fluorescence properties (FR value) in aqueous phases from the lipid extraction. The FR value at −20°C increased rapidly from 1.47 to 5.12 during the first 16 weeks of storage and then decreased to 4.20 at 20th week (). However, at −30 and −40°C, the FR value showed no significant changes during the frozen storage. The increase in FCs formation is in agreement with the primary and secondary lipid oxidation compounds formation.[Citation23] The FR value in samples at −20°C was much higher than that at −30 and −40°C, indicating a higher FCs formation at higher frozen temperatures. This indicated that the lower temperatures could protect mud shrimp from lipid oxidation. García-Soto et al.[Citation31] also reported a same trend of FCs development in crustacean lobster krill during frozen storage at −18°C.
Establishment and evaluation of the RBFNN model
The experimental values of SH, IFI, FFA, TBARS, and FC in mud shrimp stored at −20, −30, and −40°C were used to establish the RBFNN model. The spread and number of neurons in the hidden layer were decided by the testing the MSE (). In this study, 13 neurons in the hidden layer were determined by considering the smoothness of performance curve and lower MSE (0.002603). Then, the optimal parameter spreads of 0.4 were chosen with the minimum MSE from the testing process. Therefore, a 3-layer network with 13 neurons in the hidden layer was developed based on the training data. The weights and bias of the neurons and simplified algorithms derived from the optimal network are listed in Appendix.
Table 1. Mean square error (MSE) of RBFNN with different neurons and spreads in the hidden layer.
The predicted performance of RBFNN model was evaluated by comparing the predictive values and experimental values. The relative errors between predicted values and experimental values of mud shrimp at −20, −30, and −40°C are presented in . The relative errors of SH, IFI, FFA, TBARS, and FC at different temperatures were all within ±5% error. As reported by Kaymak-Ertekin and Gedik,[Citation32] a model with relative errors below 10% can be considered acceptable. The current study results indicated that the RBFNN model has a high acceptability and can be applied reliably to the estimation of the quality of frozen mud shrimp at different frozen temperatures. To evaluate the overall fitting performance of RBFNN model for each indicator at different temperatures, the MSE and the determination coefficient (r2) between experimental and predicted values were calculated. The MSE and r2 values of all indicators and temperatures varied from 0.00 to 1.26 and 0.72 to 1.00, respectively. The low MSE and high r2 in this study indicated the perfect fitting performance of RBFNN model. Therefore, the established model can be used to predict the protein and lipid quality changes in mud shrimp during frozen storage from −20 to −40°C.
Table 2. Relative errors between predicted and experimental values of SH, IFI, FFA, TBARS, and FC of mud shrimp during frozen storage at different temperatures.
Conclusions
Between the changes of protein and lipid in mud shrimpt during frozen storage, lipid changes is dominant and can be better indicators for evaluating quality of frozen mud shrimp. The lipid changes in mud shrimp are affected by both freezing duration and temperatures. Mud shrimp stored at −20°C exhibited more lipid oxidative damage due to higher FFA, PV, TBARS, and FCs compared to those stored at −30 and −40°C. While protein changes of mud shrimp are mainly influenced by the freezing duration. Protein denaturation occurred gradually with the prolongation of frozen storage time and protein oxidation mainly occurred after 12 weeks. In addition, the RBFNN model based on SH, IFI, FFA, TBARS, and FC showed excellent performance in predicting the protein and lipid changes of frozen mud shrimp stored from −20 to −40°C. This model can be potentially used to provide valuable information about the quality of mud shrimp during frozen storage.
Additional information
Funding
References
- Li, H. Y.; Ling, J. Z.; Yan, L. P.; Cheng, J. H.; Hu, F. Feeding Ecology of the Mud Shrimp Solenocera Melantho in the East China Sea. Marine Ecology 2016, 37(2), 380–391.
- Yerlikaya, P.; Gokoglu, N.; Topuz, O. K. Use of Natural Plant Extracts in Batter Coating of Shrimp and Their Effects on the Quality of Shrimp during Frozen Storage. Journal of Food Processing and Preservation 2010, 34(1), 127–138.
- Baron, C. P.; Kjærsgård, I. V. H.; Jessen, F.; Jacobsen, C. ProtR Lipid Oxidation during Frozen Storage of Rainbow Trout (Oncorhynchus Mykiss). Journal of Agricultural and Food Chemistry 2007, 55(20), 8118–8125.
- Haghshenas, M.; Hosseini, H.; Nayebzadeh, K.; Kakesh, B. S.; Mahmoudzadeh, M.; Fonood, R. K. Effect of Beta Glucan and Carboxymethyl Cellulose on Lipid Oxidation and Fatty Acid Composition of Pre-Cooked Shrimp Nugget during Storage. LWT - Food Science and Technology 2015, 62(2), 1192–1197.
- Saeed, S.; Howell, N. K. Effect of Lipid Oxidation and Frozen Storage on Muscle Proteins of Atlantic Mackerel (Scomber Scombrus). Journal of the Science of Food and Agriculture 2002, 82(5), 579–586.
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chemistry 2010, 120(4), 1025–1030.
- Eymard, S.; Baron, C. P.; Jacobsen, C. Oxidation of Lipid and Protein in Horse Mackerel (Trachurus Trachurus) Mince and Washed Minces during Processing and Storage. Food Chemistry 2009, 114(1), 57–65.
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative Study on Physicochemical Changes of Muscle Proteins from Some Tropical Fish during Frozen Storage. Food Research International 2003, 36(8), 787–795.
- Tsironi, T.; Dermesonlouoglou, E.; Giannakourou, M.; Taoukis, P. Shelf Life Modelling of Frozen Shrimp at Variable Temperature Conditions. LWT - Food Science and Technology 2009, 42(2), 664–671.
- Bao, Y.; Zhou, Z.; Lu, H.; Luo, Y.; Shen, H. Modelling Quality Changes in Songpu Mirror Carp (Cyprinus Carpio) Fillets Stored at Chilled Temperatures: Comparison between Arrhenius Model and Log-Logistic Model. International Journal of Food Science & Technology 2013, 48(2), 387–393.
- Liu, X.; Jiang, Y.; Shen, S.; Luo, Y.; Gao, L. Comparison of Arrhenius Model and Artificial Neuronal Network for the Quality Prediction of Rainbow Trout (Oncorhynchus Mykiss) Fillets during Storage at Different Temperatures. LWT - Food Science and Technology 2015, 60(1), 142–147.
- Xu, Z.; Liu, X.; Wang, H.; Hong, H.; Yu, X.; Luo, Y. Establishment of the Arrhenius Model and the Radial Basis Function Neural Network (RBFNN) Model to Predict Quality of Thawed Shrimp (Solenocera Melantho) Stored at Different Temperatures. Journal of Food Processing and Preservation 2016, 40(5), 882–892.
- Wang, H.; Kong, C.; Li, D.; Qin, N.; Fan, H.; Hong, H.; Luo, Y. Modeling Quality Changes in Brined Bream (Megalobrama Amblycephala) Fillets during Storage: Comparison of the Arrhenius Model, BP, and RBF Neural Network. Food and Bioprocess Technology 2015, 8(12), 2429–2443.
- Kong, C.; Wang, H.; Li, D.; Zhang, Y.; Pan, J.; Zhu, B.; Luo, Y. Quality Changes and Predictive Models of Radial Basis Function Neural Networks for Bbrined Common Carp (Cyprinus Carpio) Fillets during Frozen Storage. Food Chemistry 2016, 201, 327–333.
- Wu, H.; Wang, Z.; Luo, Y.; Hong, H.; Shen, H. Quality Changes and Establishment of Predictive Models for Bighead Carp (Aristichthys Nobilis) Fillets during Frozen Storage. Food and Bioprocess Technology 2014, 7(12), 3381–3389.
- Bligh, E. G.; Dyer, W. J. A. Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology 1959, 37(8), 911–917.
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative Studies on the Effect of the Freeze–Thawing Process on the Physicochemical Properties and Microstructures of Black Tiger Shrimp (Penaeus Monodon) and White Shrimp (Penaeus Vannamei) Muscle. Food Chemistry 2007, 104(1), 113–121.
- Srinivasan, S.; Hultin, H. O. Hydroxyl Radical Modification of Fish Muscle Proteins. Journal of Food Biochemistry 1994, 18(6), 405–425.
- Lee, J.; Park, J. W. Pacific Whiting Frozen Fillets as Affected by Postharvest Processing and Storage Conditions. Food Chemistry 2016, 201(15), 177–184.
- Lina, R.; Yanshun, X.; Qixing, J.; Wenshui, X.; Chunjiang, Q. Investigation on Structural Changes of Myofibrillar Proteins from Silver Carp (Hypophthalmichthys Molitrix) during Frozen Storage. Food Science and Technology Research 2013, 19(6), 1051–1059.
- Sánchez-Alonso, I.; Carmona, P.; Careche, M. Vibrational Spectroscopic Analysis of Hake (Merluccius Merluccius L.) Lipids during Frozen Storage. Food Chemistry 2012, 132(1), 160–167.
- Erkan, N.; Özden, Ö. Quality Assessment of Whole and Gutted Sardines (Sardina Pilchardus) Stored in Ice. International Journal of Food Science & Technology 2008, 43(9), 1549–1559.
- Rodríguez, A.; Cruz, J. M.; Paseiro-Losada, P.; Aubourg, S. P. Effect of a Polyphenol–Vacuum Packaging on Lipid Deterioration during an 18-Month Frozen Storage of Coho Salmon (Oncorhynchus Kisutch). Food and Bioprocess Technology 2012, 5(6), 2602–2611.
- Kashaninejad, M.; Dehghani, A. A.; Kashiri, M. Modeling of Wheat Soaking Using Two Artificial Neural Networks (MLP and RBF). Journal of Food Engineering 2009, 91(4), 602–607.
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of Gel Properties and Biochemical Characteristics of Myofibrillar Protein from Bighead Carp (Aristichthys Nobilis) Affected by Frozen and a Hydroxyl Radical-Generation Oxidizing System. Food Chemistry 2017, 223(15), 96–103.
- Qiu, C.; Xia, W.; Jiang, Q. Pressure-Induced Changes of Silver Carp (Hypophthalmichthys Molitrix) Myofibrillar Protein Structure. European Food Research and Technology 2014, 238(5), 753–761.
- Lefevre, F.; Fauconneau, B.; Thompson, J. W.; Gill, T. A. Thermal Denaturation and Aggregation Properties of Atlantic Salmon Myofibrils and Myosin from White and Red Muscles. Journal of Agricultural and Food Chemistry 2007, 55(12), 4761–4770.
- de Abreu, D. A. P.; Losada, P. P.; Maroto, J.; Cruz, J. M. Lipid Damage during Frozen Storage of Atlantic Halibut (Hippoglossus Hippoglossus) in Active Packaging Film Containing Antioxidants. Food Chemistry 2011, 126(1), 315–320.
- Veeck, A. P. L.; Klein, B.; Ferreira, L. F.; Becker, A. G.; Heldwein, C. G.; Heinzmann, B. M.; Baldisserotto, B.; Emanuelli, T. Lipid Stability during the Frozen Storage of Fillets from Silver Catfish Exposed in Vivo to the Essential Oil of Lippia Alba (Mill.) NE Brown. Journal of the Science of Food and Agriculture 2013, 93(4), 955–960.
- Ortiz, J.; Larraín, M. A.; Vivanco, J. P.; Aubourg, S. P. Rancidity Development during the Frozen Storage of Farmed Coho Salmon (Oncorhynchus Kisutch): Effect of Antioxidant Composition Supplied in the Diet. Food Chemistry 2009, 115(1), 143–148.
- García-Soto, B.; Miranda, J. A.; Barros-Velázquez, J.; Aubourg, S. P. Quality Changes during the Frozen Storage of the Crustacean Lobster Krill (Munida Spp.). European Journal of Lipid Science and Technolog 2015, 117(4), 431–439.
- Kaymak-Ertekin, F.; Gedik, A. Kinetic Modelling of Quality Deterioration in Onions during Drying and Storage. Journal of Food Engineering 2005, 68(4), 443–453.
Appendix
RBFNN: 1 hidden layer, 13 hidden neurons.
Input layer:
Hidden layer:
Output layer: