?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study describes the phytochemical profiles, antibacterial, and antioxidant properties of different solvent extracts of Solanum xanthocarpum leaves. Phytochemical analysis results revealed the presence of terpenoids, tannins, steroids, and phenols. Methanolic extract of plant had a maximum quantity of phenol (28.3 ± 2.0 mg) and flavonoids (25.2 ± 1.2 mg) than others. Similarly, the methanolic extract showed excellent antibacterial activity and exhibited the highest inhibitory effect against Pseudomonas aeruginosa (12 ± 0.5 mm), Salmonella typhi (10 ± 0.6 mm), Staphylococcus aureus (9 ± 1.0 mm), and Escherichia coli (7 ± 1.3 mm). The average minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were reported in the range of 3.2 to 6.9 µg/ml (for MIC) and 6.0 to 14.5 µg/ml (for MBC), respectively. The remarkable antioxidant activity was observed in chloroform and methanol extract on the DPPH radical scavenging activity with the lowest IC50 value of 197.245 μg/ml (chloroform) and 201.04 μg/ml (methanol) and compared with control (ascorbic acid 239.36 μg/ml). GC-MS analysis revealed the presence of six major bioactive compounds as follows; 2,Octylcyclopropene-1-Heptanol (42.81%), Hexadecanoic acid (26.63%), 1-methylene-2b Hydroxymethyl-3,3-Dimethyl-4b-(3Methylbut-2enyl)-C (9.3%), Phytol (7.5%), (1,3,3-Trimethyl-2-Hydroxymethyl-3,3-Dimethyl-4–3-Methylbut-2-Enyl)-C (7.2%). 3,7,11,15-Tetramethyl-2-Hexadecen-1-Ol (6.3%). The FT-IR spectrum reflected the presence of the twelve peaks at the range of 3746.38 cm-1 (O-H stretch alcohols), 3424.18 cm-1 (O-H stretch phenols), 2926.01 cm-1 (C-H stretch alkanes), 2857.55 cm-1 (C-H stretch alkanes), 2084.04 cm-1 (-C = C stretch alkynes), 1595.76 cm-1 (N-H bend primary amines), 1402.49 cm-1 (C-C stretch in ring aromatics) and others. This study suggests S. xanthocarpum as a potential candidature for having better antibacterial and antioxidant property and identified several bioactive compounds by GC-MS analysis.
Introduction
The medicinal properties of plants are generally desired by the presence of secondary metabolites. Among them, phenolic compounds possess multiple biological activities.[Citation1,Citation2] Reactive Oxygen Species (ROS), i.e., free radicals, are one of the major causes for the conversion of normal cell to cancerous cells.[Citation3] Herbs protect human body against ROS and reduce oxidative damage to DNA in cancer initiation or promotion. Antioxidants of herbs may mediate these biological effects by directly reacting with ROS, free radical scavenging, or catalytic metals chelating.[Citation4,Citation5] The antimicrobial resistance is growing and poses great challenge, and drug resistance, especially by bacteria and fungi, is one of the major concerns to public health and scientific communities worldwide.[Citation6] Medicinal plant extracts have a potential source for the development of new bioactive compound that acts against human infections currently difficult to treat. So in this concern antimicrobial activity of different herbal extracts has been reported against many human pathogens.[Citation7,Citation8] The medicinal values of those plants possess a chemically active substance that produces a distinct physiological action on the human body and animal health. The most important bioactive substances are alkaloid, tannin, flavonoid, and phenolic compounds.[Citation9]
Solanum xanthocarpum (Solanaceae) is a commonly growing perennial herb, very prickly, diffuses bright green perennial weed with bright green leaves and zigzag stem, and is mostly found in the arid region. The berries are green and white strips when young but yellow when matured. The flowers are purple in colour and bloom in Oct–March of the year. The berries are yellow with many seeds.[Citation10] The fruits are reported as having several biological properties such as anthelmintic, wound healing, antipyretic, laxative, anti-inflammatory, antiasthmatic, aphrodisiac activities.[Citation11] The stem, flowers and vesicular eruptions[Citation12] proved better antiasthmatic, antinociceptive, antifungal, molluscicide,[Citation13] anti spermatogenic, anti androgenic, antinociceptive, and hypoglycemic activities. The present study was designed to investigate in vitro the biological properties (antibacterial and antioxidant) of S. xanthocarpum, using different solvent crude extracts.
Materials and methods
Chemicals and media
2, 2-diphenyl- 2-picryl hydrazyl-hydrate (DPPH), Pvt. Ltd. (Mumbai, India). All other chemicals and solvents used were of analytical grade. Muller Hinton Agar and Nutrient broth were obtained from Hi-Media Laboratories, Mumbai, India. Deionized water was used throughout the bioassay. All glass wares and petriplates were washed with dilute nitric acid (HNO3) and distilled water and then dried in hot air oven.
Plant collection and extracts preparation
Fresh, disease-free, and matured plants (Solanum xanthocarpum) were collected from Mettur Dam (latitude 11.8000° N, longitude 77.8000° E), Salem District, Tamilnadu, India. The plant herbaria [voucher specimens: (PU/DBT/NDRL//2010/07)] were deposited in Natural Drug Research Laboratory (NDRL), Department of Biotechnology, Periyar University, Salem, Tamil Nadu, India. The leaves were washed with fresh running tap water and shade-dried for 3 weeks. Dried leaves were powdered uniformly using a mechanical grinder. The powder was extracted in various solvents (hexane, acetone, ethyl acetate, chloroform, and methanol) separately using a Soxhlet apparatus. These extracts were concentrated at 40°C under reduced pressure (72 m bar) in rotary evaporator and dried using lyophilizer. Dried extracts were collected in air tight container and stored at 4°C until further use.
Qualitative phytochemical analysis
The crude extract of plant samples was dissolved in respective solvents used for qualitative confirmation of major phytochemicals such as alkaloids, flavonoids, phenolics, saponins, steroids, tannins, and carbohydrate.[Citation14,Citation15]
Protein and carbohydarate
The crude extract of this plant was boiled with 2 ml of 0.2% solution of Ninhydrin, and the violet colour development indicates the presence of amino acids and proteins. Crude extract was mixed with 2 ml of iodine solution. A dark blue or purple colour formation indicates the presence of carbohydrate.
Steroids, terpenoids, and alkaloids
Crude extract was mixed with 2 ml of chloroform, and concentrated H2SO4 was added sidewise. A red colour produced in the lower chloroform layer indicates the presence of steroids. Crude extract was dissolved in 2 ml of chloroform and evapourated to dryness. To this, 2 ml of concentrated H2SO4 was added and heated for about 2 min. A greyish colour indicates the presence of terpenoids. Crude extract was mixed with 2 ml of 1% HCl and heated gently. Mayer’s and Wagner’s reagents were added to the mixture. Turbidity of the resulting precipitate was taken as evidence for the presence of alkaloids.
Phenols, flavonoids, and tannins
Crude extract was mixed with 2 ml of 2% solution of FeCl3. A blue-green or black colour formation indicates the presence of phenols and tannins. Crude extract was mixed with 2 ml of 2% solution of NaOH. An intense yellow colour was formed which turned into colourless by the addition of few drops of diluted acid, and this indicates the presence of flavonoids.
Saponins and glycosides
Crude extract was mixed with 5 ml of distilled water in a test tube and was shaken vigorously. The formation of stable foam was taken as an indicator for the presence of saponins. Crude extract was mixed with 2 ml of chloroform and 2 ml of acetic acid. The mixture was cooled in ice, and then the concentrated H2SO4 was carefully added. A colour change from violet to blue to green indicates the presence of steroidal nucleus (i.e., glycine portion of glycoside).
Quantitative phytochemical analysis
Determination of glycosides
Glycosides of S. xanthocarpum plant extracts were quantitatively determined by the modified method of Solich et al.[Citation16] 10% of each extracts were mixed with 10 ml freshly prepared Baljet’s reagent (95 ml of 1% picric acid + 5 ml of 10% NaOH). After an hour, the mixture was diluted with 20 ml distilled water, and the absorbance was measured at 495 nm by Shimadzu UV/Vis spectrophotometer model 160A (Kyoto, Japan).
Determination of flavonoids
Flavonoids were quantitatively determined according to the method of Harborne.[Citation17] The total flavonoid content was determined by the aluminium chloride colourimetric method. 0.5 ml aliquots of various extracts (1 mg/ml) were mixed with 1.5 ml of methanol, followed by the addition of 0.1 ml of 10% aluminum chloride, 0.1 ml of potassium acetate (1 M), and 2.8 ml of distilled water. The reaction mixture was kept at room temperature for 30 min. The absorbance of the reaction mixture was recorded at 415 nm. The calibration curve (0–8 μg/ml) was plotted using rutin as a standard.
Determination of protein
The S. xanthocarpum sample was extracted by stirring with 50 ml of 50% methanol (1:5 w/v) at 25°C for 24 h and centrifuged at 8,000 rpm for 10 min. 0.2 ml of extract was pipette out, and the volume was made into 1.0 ml with distilled water. 5.0 ml of alkaline copper reagent is added to all the tubes and allowed to stand for 10 min. Then, 0.5 ml of Folin’s Ciocalteau reagent is added and incubated in dark condition for 30 min. The intensity of the colour was developed, and the absorbance was read with a spectrophotometer at 660 nm.[Citation6]
Determination of carbohydrates
A total of 100 mg of different extract sample was hydrolysed in a boiling tube with 5 ml of 2.5 N HCl in a boiling water bath for a period of 3 h. It was cooled to room temperature, and solid sodium carbonate was added until agitation ceases. The contents were centrifuged, and the supernatant was made with 100 ml using distilled water. From this 0.2 ml of sample was pipette out and made up to 1 ml with distilled water. Then 1.0 ml of phenol reagent was added and followed by 5.0 ml of sulphuric acid. The tubes were kept at 25–30°C for 25 min. The absorbance was read with a spectrophotometer at 490 nm.[Citation7]
Determination of total steroids
The estimation of total steroids in the different extracts of S. xanthocarpum was carried out by the modified method of Sabir et al.[Citation18] Fifty grams of extracts was dissolved in 200 ml Aceto nitrile and incubated at 50–60°C for 2 h. It was filtered through Whatman No.1 filter paper, and 200 ml of methanol was added. Then, 3 ml of the extract was taken in a test tube with three replicates, and one blank. Then, 2 ml of Liberman-Burchard reagent was added to each tube. The reference standard solution was prepared by dissolving 10 gm of standard cholesterol in 10 ml chloroform. This was taken in five test tubes (1, 1.5, 2, 2.5, and 3.0 ml), and chloroform was used as blank. 2 ml of Liberman-Burchard reagent was added to each of them to develop the green colour, which is an indication of steroids. The final volume was made up to 10 ml by adding methanol and incubated in the dark for 15 min. The optical density of the standard solutions was determined with a spectrophotometer (Shimadzu) at 640 nm. The amount of steroids in the plant sample was determined by plotting the standard graph as mg/gm.
Determination of tannins
Tannin determination of sample was done according to the method of Van-Burden and Robinson.[Citation19] 50 ml of distilled water was added to 500 mg of the sample taken in a 500 ml flask and kept in a shaker for 1 h. It was filtered into a 50 ml volumetric flask, and then 5 ml of the filtrate was pippette out into a test tube and mixed with 2 ml (10 fold diluted) of 0.1 M FeCl3 in 0.1 N HCl and 0.008 M potassium ferrocyanide. Within 10 min, the absorbance of sample was measured with a spectrophotometer at 605 nm.
Determination of total phenolic content
The amount of total phenolic content of sample was determined as per the method of Velioglu et al.[Citation20] using the Folin–Ciocalteu reagent. Aliquot of 0.1 ml of various extracts (4 mg/ml) was mixed with 0.75 ml of Folin–Ciocalteu reagent (10-fold diluted with dH2O). The mixture was kept at room temperature for 5 min, and 0.75 ml of 6% sodium carbonate was added. After 90 min of reaction, its absorbance was recorded at 725 nm. The standard calibration (0–25 μg/ml) curve was plotted using gallic acid. The total phenolics were expressed as mg gallic acid equivalent/gram dry weight. Negative control was prepared by adding 0.1 ml of DMSO instead of extracts.
Determination of saponins
Quantitative determination of saponin of samples was done according to Obadoni and Ochuko.[Citation21] The samples were ground, and 10 g of each sample were put into a conical flask, and 100 cm3 of 20% aqueous ethanol was added. The samples were heated over a hot water bath for 4 h with continuous stirring at 55°C. The mixture was filtered, and the residue was re-extracted with another 200 ml 20% ethanol. The combined extracts were reduced to 40 ml over a water bath at 90°C. The concentrate was transferred into a 250 ml of separating funnel, and 20 ml of diethyl ether was added and shaken vigorously and the aqueous layer was recovered. The purification processes were repeated, and 60 ml of n-butanol was added. The combined various extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evapouration, the samples were dried in the oven to a constant weight; the saponin content was calculated.
Determination of quinones
50 mg of the fine powder sample was soaked in 50 ml of distilled water for 16 h. This suspension was heated in a water bath (at 70°C) for 1 h. After the suspension was cooled, 50 ml of 50% methanol was added, and it was filtered. The clear solution was measured by spectrophotometer at a wavelength of 450 nm and compared with a standard solution containing 1 mg/100 ml of purpurin with a maximum absorption by a spectrophotometer at 450 nm.
Antibacterial activity
Bacterial strains: Multidrug resistant Escherichia coli and Staphylococcus aureus bacteria were previously isolated in our laboratory.[Citation12] The strains were subcultured and used throughout the study. Two bacterial strains Salmonella typhi and Pseudomonas aeruginosa were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India.
Preparation of inoculums
The inoculum was prepared as described by Tereshuck et al.[Citation22] from 24-h-old cultures by picking numerous colonies and suspending with sterile saline (NaCl, 0.9 %) solution. Absorbance was read at 530 nm and adjusted with the saline solution to match to that of a 0.5 McFarland standard solution, corresponding to about 1.5 × 108 Colony Forming Units (CFU). The bacterial cultures were maintained at −20 °C in glycerol stock and were sub-cultured in nutrient broth for 24 h at 30°C before it is used for experimental purpose.
Agar well diffusion method
Antibacterial activity of S. xanthocarpum extracts was carried out by the agar well diffusion assay discussed by Natarajan et al.[Citation23] 25 ml of the molten agar (45°C) were poured into sterile petri dishes. The working cell suspensions were prepared, and 100 μl was evenly spread on the surface of the agar plates of Mueller-Hinton agar (Hi-Media, India). Once the plates had been aseptically dried, 5 mm wells were punched into the agar with a sterile Pasteur pipette. The residual extracts were dissolved in their extracting solvents to yield the final concentration - 1 mg/ml - and sterilized by filtration (filter pore size 0.45 μm). A total of 100 µl (stock 1 mg/ml) of extracts was placed into the wells, and the plates were incubated at 37°C for 24 h. The respective wells 10% DMSO were used as negative control, and chloramphenicol (10 μg/ml) antibiotic was used as positive control. The diameter of growth inhibition zones around the well was noted. All the tests were performed in triplicates.[Citation15]
Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC)
The MICs of the plant extracts were determined in sterile 96-well microplates using the broth microdilution method recommended by the Clinical and Laboratory Standards Institute, M07-A8,[Citation24] as the lowest extract concentration suppresses the growth of microorganisms after 24 h of incubation at 37°C, whereas the MBC values defined as the lowest concentration of an antibacterial agent needed to inhibit the growth of a certain bacteria followed by subculturing using antibiotic-free media. In brief, extracts (100 µg/ml dissolved in 10% DMSO) and two-fold serial dilution were prepared in a 96-well microplate. Chloramphenicol was used as positive control. The solution without extract served as a blank and negative control. Each microplate well included 40 µl of the growth medium, 50 µl of the diluted sample extracts, and 10 µl of the inoculums (106 CFU/ml). The wells containing no growth in the plate were taken as MBC.
DPPH free radical scavenging activity
The plant extracts were diluted with methanol to make 20, 40, 60, 80, and 100 μl/ml dilutions. Two milliliters of each dilution was mixed with 1 ml of DPPH solution (0.2 mM/ml in methanol) and mixed thoroughly. The mixture was incubated in dark at 20°C for 40 min. Absorbance was measured at 517 nm by UV–Visible spectrophotometer with methanol as blank.[Citation25] Each experiment was performed in triplicates. The percentage scavenging of DPPH was calculated according to the following formula:
where Abs control is the absorbance of the control, and Abs sample is the absorbance of test.
Fourier transform-infrared (FT-IR) spectroscopy analysis
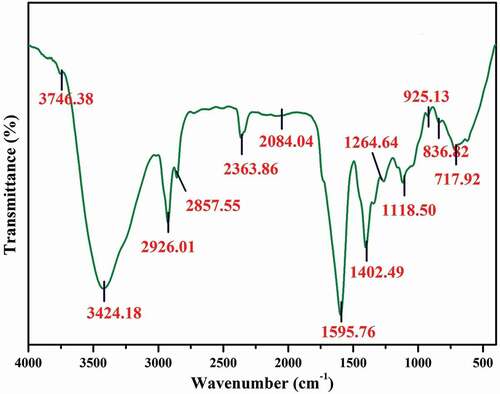
The FT-IR analysis of methanolic extract has been carried out as per the method of Jagmohan.[Citation26] The powdered plant samples were mixed with potassium bromide (KBr pellet) and subjected to a pressure of about 5 × 106 Pa to produce a clear transparent disc (diameter 13 mm) and thickness (1 mm). IR spectra in frequency region 400–4000 cm−1 were recorded at room temperature on a PerkinElmer Fourier Transform Spectrometer equipped with an air-cooled deuterated triglycine sulphate (DTGs) detector. For each spectrum, 100 scans were co-added at a spectral resolution of 4 cm. The frequencies for all sharp bands were accurate to 0.01 cm. All the spectral values were expressed in percentage (%) transmittance.[Citation27]
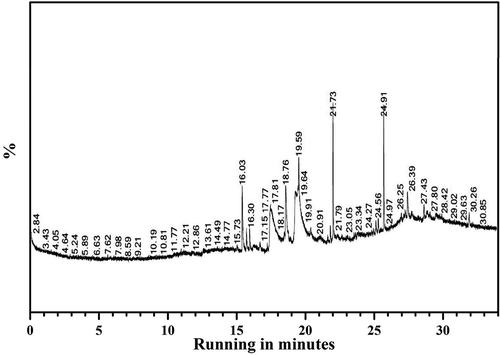
Gas chromatography-mass spectrometry (GC-MS) analysis
The chemical composition of leaf extract from S. xanthocarpum was determined by GC-MS using Hewlett-Packard series II gas chromatography equipped with HP-5 capillary columns (30 mm x 0.25 mm, 0.25 µm film thicknesses). Injector temperature was 280°C, with the rate of 1°C/min. Helium was used as a carrier gas with the flow rate of 1.0 ml/min. The identification of the constituents was achieved by the comparison of their retention indices and mass spectra with data generated under identical experimental conditions. The identification of compounds is mainly based on the comparison of their mass spectra and retention indices (RIs) with those recorded in the Wiley and NIST mass spectral databases and additional library data of the GC-MS system and literature data. The retention indices determined in relation to homologous n-alkanes series (C8-C24) under the same operating conditions. Components relative concentrations were obtained by peak area normalization, which consisted of an auto sampler and gas chromatography interfaced to a mass spectrometer (GC–MS) instrument employing with the following condition: capillary column−624 ms (30 m × 0.32 mm×1.8 µm) operating in an electron mode at 70 eV; helium (99.99%) was used as carrier gas at a constant flow of 1.491 ml/min and injection volume of 1.0 ml, injector temperature was 140°C; ion source temperature of 200°C. The oven temperature was programmed from 45°C. Mass spectra were taken at 70 eV.[Citation28,Citation29]
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using the SPSS 20.0 software.
Results
The phytochemical screening of S. xanthocarpum showed the presence of various types of chemical constituents. The flavonoid was present in all the extracts, whereas quinones was present in all the extracts except chloroform extract. Steroids and terpenoids were absent in all the extracts tested. Phenols and glycosides are occurred in acetone, ethyl acetate, hexane, and chloroform extract except methanol extract (). Preliminary phytochemical analysis is very important for the quantitative estimation of pharmacologically active natural compounds. The results of various quantitative phytochemical analyses of different solvent extracts of S. xanthocarpum are presented in . The level of carbohydrate was found higher in the methanol extract (7.2 mg/g) and lower in hexane extract (1.8 mg/g). The methanol extracts of this plant shown maximum amount of phenol (28.3 mg/g), flavonoids (25.2 mg/g), steroids (8.2 mg/g), quinones (7.3 mg/g), and terpenoids (6.3 mg/g) than other extracts.
Table 1. Phytochemicals content of S. xanthocarpum extracts.
Table 2. Quantitative phytochemical estimation of S. xanthocarpum leaf extracts.
The solvent extracts of S. xanthocarpum were tested against five bacterial pathogens using agar well diffusion method. The results show methanol extract have significant antibacterial activity against P. aeruginosa (12 ± 0.5 mm) followed by S. typhi, S. aureus, E. coli, and C. diphtheriae. Ethyl acetate extracts exhibit moderate activity against Pseudomonas aeruginosa (8 ± 1.2 mm) and Staphylococcus aureus (7 ± 1.0 mm) having broad spectrum activity, and no inhibition was found in S. typhi. Chloroform, hexane, and acetone extract exhibit least antibacterial activity against S. aureus, P. aeruginosa, and C. diphtheriae (). The MIC is interpreted as the lowest concentration that inhibits visible bacterial growth, whereas the minimum bactericidal concentration (MBC) is interpreted as the lowest concentration that can completely remove the microorganisms. The MIC and MBC values of bacteria were examined after 24 h of incubation. The MIC and MBC values of most of the test organisms were reported in the ranges of 3.2 to 6.9 µg/ml (for MIC) and 6.0 to 14.5 µg/ml (for MBC), respectively. The outcome from this study shows S. typhi and C. diptheriae having been reported as higher MIC as well as MBC values ().
Table 3. Antibacterial activity of different solvents extracts of S. xanthocarpum by agar well diffusion method.
Table 4. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of S. xanthocarpum different solvent extracts.
The DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging activity of the different extracts of S. xanthocarpum is shown in . This activity was increased by increasing the concentration of the samples. Antioxidant property of DPPH was mainly based on the ability of 2, 2- Diphenyl-1- picrylhydrazyl (DPPH), and a stable-free radical, to decolourize in the presence of antioxidants. The DPPH radical contains an odd electron, which is responsible for the absorbance at 517 nm and also for a visible deep purple colour. When DPPH accepts an electron donated by an antioxidant compound, the DPPH is decolourized, which can be quantitatively measured from the changes in absorbance. The IC50 value of the chloroform extract was found to be 197.245 µg/ml and showed the maximum radical scavenging activity. The free radical scavenging inhibition effects of various solvents extracts reported in the following order: chloroform> methanol> ethyl acetate > acetone >hexane. The IC50 value for ascorbic acid was found to be 239.36 μg/ml. IC50 values of S. xanthocarpum extracts were calculated and compared with standard value. Among different crude extracts tested, the chloroform extract exhibited better radical scavenging activity at 20 µg/ml concentration (73.84%) followed by other extracts.
Table 5. Percentage of DPPH radical scavenging activity of S. xanthocarpum.
The FTIR spectrum of the sample contains 12 major peaks at the range of 3746.38 cm−1 (O-H stretch alcohols), 3424.18 cm−1 (O-H stretch phenols), 2926.01 cm−1 (C-H stretch alkanes), 2857.55 cm−1 (C-H stretch alkanes), 2084.04 cm−1 (-C = C stretch alkynes), 1595.76 cm-1 (N-H bend primary amines), 1402.49 cm−1 (C-C stretch in ring aromatics), 1264.64 cm-1 (C-N stretch aromatic amines), 1118.50 cm-1 (C-N stretch aliphatic amines), 925.13 cm-1 (O-H bend carboxylic acids), 836.82 cm-1 (C-Cl stretch alkaloids), 717.92 cm-1 (C-H rock alkanes) cm-1 (; ). The chemical profile of the bioactive methanolic extract of S. xanthocarpum was done by GC-MS analysis. Totally six major bioactive compounds were identified, that is, 2,Octylcyclopropene-1-Heptanol (42.81%), Hexadecanoic acid (26.63%), 1-methylene-2b Hydroxymethyl-3,3-Dimethyl-4b-(3Methylbut-2enyl)-C (9.3%), Phytol (7.5%), (1,3,3-Trimethyl-2-Hydroxymethyl-3,3-Dimethyl-4–3-Methylbut-2-Enyl)-C (7.2%) and 3,7,11,15-Tetramethyl-2-Hexadecen-1-Ol (6.3%), respectively (; ).
Table 6. FTIR analysis of methanolic extract of S. xanthocarpum.
Table 7. GC-MS analyis of methanolic extract of S. xanthocarpum.
Discussion
Herbal plants and plant-derived drugs have been widely used in traditional cultures everywhere in the world and gained popularity in modern society as natural alternatives to produce new potential therapeutic agents for combating diseases.[Citation30] The qualitative analyses of phytoconstituents (alkaloids, flavonoids, phenolics, saponins, steroids, tannins, carbohydrate, glycosides, terpenoids, and quinones) of S. xanthocarpum were carried out from present study. Similarly, Yadav et al.[Citation31] and Jaberian et al.[Citation32] analyzed the phytochemical nature of various medicinal plants, and their results were supported with the findings of current investigation.
The methanolic extract of S. xanthocarpum exhibits the maximum number of phytochemicals than other extracts, which may be due to the polar nature of the solvents. This result was supported by Narender et al.,[Citation33] which found that the methanol used as leading solvent for extraction of variety of plant constituents than other solvents. Kalita et al.[Citation34] stated that the flavonol compounds from medicinal plants exhibited better antioxidant property. The outcome of present study observed the maximum amount of total flavonoid (25.2 mg/mg) and phenol (28.3 ± 2.0 mg/mg) contents from the leaf of S. xanthocarpum.
Many studies suggest the role of phenolics and saponins obtained from plants acted as potent antibacterial agents against human pathogenic bacteria.[Citation34,Citation35] The present study clearly focused on crude extracts of S. xanthocarpum that were evaluated for antibacterial activity against selected bacterial cultures. The significant zone of inhibition was noticed in the crude methanol and ethyl acetate extracts against P. aeruginosa and S. aureus. Similarly, Nithya et al.[Citation36] reported better inhibition zones (from the ethanol extracts of Thaaleesadhi chooranam) against E. coli, S. aureus, K. pneumoniae (around 12 mm). The highest antibacterial activity (14 mm) was observed in B. subtilis, and least activity was recorded in P. aeruginosa (8 ± 1.2 mm). Likewise, Aisha Ashraf et al.[Citation37] reported the methanol and hexane extracts showed an excellent antimicrobial activity against A. niger. The minimum inhibitory action (5 mm) was observed by methanol extract against E. coli. The MIC and MBC values of selected bacteria were observed after 24 h incubation and resulted better values, i.e., 3.2 to 6.9 µg/ml (for MIC) and 6.0 to 14.5 µg/ml (for MBC). The organism like S. typhi and C. diptheriae showed higher values for MIC as well as MBC. Similarly, Mahfuzul Hoque et al.[Citation38] reported S. aromaticum extract was found to be effective with a minimal inhibitory concentration (10 mg/ml) against B. cereus, S. aureus, E. coli, and P. aeruginosa. On the other hand, Mahboubi and Haghi[Citation39] reported Mentha pulegium plant as an effective antibacterial activity with least MIC and MBC values.
The antioxidant activity of different extracts of S. xanthocarpum was determined using DPPH assay. It showed the highest activity was obtained in the methanolic extracts. Previously, Patil Dinanath et al.[Citation40] reported the antioxidant activity of ethanol, chloroform, and ethyl acetate extracts of leaves and stems of S. xanthocarpum. Among them, ethanol extract of leaves and stem show better antioxidant activity. Likewise, Hoshyar et al.[Citation41] proved the antioxidant activity of lemon exhibited the highest antioxidant activity in all three extractions. Similarly, Saumya et al.[Citation42] reported Panax ginseng and Lagerstroemia speciosa, the percentage of inhibition was mainly dose-dependent manner, showing the IC50 value of 3.18 μg/ml and 6.15 μg/ml, respectively, when compared to control (IC50 value 3.35 μg/ml).
Conclusion
Solanum xanthocarpum contains many phytoconstituents such as flavonoids, carbohydrates, tannins, and phenols. Methanol extract of plant exhibited strong antibacterial effects against tested bacteria followed by other extracts due to the presence of high amount phenolics and flavonoids, and it was confirmed based on low minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC) values. The same extracts was found to better antioxidant activity. Totally six major bioactive compounds and their functional groups have been identified by GC-MS and FT-IR spectral analysis, respectively. The outcome of the study is strongly recommends further isolation and structural elucidation of bioactive compounds from the plant and their potential against the different biological action.
Acknowledgements
The authors wish to thank to the Department of Biotechnology, Periyar University for providing the necessary laboratory facilities. We thank VIT, Vellore for their instrumental support (GC-MS) for analysis of plant samples. The authors declare that they have no conflict of interest. CR and DN designed the related subject, managed all of the processes, conceived this study, and drafted the manuscript. MN and CR carried out the experiments and organized the data. CR and DN coordinated the project and discussed the results. All authors read and approved the final manuscript.
References
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T. C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease and Cancer. Pharmacology Review 2000, 52, 673–751.
- Nichols, J. A.; Katiyar, S. K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Archive Dermatology Research 2010, 302, 71–83. DOI: 10.1007/s00403-009-1001-3.
- Rajkumar, V.; Guha, G.; Kumar, A. Antioxidant and Antineoplastic Activities of Picrorhiza Kurroa Extracts. Food Chemistry and Toxicology 2011, 49, 363–369. DOI: 10.1016/j.fct.2010.11.009.
- Kaliora, A. C.; Dedoussis, G. V. Z.; Schmidt, H. Dietary Antioxidants in Preventing Atherogenesis. Atherosclerosis 2006, 187, 1–17. DOI: 10.1016/j.atherosclerosis.2005.11.001.
- Koncic, M.; Kremer, D.; Karlovic, K.; Kosalec, I. Evaluation of Antioxidant Activities and Phenolic Content of Berberis Vulgaris L. And Berberis Croatica Horvat. Food Chemistry and Toxicology 2010, 48, 2176–2180. DOI: 10.1016/j.fct.2010.05.025.
- Dismukes, W. E. Antifungal Therapy: Lessons Learned over the Past 27 Years. Clinical Infectious Disease 2006, 42, 1289–1296. DOI: 10.1086/503043.
- Chung, P. Y.; Chung, L. Y.; Ngeow, Y. F.; Goh, S. H.; Imiyabir, Z. Antimicrobial Activities of Malaysian Plant Species. Pharma Biology 2004, 42, 292–300. DOI: 10.1080/13880200490511837.
- Nair, R.; Chanda, S. V. Antibacterial Activity of Some Medicinal Plants of Saurashtra Region. Journal of Cell Tissue and Research 2004, 4, 117–120.
- Edeoga, H. O. Phytochemical Constituents of Some Nigerian Medicinal Plants. African Journal of Biotechnology 2005, 4(7), 685–688. DOI: 10.5897/AJB2005.000-3127.
- Parmar, S.; Gangwal, A.; Sheth, N. Solanum Xanthocarpum (Yellow Berried Night Shade): A Review. Scholars Research Library 2010, 2(4), 373–383.
- Kumar, N.; Prakash, D.; Kumar, P. Wound Healing Activity of Solanum Xanthocarpum Schrad. And Wendl. Fruits. Indian Journal of Natural Products and Resources 2010, 1, 470–475.
- Kar, D. M.; Maharana, L.; Pattnaik, S.; Dash, G. K. Studies on Hypoglycaemic Activity of Solanum Xanthocarpum Schrad. & Wendl. Fruit Extract in Rats. Journal of Ethanoparmacology 2006, 108(2), 251–256. DOI: 10.1016/j.jep.2006.05.016.
- Bhutani, K.; Paul, A. T. K.; Fayad, W.; Linder, S. Apoptosis Inducing Activity of Steroidal Constituents from Solanum Xanthocarpum and Asparagus Racemosus. Phytomedicine. 2010, 17(10), 789–793. DOI: 10.1016/j.phymed.2010.01.017.
- Harborne, J. B. Phytochemical Methods- A Guide to Modern Techniques of Plant Analysis, 2nd ed.; Chapman and Hall: London, 1984; pp 4–6.
- Kuppusamy, P.; Yusoff, M. M.; Pragas Maniam, G.; Jauhari Arief Ichwan, S.; Soundharrajan, I.; Govind, N. Nutraceuticals as Potential Therapeutic Agents for Colon Cancer: A Review. Acta Pharmaceutica Sinica B. 2014, 4(3), 173–181. DOI: 10.1016/j.apsb.2014.04.002.
- Solich, P.; Sedliakova, V.; Karlicek, R. Spectrophotometric Determination of Cardiac Glycosides by Flow-Injection Analysis. Analytica Chimica Acta 1992, 269(2), 199–203. DOI: 10.1016/0003-2670(92)85403-S.
- Harborne, J. B. Phytochemical Methods – A Guide to Modern Techniques of Plant Analysis, 3rd ed. Springer Pvt. Ltd: New Delhi, 2005.
- Sabir, S. M.; Hyat, I.; Gardezi, S. D. Estimation of Sterols in Edible Fats and Oils. Pakistan Journal of Nature 2003, 2, 178–181. DOI: 10.3923/pjn.2003.178.181.
- Van-Burden, T. P.; Robinson, W. C. Formation of Complexes between Protein and Tannin Acid. Journal of Agricultural and Food Chemistry 1981, 1, 77–82.
- Velioglu, Y. S.; Mazza, G.; Gao, L.; Oomah, B. D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. Journal of Agricultural and Food Chemistry 1998, 46, 4113–4117. DOI: 10.1021/jf9801973.
- Obadoni, B. O.; Ochuko, P. O. Phytochemical Studies and Comparative Efficacy of the Crude Extracts of Some Haemostatic Plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Sciences 2001, 8, 203–208.
- Tereshuck, M. L.; Riera, M. V. Q.; Castro, G. R.; Abdala, L. R. Antimicrobial Activity of Flavonoid from Leaves of Tagetes Minuta. Journal of Ethnopharmacology 1997, 56, 227–232. DOI: 10.1016/S0378-8741(97)00038-X.
- Natarajan, D.; John Britto, S.; Srinivasan, K.; Nagamurugan, N.; Mohanasundari, C.; Perumal, G. Antibacterial Cctivity of Euphorbia Fusiformis - A Rare Medicinal Herb. Journal of Ethnopharmacology 2005, 102(1), 123–126. DOI: 10.1016/j.jep.2005.04.023.
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Wayne: Clinical Laboratory Standards Institute. 2009. [Online] Available from: http://antimicrobianos.com.ar/ATB/wpcontent/uploads/2012/11/03-CLSI-M07-A9-2012.pdf [Accessed on 21st March, 2014]
- Krishnaveni, S.; Balasubramanian, T.; Sadasivam, S. Phenol Sulphuric Acid Method. Food Chemistry 1984, 15, 229–232. DOI: 10.1016/0308-8146(84)90007-4.
- Mohan, J. Organic Spectroscopy Principles and Applications, 2nd ed.; Narosa Publishing House: Daryagani, Delhi, 2005.
- Al-Mekhlafi, F. A.; Abutaha, N.; Ashraf, M. A.; Mashaly Fahd, A.; Nasr Khalid, E.; Ibrahim Mohamed, A.; Wadaan, H. Biological Activity of Xanthium Strumarium Seed Extracts on Different Cancer Cell Lines and Aedes Caspius, Culex Pipiens (Diptera: Culicidae). Saudi Journal of Biological Sciences 2017, 24, 817–821. DOI: 10.1016/j.sjbs.2016.07.003.
- Suman, T. Y.; Radhika Rajasree, S. R.; Jayaseelan, C.; Regina Mary, R.; Gayathri, S.; Aranganathan, L.; Remya, R. R. GC-MS Analysis of Bioactive Components and Biosynthesis of Silver Nanoparticles Using Hybanthus Enneaspermus at Room Temperature Evaluation of Their Stability and Its Larvicidal Activity. Environmental Science and Pollution Research 2016, 23, 2705–2714. DOI: 10.1007/s11356-015-5468-5.
- Hussaina, A. I.; Shahid Chathaa, S. A.; Mustafa Kamal, G.; Adnan Alia, M.; Asif Hanif, M.; Ibrahim Lazhar, M. Chemical Composition and Biological Activities of Essential Oil and Extracts from Ocimum Sanctum. International Journal of food properties 2017, 20(7), 1569–1581. DOI: 10.1080/10942912.2016.1214145.
- Azab, S.; Abdel-Daim, M.; Eldahshan, O. A. Phytochemical, Cytotoxic, Hepatoprotective and Antioxidant Properties of Delonix Regia Leaves Extract. Medicinal Chemistry Research 2013, 22, 4269–4277. DOI: 10.1007/s00044-012-0420-4.
- Yadav, D. K.; Singh, N.; Dev, K.; Sharma, R.; Sahai, M.; Palit, G.; Maurya, R. Anti-Ulcer Constituents of Annona Squamosa Twig. Fitoterapia. 2011, 82, 666–675. DOI: 10.1016/j.fitote.2011.02.005.
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical Composition and in Vitro Antimicrobial and Antioxidant Activities of Some Medicinal Plants. Food Chemistry. 2013, 136, 237–244. DOI: 10.1016/j.foodchem.2012.07.084.
- Narender, P. D.; Ganga, R.; Sambasiva, E.; Mallikarjuna, T.; Praneeth, V. S. Quantification of Phytochemical Constituents and in Vitro Antioxidant Activity of Mesua Ferea Leaves. Asian Pacific Journal of Tropical Biomedicine 2012, 2, 539–542. DOI: 10.1016/S2221-1691(12)60269-X.
- Kalita, P.; Barman, T. K.; Pal, T. K.; Kalita, R. Estimation of Total Flavonoids Content (TFC) and Antioxidant Activities of Methanolic Whole Plant Extract of Biophytum Sensitivum Linn. Journal of Drug Delivery Therapeutics 2013, 3, 33–37. DOI: 10.22270/jddt.v3i4.546.
- Maddox, C. E.; Laur, L. M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella Fastidiosa. Current Microbiology 2010, 60, 53–58. DOI: 10.1007/s00284-009-9501-0.
- Nithya, V.; Dahineeswari, R.; Chowdhury, S. R.; Sivakumar, S. Antibacterial Activity of Thaaleesaadhi Chooranam against Human Pathogens. International Journal of Drug Discovery and Herbal Research 2011, 1, 4.
- Aisha, A.; Raja Sarfraz, A.; Rashid, M. A.; Shahid, M. Antioxidant, Antimicrobial, Antitumor, and Cytotoxic Activities of an Important Medicinal Plant (Euphorbia Royleana) from Pakistan. Journal of Food Drug analysis 2015, 23, 109–115. DOI: 10.1016/j.jfda.2014.05.007.
- Mahfuzul Hoque, M.; Bari, M. L.; Juneja, V. K.; Kawamoto, S. Antimicrobial Activity of Cloves and Cinnamon Extracts against Food Borne Pathogens and Spoilage Bacteria and Inactivation of Listeria Monocytogenes in Ground Chicken Meat with Their Essential Oils. Journal of Food Science and Technology 2007, 72, 9–21.
- Mahboubi, M.; Haghi, G. Antimicrobial Activity and Chemical Composition of Mentha Pulegium L. Journal of Ethnopharmacology 2008, 119, 325–327. DOI: 10.1016/j.jep.2008.07.023.
- Dinanath, P. Antioxidant Effect of the Stem and Leaves of Solanum Xanthocarpum. Unique Journal of Ayurvedic Herbal Medicine 2013, 01(03), 68–70.
- Hoshyar, R.; Mostafavinia, S.; Zarban, A.; Hassanpour, M.; Partovfari, M.; Taheri, A.; Pouyan, M. Correlation of Anticancer Effects of 12 Iranian Herbs on Human Breast Adenocarcinoma Cells with Antioxidant Properties. Free Radicals and Antioxidants 2015, 5(2), 65–73. DOI: 10.5530/fra.
- Saumya, S. M.; Basha, M. In Vitro Evaluation of Free Radical Scavenging Activities of Panax Ginseng and Lagerstroemia Speciosa: A Comparative Analysis. International Journal of Pharmacy Pharmaceutical Sciences 2011, 3, 1.
- Rajeswari, G.; Murugan, M.; Mohan, V. R. GC-MS Analysis of Bioactive Components of Hugonia Mystax L. (Linaceae). Research Journal of Pharmaceutical and Bio Chemical Science 2012, 3(4), 301–308.
- Hamidi, N.; Ziane, L.; Djellouli, M.; Lazouni, H. A. Chemical Characterization by GC-MS from the Aerial Parts of Fagonia Longispina (Zygophyllaceae). Asian Journal of Pharmaceutical and Clinical Research 2016, 9(1), 175–176.
- Das, M.; Himaja, M. Phytochemical Screening, GC-MS Analysis and Biological Activities of Ipomoea Eriocarpa Leaf Extracts. International Journal of Pharmaceutical Sciences 2014, 6(4), 592–594.
- Omnia Gamal, E. D.; Eman, S.; Amal, H.; Rokia, A.; Abdallah, M.; Nabil, A.; Abdel, S. Phytochemical and Biological Investigation of Spergularia Marina (L.) Griseb. Growing in Egypt. Natural Product Science 2014, 20(3), 152–159.
- Mercy Jasmine, J. K.; Latha, K.; Vanaja, R. Determination of Bioactive Components of Decholestrate, a Polyherbal Formulation by GC-MS Analysis. New York Science Journal 2013, 6(5), 1–5.
- Susitra Manjari, M.; Karthi, S.; Ramkumar, G.; Muthusamy, R.; Natarajan, D.; Shivakumar, M. S. Chemical Composition and Larvicidal Activity of Plant Extracts from Clausena Dentata (Willd) (Rutaceae) against Dengue, Malaria, and Filariasis Vectors. Parasitology Research 2014, 113, 2475–2481. DOI: 10.1007/s00436-014-3896-7.
- Anupama Prasad, D.; Rajendra Prasad, B.; Krishna Prasad, D.; Puneeth Shetty, K.; Sunil Kumar, N. GC-MS Compositional Analysis of Essential Oil of Leaf and Fruit Rind of Citrus Maxima (Burm.) Merr. From Coastal Karnataka. Indian Journal of Applied Pharmaceutical Science 2016, 6(5), 68–72. DOI: 10.7324/JAPS.2016.60511.
- Peng, W.; Dongli, L.; Zhang, M.; Shengbo, G.; Bo, M.; Shasha, L.; Ohkoshi, M. Characteristics of Antibacterial Molecular Activities in Poplar Wood Extractives. Saudi Journal of Biological Sciences 2015, 24(2), 399–404. DOI: 10.1016/j.sjbs.2015.10.026.