ABSTRACT
The formation and stabilization of fat crystals in palm oil (PO) by the templating effects of sorbitan tristearate (STS) and sucrose stearate (S-370) were examined. We observed that the crystallization mechanism occurred via heterogeneous nucleation, which was induced by co-crystallization or seeding, after the addition of STS or S-370, respectively. Overall, both emulsifiers, STS and S-370, caused an increase in the hardness of PO, which was related to changes in the microstructure. In addition, the intensity of this effect was shown to be dependent on the emulsifiers concentration. In differential scanning calorimetry analysis, S-370 proved to be associated predominantly to the crystallization of high-melting triacylglycerols (TAGs), while STS showed to actuate the crystallization of high and low-melting TAGs. Moreover, the addition of STS improved the polymorphic stability of the PO, while the use of S-370 resulted in a soft texture at lower temperatures.
Introduction
Crystallization behavior of the palm oil (PO) is extremely important from an industrial perspective, as it presents the crystal habit β′, which is usually the most functional polymorph in many fat products. This characteristic combined with PO properties, such as plasticity and oxidative stability, ensures its application in margarines, spreads, bakery fat, confectionery, and shortenings. Although PO is a β′ fat, the crystals of PO require a long time for the transition α → β′, which is an inappropriate characteristic from the perspective of an industrial process.[1] Therefore, depending on the processing conditions and storage, PO has the potential to change the crystalline behavior of the product (i.e., β′ → β).[2] In this context, ensuring the stability of the β′ polymorph in PO-based products is of special interest for industry, because of the great economic importance associated with the use of this raw material.[3]
It is known that adding foreign materials (referred to as templates or additives) in the crystallizing liquid is widely applied to modify the crystallization behavior of inorganic and organic substances, including fat crystals.[4] In addition to their well-known functions of emulsification and stabilization of emulsions, emulsifiers can act as a template, performing a dynamic control of the crystallization process in natural fats.[5]
In general, the effect of emulsifiers on the fat crystallization process is related to different crystalline organizations and to the creation of imperfections. Some emulsifiers can delay transformations via steric hindrance, while others lead to these transformations, thus favoring polymorphic transitions.[6] Two different procedures have been reported in the literature to analyze the effect of emulsifiers on fat crystallization. The first mechanism describes the action of these additives as heteronuclei, whereby the crystallization is accelerated by direct catalytic action as impurities. Accordingly, during the crystal growth, emulsifiers would be adsorbed at the surface of the crystals, thus modifying the incorporation rate of triacylglycerols (TAGs) and crystal morphology (seeding effect). The second mechanism considers that TAGs and emulsifiers are susceptible to co-crystallization, due to the similarity between their chemical structures (co-crystallization effect).[3,7,8] By considering bulk crystallization properties, the predominant factor in the crystal structure of emulsifiers is the hydrophilic portion, which is the larger portion of the molecule. The size of the hydrophilic group, along with the extension and spatial distribution of hydrogen bonding among adjacent groups, has a much larger influence on the molecular packing of the crystal than the nature of the fatty acid chain.[9]
Sorbitan tristearate (STS or Span 65) emulsifier has been widely used for its ability to efficiently modify the crystal morphology and consistency of fats, for example, as an anti-bloom agent in confectionery products containing cocoa butter and in cocoa butter substitutes, thereby being indicated as a potential controller of crystallization. The STS emulsifier also showed to be particularly effective in stabilizing the polymorph β′ of margarines and modifying the solid fat content (SFC), promoting fusion profiles appropriate for the body temperature.[10] Moreover, in the pursuit of healthier structured fats, STS has also been applied in the production of organogels.[11]
In addition to STS, sucrose esters have also been employed as emulsifiers in the food industry, albeit to a lesser degree. As application examples, higher substitution esters (e.g., hexa-, hepta-, and octa-esters) are used as fat replacers, and lower substitution esters (e.g., mono-, di-, and tri-esters) find use as oil-in-water as well as water-in-oil emulsifiers. This offers advantages over other commercially available emulsifiers. In addition to the emulsification function, sucrose esters can be used in starch interaction, protein interaction, sugar crystallization, aeration, and foam stabilization. These functions can affect production and the quality of the end product.[12] On the other hand, few studies explore the effect of sucrose esters on the induction period and the rate of crystallization and development of polymorphic forms in fatty systems. In Refs. [9,13], Domingues et al. investigated some effects of the sucrose esters S-370 and B-370 on the crystallization properties of interesterified fat and the palm mid fraction, respectively.
In this study, we compared the effects of structure (sorbitan vs. sucrose) and examined the template effect of STS and S-370 on the formation and stabilization of fat crystals in PO. In doing so, we evaluated the microstructure and the three-dimensional structure, compatibility diagrams, hardness, melting profile, isothermal and non-isothermal crystallization, and thermal and polymorphic behavior of the PO and its blends with of the emulsifiers. In addition, we also investigated the crystallization characteristics of the pure emulsifiers.
Material and methods
Raw materials
Refined, bleached, and deodorized PO was supplied by Agropalma (Belém-PA, Brazil). This PO contains 6.21% of diacylglycerols and is composed mainly of the following fatty acids: 40.26% palmitic acid (P), 4.83% stearic acid (St), 41.00% oleic acid (O), and 8.67% linoleic acid (L). The emulsifiers, STS Grinsted-B-30 in powder (hydrophilic-lipophilic balance [HLB]—2.1; melting point, 52°C; DuPont, Brazil) and S-370 in powder (HLB—3; melting point, 66°C; Mitsubishi-Kagaku Foods, Japan), were used in proportions of 1%, 3%, and 5% w/w.
Sample preparation
The PO was heated to the complete of melting point. Then, samples of PO containing 1%, 3%, and 5% of STS and 1%, 3%, and 5% of S-370 were prepared. Each melted sample at 70°C was blended manually with a glass stick until the emulsifier completes dissolution.
Composition of tags
The TAG composition was determined according to the AOCS method Ce 5-86 (2009) using the gas chromatograph GC CGC AGILENT 6850 Series GC System and capillary column DB-17 HT AGILENT (50% phenyl-methylpolysiloxane, 15 m long, 0.25 mm internal diameter, 0.15 mm film). The analysis conditions were as follows: split ratio, 1:100; column temperature, 250°C, programmed up to 350°C at a rate of 5°C/min; carrier gas, helium at a flow rate of 1 mL/min; injector temperature, 360°C; detector temperature, 375°C; injection volume, 1 μL; sample concentration, 100 mg/5 mL tetrahydrofuran. The TAG groups were identified by comparing the retention times as described by Antoniosi Filho, Mendes, and Lanças.[14] The analysis was performed in triplicate.
Regiospecific distribution
The regiospecific distribution of the samples was determined by high-resolution 13C nuclear magnetic resonance (NMR) spectroscopic analysis of the TAG acyl chains. The analyses were performed on an NMR spectrometer (Bruker Advance DPX 300; Silberstreifen, Rheinstetten, Germany). The determination of 13C was performed at a frequency of 75.8 MHz with a 5-mm multiple nuclear probe operating at 30°C, as previously reported by Vlahov.[15,16] The results are presented as the compositions of oleic and linoleic acids at the sn-2 and sn-1,3 positions of TAGs. The analysis was performed in triplicate.
Compatibility diagrams and melting point
First, the SFC was determined using an NMR spectrometry (Bruker Minispec PC120) and a TCON 2000 high precision dry bath (0–70°C) (Duratech, USA), according to the AOCS method Cd 16b-93.[17] The readings of the SFC were performed at temperatures of 0, 10, 15, 20, 25, 30, and 40°C. The analysis was performed in triplicate. Then, the melting point was measured by a polynomial equation at the temperature at which the SFC is the same as 4%.[18] The compatibility diagrams were constructed by plotting the SFC versus emulsifier ratio for each temperature.
Hardness measurement
The hardness was determined using the PC-controlled texture analyzer TA-XT2i (Stable Micro Systems). The samples were completely melted for the destruction of the crystalline history. Then, the samples were conditioned at 5°C for 24 h and stabilized at the analysis temperature for 24 h. The conditioning was carried out in an incubator (Biosystems) with temperature control, and the analysis was performed at the following temperatures: 10, 15, 20, 25, and 30°C. For the analysis, an acrylic cone with a non-truncated tip at an angle of 45° was used. The determinations were carried out in triplicate for each sample under the following conditions: distance = 10 mm; speed = 2 mm/s; time = 5 s.[19] From these conditions, the compressive force was obtained in Newton (N). The hardness diagrams were constructed by plotting the force versus emulsifier ratio for each temperature.
Microstructure
The microstructure (crystal size and morphology) of the samples was determined using polarized light microscopy. The samples were melted at 70°C in an oven to destroy the crystalline history. Using a capillary tube, a drop of the sample was placed on a preheated slide under a controlled temperature (similar to the temperature used to melt the crystals) and covered with a coverslip. The slides were prepared in duplicate and crystallized at 20°C in an oven for 2 h. The crystal morphology was evaluated using a polarized light microscope (BX51, Olympus) coupled to a digital video camera (MicroPublisher 5.0 Mpixel, Media Cybernetics). The slides were placed on the heating plate support and maintained at the same temperature used for crystallization. Images were captured using the software Image-Pro Plus version 7.01 (Media Cybernetics), with polarized light at 40× magnification. Three visual fields were selected for each slide, of which only one was chosen to evaluate the crystals. The parameters selected from Image Pro-Plus software for the analysis of images include the following: mean value of the crystals diameter, fractal dimension, and percentage of the crystallized area. Microstructure of the pure emulsifiers: the STS and S-370 emulsifiers were melted directly on the slide at 100°C. Then, the slides were covered with a coverslip and immediately analyzed at 25°C. The slides were prepared in triplicate.
Three-dimensional crystalline network
The samples were melted and placed in 15-mL plastic capsules with hermetic lids. Then, they were stabilized for 24 h at 5°C, followed by an additional 24 h at 20°C. In the analysis, the capsules containing the samples were submerged in liquid nitrogen to spontaneous fracture of the samples. Then, the fractured areas of the samples were immediately analyzed using a scanning electron microscope (Hitachi TM-3000) with 1000× magnification and 15 kV.[9]
Isothermal crystallization
The blends were melted and kept at 70°C/15 min in a high precision dry bath (TCON 2000, Duratech, Carmel, USA) for the complete destruction of their crystalline history. The increase in SFC due to crystallization time was monitored by a NMR spectrometer Bruker pc120 Minispec (Silberstreifen, Rheinstetten, Germany), with the reading compartment stabilized at 20°C. The data acquisition was automatic, with measurements taken every minute, during 90 min. The STS and S-370 emulsifiers were completely melted at 100°C and immediately analyzed at 25°C, with manual data acquisition during 150 s.
Thermal behavior
Thermal analysis of the samples and pure emulsifiers by differential scanning calorimetry (DSC) was performed using a calorimeter (Model Q2000, TA Instruments) with indium (TA Instruments) as the calibration standard. The samples were weighed (4–10 mg) in aluminum hermetic pans. The analysis was performed in triplicate, using the AOCS method Cj 1-94 (2009)[17] under the following conditions:
Crystallization: (1) thermal equilibrium at 80°C (10 min) and (2) a cooling ramp of 10°C/min to −60°C.
Melting: (1) thermal equilibrium at −40°C (30 min) and (2) a heating ramp of 5°C/min to 80°C.
X-ray diffraction
The polymorphic form of the fat crystals was determined using X-ray diffraction in accordance with the AOCS method Cj 2-95.[17] The analyses were performed on a Philips (PW 1710) diffractometer using the Bragg–Brentano geometry (ϴ:2ϴ) with CuKα radiation (λ = 1.54056 Å, 40 kV, 30 mA). The measurements were done using steps of 0.02° and 2ϴ, time of acquisition of 2 s, and scans from 15° to 27° (2ϴ scale). The samples were melted until complete destruction of the crystalline historical and kept at 25°C for 24 h in an oven for stabilization. The pure emulsifiers were only stabilized at 25°C for 24 h. The polymorphic forms were identified using the short-spacing characteristics of the crystals. The contents of the different types of crystals were estimated from the relative intensity of the short spacings (β′ = 3.80 and 4.20 Å, and β = 4.60 Å).[17,20,21]
Statistical analysis
Statistical analysis for the melting point, mean crystal diameter, and fractal dimension involved the analysis of variance and Tukey’s test to determine significant differences between the means at 5% significance level (p < 0.05) using Statistica 8.0 (Statsoft, USA).
Results and discussion
TAG composition and regiospecific distribution
The TAG composition of the PO is characterized by a mixture of TAGs with low, medium, and high melting points. The trisaturated TAGs (SSS) with high melting points represent 16.72% of the composition and are mainly composed of MPP, PPP, and PPSt species, which are considered as crystallization inducers in PO ().[22] According to Calliauw et al.,[23] PPP is an excellent inductor for POP; however, it presents limited miscibility between its molecules, which often leads to the formation of two distinct crystalline fractions. The high concentration of PPP species can favor the formation of POP, POSt, and SOS crystals, which are dense crystals with a high melting point.[2,24]
Table 1. Triacylglycerol composition and regiospecific distribution of the RDB palm oil.
The fraction of the intermediate melting point, represented by the symmetric disaturated species (SUS), is about 40.05%, consisting of MOP, POP, PLP, and POS. The symmetry of this group of TAGs is verified using regiospecific distribution analysis, as shown in , wherein approximately 72% of the saturated FAs are in the sn-1,3 position. The higher content of symmetric TAGs in the PO combined with the considerable proportion of diacylglycerols (6.21%) can result in a slow crystallization process.[25] In addition, crystal lattice stability also is compromised because of the undesirable increase in the crystal size by the limited miscibility between POP and POO species, resulting in the formation of large POP crystals surrounded by POO.[26]
Compatibility diagrams and hardness
As well known, PO presents a wide melting range as compared to the other vegetable oils, which makes it a suitable raw material for fractionation. According to Smith,[27] TAGs, such as PPP, present a high melting point, melting at 69°C, and OOO presents a low melting point, melting at 4°C. While other TAGs, such as POP, melt in intermediate temperatures of around 38°C. Therefore, PO is semi-solid at room temperature, with a melting range between 35 and 45°C.[27]
The PO sample shows a final melting point of 34.55°C. In , we can observe that the addition of 1%, 3%, and 5% of S-370 increases the final melting temperature of PO to 36.32, 39.02, and 41.58°C, respectively. On the other hand, pure STS, which presents a high melting point, does not significantly modify the melting point of the PO crystals. These results agree with those found in Ref. [28], wherein STS acts as a crystal modifier in some fats, thereby avoiding the formation of crystals with a high melting point. The higher melting point of PO containing S-370 might be related to the fact that the sucrose esters with a low HLB value present a higher recrystallization rate.[29]
Figure 1. Compatibility diagrams (a, c), hardness (b, d), and melting point of the palm oil (PO) and its blend with STS and S-370.
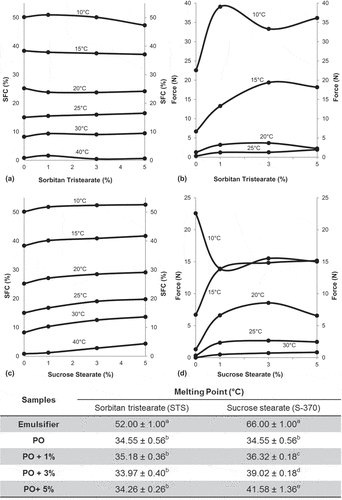
In and , we can visualize the compatibility diagrams of PO containing the STS and S-370 emulsifiers. Either an isothermal solid or a compatibility diagram denoting the SFC values at different temperatures in relation to the compositions of the blend is useful to understanding the interactions between of the mixture components.[30] We can observe that the SFC value increases for the sample containing 1% of STS at 10, 25, 30, and 40°C and decreases at temperatures such as 15 and 20°C, which causes depressions in the curves of the diagram. Moreover, the addition of 3% and 5% of STS promotes a slight increase in the SFC value at the temperature of 25 and 30°C. This interaction can be attributed to the preferential action of STS on the MPP, PPP, and PPSt TAGs, which are responsible for the increasing the SFC in the palm-based fats.[31]
The cloud point is an important property for PO fractions, in special for palm olein. At approximately 20°C, some TAGs crystallize out of solution and the oil becomes cloudy. At lower temperatures, the cloudy oil then becomes solid. According to Smith,[27] many emulsifiers have an influence on the clouding of the PO. Note that STS influenced the cloud point and shows resistance to crystallize at 20°C. On the other hand, the addition of S-370 resulted in higher SFC values, which indicated the clouding of the PO at all temperatures studied herein. The results observed in the compatibility diagram () show that a lesser degree of cooling can be sufficient for the crystallization of the mixture of PO + S-370. This phenomenon modifies the crystal morphology and reduces the viscosity of the melted material, which could be a potential solution for the TAGs intersolubility problem.[32]
In and , we can observe that the hardness of the PO increased with addition either of STS or S-370, which improves the technological characteristics of the PO. At temperatures of 10, 15, 20, and 25°C, the STS concentrations lead to hardness increase of the PO, despite a lower solids formation compared to PO. On the other hand, the addition of S-370 decreases the hardness of PO at 10°C and increases it at 15, 20, 25, and 30°C. According to Campos, Narine, and Marangoni,[33] the mechanical strength of a material is a function of the number of interactions between the particles, which in turn depends on the SFC and morphology of crystals.[34] If we consider two identical fat systems with equivalent bulk but different sizes and proportions of crystals, the interactions between the particles will be different. We can consider that, due to use static crystallization and slow cooling, the addition of S-370 at 10°C promotes the growth of large size crystal, and it is known that larger particles would be stabilized by weak attractive forces. Domingues et al.[9] have found similar results studying the effect of STS and S-370 on the interesterified fat crystallization. Puppo et al.[35] found that blends of milk-fat fraction and sunflower oil containing S-170 had lower penetration force at 10°C.
Microstructure and three-dimensional structure
shows the results of the microstructural elements of the PO sample and its blends with STS and S-370 at concentrations of 1%, 3%, and 5%. We observed that the addition of both emulsifiers increases the crystalline elements and reduces the mean crystal diameter. The addition of STS induces the formation of many crystals, which are more uniform and have smaller diameters (4.50–5.91 μm), which indicates the development of a well-structured network. Thus, the addition of STS in PO can have a role of crystals templates, promoting a change in habit of the PO crystals. A large number of small crystals result from the adsorption of the TAGs over the STS surface, followed by the crystallization process. This result implies a higher proportion of the crystallized area and better spatial distribution, thereby increasing the fractal dimension of the samples with the addition of STS (1.81–1.82). At temperature 20°C, the crystals formed by the addition of STS showed an intermediate melting point. Basso et al.[36] obtained similar results in relation to the number of crystals formed from the addition of monopalmitin in the PO. However, the crystal diameter was in the range of 7–99 μm, and this variance in the diameter suggests that the addition of monopalmitin did not lead to the formation of a well-structured network.
Figure 2. Microstructure (A1, B1, C1, D1, E1, F1, G1), three-dimensional structure (A2, B2, C2, D2, E2, F2, G2), and microstructural parameters (mean diameter, fractal dimension, and percentage of the crystallized area) of the palm oil (PO) and its blend with STS and S-370 at 20°C. The bar represents 200 μm.
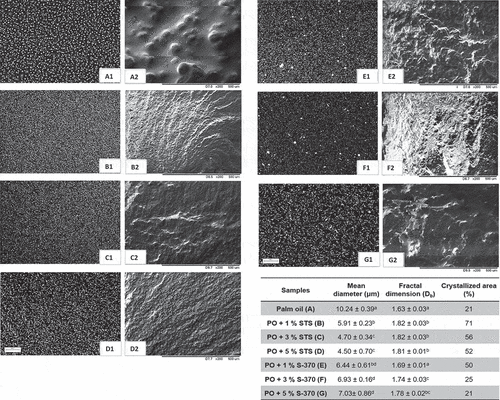
The addition of S-370 leads to the formation of fewer crystals with smaller diameters (6.44–7.03 μm) when compared with the PO crystals. The S-370 emulsifier improves the spatial distribution of the elements in the network by increasing the fractal dimension of the sample; however, only the addition of 1% S-370 influences the proportion of the crystallized area. As seen in , S-370 promotes the formation of heterogeneous crystals in size and form. We found that the S-370 played a seeding effect for PO crystallization.
The ability of a crystal network to immobilize liquid oil greatly depends on the crystal size. According to Campos, Narine, and Marangoni,[33] a large number of small particles would be attached by stronger stabilizing forces, and these interactions might lead to increased resistance to penetration. These results are in agreement with hardness ( and ), wherein the addition of either STS or S-370 resulted in greater hardness when compared to the pure PO (excepting for 10°C).
The texture properties, i.e., the hardness or mouthfeel of fat-based products, are closely related to the crystals organization in the crystalline lattice. In , we can observe the three-dimensional structure of the PO and its blends containing STS and S-370. Note that large crystals are formed in the PO, and changes are observed in the three-dimensional structure of the blends. The STS emulsifier promotes a compact structure and is better organized. In contrast, S-370 produces overlapping structures, indicative of a greater disorder in the formation of the three-dimensional network. Domingues et al.[9] observed that STS addition in interesterified fat improves the interaction between the crystals, which provides a cohesive three-dimensional structure. On the other hand, the addition of sucrose ester promotes the formation of loose crystals, resulting in a more heterogeneous structure. A similar analysis was performed by Błaszczak and Fornal,[37] who reported images of shortenings of margarine.
Isothermal and non-isothermal crystallization behavior
The crystallization behavior of the PO was modified by the addition of STS and S-370. shows that both emulsifiers accelerate crystallization of PO in either isothermal or non-isothermal condition. We highlight that the induction period is an important crystallization parameter and usually associated with the nucleation rate.[32] At a temperature of 20°C, the induction period of PO is approximately of 13 min ( and ), whereas the PO containing 1%, 3%, and 5% of S-370 shows induction periods of 4, 2, and 2 min, respectively. On the other hand, the formation of stable nuclei is observed in PO containing 1%, 3%, and 5% of STS only from induction periods of 7, 5, and 4 min, respectively.
Figure 3. Isothermal crystallization of palm oil (PO) and its blend with either STS or S-370 (a, b), at temperature 20°C, and non-isothermal crystallization of the palm oil (PO) and its blend with either STS and S-370 (c, d).
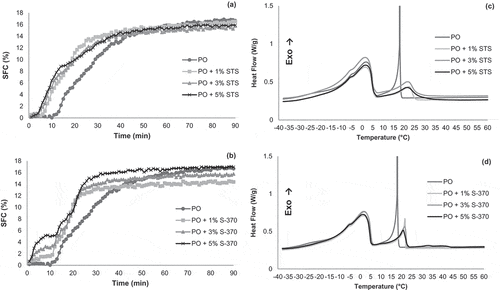
The addition of STS leads to a maximum SFC lower than of the PO. In contrast, the addition of S-370 induces to a two-step crystallization behavior, which exhibiting a slower crystallization process compared to that observed from the addition of STS. Nevertheless, the addition of 5% of S-370 reaches the highest SFC among all samples. According to Chen et al.,[38] the two-step crystallization occurs possibly owing to the formation of two distinct polymorphs. In the first step, the crystallization temperature of 20°C is below the melting of metastable polymorphic form; therefore, the nucleation rate is high. In the second step, a more stable polymorph is formed. On the other hand, the PO containing STS reaches the polymorphic stability and does not exhibit the first crystallization step.
In and , we can observe the formation of two crystallization events of the PO and its blends either STS or S-370, during the crystallization in non-isothermal conditions. The first crystallization event in the PO is between 20 and 5°C, reaching a maximum of crystallization between 20 and 15°C, which represents the crystallization of the high melting point TAGs such as the MPP, PPP, and PPSt. We also considered that a small part of the symmetric TAGs such as the POP, PLP, and POSt are crystallized in this first event. The second event occurs between 5 and −30°C, representing the unsaturated TAGs crystallization, such as the POO, POL, and OOO, besides the remaining of symmetric TAGs. These findings are according to Basso et al.[36] and Saadi et al.,[39] who reported the formation of two crystallization peaks in the DSC analysis of PO.
In the presence of STS, differences in the crystallization temperatures are observed. At 1%, 3%, and 5% STS, the onset of the first crystallization event occurs at approximately 25, 33, and 36°C, respectively, thereby indicating that the addition of STS actually influences the crystallization of the saturated TAGs. According to Miskandar et al.,[40] the greater effect of STS on the crystallization of the PO occurs in the PPP, PPSt, and MPP TAGs, thereby increasing the formation of crystals with homogeneous sizes. Thus, we note that STS not only controls the development of crystals with high melting points[28] but also accelerates the crystallization of these TAGs. In addition, the STS emulsifier addition accelerates the onset of the second crystallization event of the PO, which is represented by the more unsaturated TAGs.
The results of the addition of S-370 for the first crystallization event are similar to those observed in the addition of STS. However, the onset of crystallization occurs at lower temperatures for the PO containing 1%, 3%, and 5% S-370, 22, 23, and 25°C, respectively. Almost no difference is observed in the second crystallization event, thereby indicating that S-370 has no great influence on the unsaturated TAGs crystallization.
As seen in , between 46 and 23°C, an isolated crystallization is observed for samples containing 3% and 5% of S-370. Thus, it is important to emphasize that the S-370 emulsifier crystallizes at high temperatures, accelerating the crystallization of TAGs with high melting points, no great effects on the either middle or olein fractions.
Melting and polymorphic behavior
In , we can observe the melting events and polymorphic behavior of PO and its blend containing either STS or S-370. The first event represents the TAGs with low melting point, whereas the second event corresponds to the TAGs with a high melting point. Three sub-peaks can be observed in the first melting event in the range from 0 to 9°C. Braipson-Danthine and Gibon[41] found that the relative intensity of these sub-peaks is variable, while their positions are related to the TAG composition of the PO, where the first and second sub-peaks are related to the SUU content, and third sub-peak might be related to the SUS content. We can observe a broad peak in the second melting event. According to Calliauw et al.,[23] the peak width of the second melting event suggests the presence of a solid solution formed from the interaction between PPP and POP TAGs. Moreover, this melting behavior is also related to the polymorphic behavior of the PO.
Figure 4. Melting behavior (a, c) and polymorphic behavior of the palm oil (PO) and its blend with either STS and S-370 (b, d).
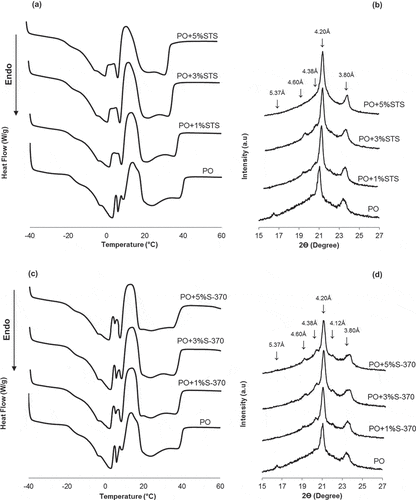
In , note that the addition of STS influences the first melting event, modifying the melting of the TAGs unsaturated and monosaturated. According to Garti,[42] the sorbitan esters has a strong influence on the melting behavior of the TAGs with the lowest melting point. However, the second melting event is also modified, by shifting to the left, which indicates a decrease in the final melting temperature of the PO containing STS.
Contributing to these results, shows that the addition of STS changes the polymorphic behavior of the PO. The addition of 1% and 3% of STS produces a peak in 4.60 Å, characteristic of the β polymorph. However, the higher intensity of the short spacings at 4.20 and 3.80 Å indicates the predominance of the β′ polymorph. The addition of 5% of STS stabilizes the β′ form in the PO, which exhibits only two intense short spacings of 4.20 and 3.80 Å. Initial studies indicate that the STS stabilizes the β′ polymorph by steric hindrance.[43] According to Rousseau et al.,[44] the ability of the emulsifier to prevent the transition to the β form not only depends on the particular chemical structure but also on the structural adjustment, which affords the interposition of the additive between the fat molecules, thereby minimizing the defects, and forming hydrogen bonds between the functional groups.[45] In this context, the addition of 5% of STS is enough to the organization of the TAGs of the PO in the most stable orientation.
In , we can observe that the addition of S-370 produces a small change in the sub-peaks present in the first melting event, indicating a fewer influence on the melting of unsaturated, monosaturated TAGs than STS. The second melting event of the PO is also slightly affected by the presence of S-370, thereby exhibiting a small shift of the peak to the left; however, the maximum melting temperature remained at around 23°C for all samples.
The addition of S-370 affords a similar polymorphic behavior to the PO, allowing the transition from β′ to β, independent of the concentration used (). Domingues et al.[13] found that the addition of B-370 (sucrose behenate) promotes the initial formation of polymorph β in PO. In turn, Cerdeira et al.[8] found no changes in the polymorphic behavior when the sucrose esters (S-170 and P-170) were added to milk fat. The presence of liquid oil and the crystal diameter size are factors that contribute to this polymorphic behavior of the PO containing S-370. Apparently, the liquid oil facilitates the conversion from β′ to β form in some fats. Moreover, higher crystal diameters indicate a tendency for forming β crystals.[46]
Crystallization mechanisms of the pure emulsifiers
We investigated the crystallization characteristics of both STS and S-370 emulsifiers to relate their effects on PO crystallization. In this context, we feature the isothermal crystallization (a), the microstructure (c), and the thermal and polymorphic behavior (b, d) of each emulsifier. The STS and S-370 emulsifiers acted as templates of the crystallization, though some differences between them can be verified ().
Figure 5. Crystallization characteristics of pure STS and S-370. Isothermal crystallization (a); thermal behavior (b); microstructure (c), the bar represents 10 μm; and polymorphic behavior (d).
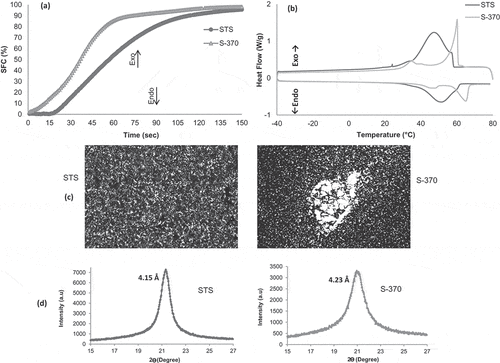
Based on the isothermal crystallization, we can evidence a great similarity between the shape of curves crystallization of the pure emulsifiers and of the PO containing the emulsifiers. A similar behavior was evidenced for the non-isothermal crystallization, corroborating the co-crystallization effect of STS and seeding effect of S-370.
Templating effect of STS occurs by a molecular similarity among STS and TAGs of the palm oil, which allowed them to co-crystallize into uniform crystals.[47] Note that although the STS starts crystallization at around 60°C, no peak is observed at this temperature for crystallization of the PO + STS blends. On the other hand, S-370 acts as seeding, providing a template on which further molecules can self-assemble. We can observe that the microstructure of STS shows homogeneous shape and size crystals, whereas S-370 shows large crystalline clusters. Energetically, S-370 addition is more favorable due to the molecules adsorption to already existing crystal plane than to create new nuclei from co-crystallization, as in the case of STS.
The polymorphic behavior of STS (4.15 Å) and S-370 (4.23 Å) is attributed to the alpha polymorph, which contains a hexagonal subcell structure. Although show polymorphic similarities, we observed differences in the action mode for each emulsifier. In the STS emulsifier, this hexagonal structure allows it to act as a dynamic controller of polymorphic transitions in the fat, due to its ability to create hydrogen bonds with neighboring TAGs. This phenomenon is called of The Button Syndrome, whereby the presence of a specific emulsifier does not form a preferred polymorph but rather controls the degree of mobility of the molecules and their potential to undergo configurational changes. In this process, emulsifiers can modulate the polymorphic transformations in the solid state or via the liquid state, controlling the physical state of crystals and extension of the mobility of the molecules, thereby regulating the rate of polymorphic transformation.[45] Accordingly, STS contributes to the formation more stable polymorph in PO, as β′ crystals.
The wide-angle peak at 4.23 Å in S-370 also is typical for the hexagonal subcell, where chain–chain interactions are nonspecific and the lattice is loosely packed. However, we should consider that the sucrose molecule shows a considerable conformational freedom and many functional hydrogen bonds, which suggest the possibility or tendency for the existence of multiple crystal forms. Thus, the seeding effect creates a resulting crystal which will inherit many of the characteristics of S-370 seed from which is originated.
Conclusion
This work showed that the incorporation of STS and S-370 within the crystal lattice of the PO presents a template effect. This incorporation phenomenon is shown to be dependent on the structure and concentration of those emulsifiers. The results indicated that both STS and S-370 emulsifiers increase the SFC, improve the hardness, and assist the formation of a more organized crystal network. Owing to the co-crystallization mechanism, the addition of STS resulted in a faster crystallization, thus developing crystals with an intermediate melting point, promoting the stability of the β′ polymorph, and conferring to PO better thermal stability during the melting event. The seeding effect of S-370 was confirmed by a two-step crystallization, thereby forming crystals with characteristics very similar to S-370, allowing the formation of the β′ and β polymorphs, and increasing the maximum melting temperature.
References
- Chong, C.-L.; Kamarudin, Z.; Lesieur, P.; Marangoni, A.; Bourgaux, C.; Ollivon, M. Thermal and Structural Behaviour of Crude Palm Oil: Crystallisation at Very Slow Cooling Rate. European Journal of Lipid Science and Technology 2007, 109(4), 410–421. DOI:10.1002/ejlt.200600249.
- Tanaka, L.; Miura, S.; Yoshioka, T. Formation of Granular Crystals in Margarine with Excess Amount of Palm Oil. JAOCS, Journal of the American Oil Chemists' Society 2007, 84(5), 421–426. DOI:10.1007/s11746-007-1064-2.
- Domingues, M.-A.-F.; Ribeiro, A.-P.-B.; Kieckbusch, T.-G.; Gioeilli, L.-A.; Grimaldi, R.; Cardoso, L.-P.; Cardoso, L.-A.-G.-G. Advances in Lipids Crystallization Technology. In Advanced Topics in Crystallization; Mastai, Y., Ed.; Intech: Croatia, 2015; pp. 105–132.
- Sato, K.; Ueno, S. Crystallization, Transformation and Microstructures of Polymorphic Fats in Colloidal Dispersion States. Current Opinion in Colloid & Interface Science 2011, 16(5), 384–390. DOI:10.1016/j.cocis.2011.06.004.
- Sato, K.; Bayés‐García, L.; Calvet, T.; Cuevas‐Diarte, M.-À.; Ueno, S. External Factors Affecting Polymorphic Crystallization of Lipids. European Journal of Lipid Science and Technology 2013, 115(11), 1224–1238. DOI:10.1002/ejlt.201300049.
- Aronhime, J.-S.; Sarig, S.; Garti, N. Mechanistic Considerations of Polymorphic Transformations of Tristearin in the Presence of Emulsifiers. JAOCS, Journal of the American Oil Chemists' Society 1987, 64(4), 529–533. DOI:10.1007/BF02636388.
- Food Emulsifiers:, G. N.;. Structure–Reactivity Relationships, Design, and Applications. In Physical Properties of Lipids; Marangoni, A. G., Narine, S. S., Eds.; Marcel and Dekker: New York, 2002; pp. 265–340.
- Cerdeira, M.; Pastore, V.; Vera, L. V.; Martini, S.; Candal, R. J.; Herrera, M.-L. Nucleation Behavior of Blended High-Melting Fractions of Milk Fat as Affected by Emulsifiers. European Journal of Lipid Science and Technology 2005, 107(12), 877–885. DOI:10.1002/ejlt.200500257.
- Domingues, M.-A.-F.; Ribeiro, A.-P.-B.; Chiu, M.-C.; Gonçalves, L.-A.-G. Sorbitan and Sucrose Esters as Modifiers of the Solidification Properties of Zero Trans Fats. LWT - Food Science and Technology 2015, 62(1), 122–130. DOI:10.1016/j.lwt.2015.01.008.
- O’brien, R.-D.;. Fats and Oils Formulation. In Fats and Oils - Formulating and Processing for Applications; O’brien, R.-D., Ed.; CRC Press: New York, 2004; pp. 235–287.
- Pernetti, M.; Van Malssen, K.-F.; Flöter, E.; Bot, A. Structuring of Edible Oils by Alternatives to Crystalline Fat. Current Opinion in Colloid and Interface Science (2007, 12(4–5), 221–231. DOI:10.1016/j.cocis.2007.07.002.
- Nelen, B.-A.; Cooper, J.-M. Sucrose Esters. In Emulsifiers in Food Technology; Whitehurst, R.-J., Ed.; Blackwell Publishing: Oxford, 2004; pp. 131–161.
- Domingues, M.-A.-F.; Da Silva, -T.-L.-T.; Ribeiro, A.-P.-B.; Chiu, M.-C.; Gonçalves, L.-A.-G. Sucrose Behenate as a Crystallization Enhancer for Soft Fats. Food Chemistry 2016, 192, 972–978. DOI:10.1016/j.foodchem.2015.07.109.
- Antoniosi Filho, N.; Mendes, O. L.; Lanças, F.-M. Computer Predition of Triacylglycerol Composition of Vegetable Oils by HRGC. Chromatographia. 1995, 40, 557–562. DOI:10.1007/BF02290268.
- Vlahov, G.;. Use of 13C Nuclear Magnetic Resonance Spectroscopy to Study the Triglyceride Fraction of Vegetable Oils. Rec. Res. Dev. Oil Chem. 1998, 189–202. DOI:10.1007/BF02523409.
- Vlahov, G.;. 13C Nuclear Magnetic Resonance Spectroscopy to Check 1, 3-Random, 2-Random Pattern of Fatty Acid Distribution in Olive Oil Triacylglycerols. J. Spectroscopy. 2005, 19(2), 109–117. DOI:10.1155/2005/765236.
- AOCS. American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society,6th Ed. AOCS Press: Champaign, IL, USA, 2009.
- Karabulut, I.; Turan, S.; Ergin, G. Effects of Chemical Interesterification on Solid Fat Content and Slip Melting Point of Fat/Oil Blends. European Journal of Lipid Science and Technology 2004, 218(3), 224–229. DOI:10.1007/s00217-003-0847-4.
- Rodrigues, J.-N.; Gioielli, L.-A.; Anton, C. Propriedades Físicas De Lipídios Estruturados Obtidos De Misturas De Gordura Do Leite E Óleo De Milho. Ciência E Tecnol. Ciência e Tecnol Aliment 2003, 23, 226–233. DOI:10.1590/S0101-20612003000200022.
- Yap, P.-H.; deMan, J.-M.; deMan, L. Polymorphism of Palm Oil and Palm Oil Products. JAOCS, Journal of the American Oil Chemists' Society 1989, 66, 693–697. DOI:10.1007/BF02669954.
- Schenck, H.; Peschar, R. Understanding the Structure of Chocolate. Radiation Physics and Chemistry 2004, 71, 829–835. DOI:10.1016/j.radphyschem.2004.04.105.
- Sulaiman, M.-Z.; Sulaiman, N.-M.; Kanagaratnam, S. Triacylglycerols Responsible for the Onset of Nucleation during Clouding of Palm Olein. Journal of the American Oil Chemists' Society 1997, 74(12), 1553–1558. DOI:10.1007/s11746-997-0076-2.
- Calliauw, G.; Fredrick, E.; Gibon, V.; De Greyt, W.; Wouters, J.; Foubert, I.; Dewettinck, K. On the Fractional Crystallization of Palm Olein: Solid Solutions and Eutectic Solidification. Food Research International 2010, 43(4), 972–981. DOI:10.1016/j.foodres.2010.01.002.
- Jin, Q.; Gao, H.; Shan, L.; Liu, Y.; Wang, X. Study on Grainy Crystals in Edible Beef Tallow Shortening. Food Research International 2007, 40(7), 909–914. DOI:10.1016/j.foodres.2007.03.006.
- Da Silva, R.-C.; Soares, F.-A.-S.-D.-M.; Roque Dagostinho, N.; Maruyama, J.-M.; Silva, Y.-A.; Ract, J.-N.-R.; Gioielli, L. A. Crystallization of Monoacylglycerols and Triacylglycerols at Different Proportions: Kinetics and Structure. International Journal of Food Properties 2017, 20(1), 385–398. DOI:10.1080/10942912.2017.1297950.
- Miura, S.; Yamamoto, A.; Konishi, H. Effect of Agglomeration of Triacylglycerols on the Stabilization of a Model Cream. European Journal of Lipid Science and Technology 2002, 104(4), 222–227. DOI:10.1002/1438-9312(200204)104:4<222::AID-EJLT222>3.0.CO;2-T.
- Smith, K. W.;. Crystallization of Palm Oil and Its Fractions. In Crystallization Processes in Fat and Lipid Systems; Garti, N., Sato, K., Eds.; Marcel and Dekker: Switzerland, 2001; pp. 357–380.
- Krog, N.-J.; Sparso, F.-V. Food Emulsifiers: Chemical and Physical Properties. In Food Emulsions; Friberg, S., Larsson, K., Sjoblom, J., Eds.; Marcel and Dekker: New York, 2003; pp. 141–188.
- Szűts, A.; Makai, Z.; Rajkó, R.; Szabó-Révész, P. Study of the Effects of Drugs on the Structures of Sucrose Esters and the Effects of Solid-State Interactions on Drug Release. Journal of Pharmaceutical and Biomedical Analysis 2008, 48(4), 1136–1142. DOI:10.1016/j.jpba.2008.08.028.
- Meng, Z.; Liu, Y.; Shan, L.; Jin, Q.; Wang, F.; Wang, X. Specialty Fats from Beef Tallow and Canola Oil: Establishment of Reaction Conditions, Characterization of Products, and Evaluation of Crystal Stability. Food Biophysics 2011, 6(1), 115–126. DOI:10.1007/s11483-010-9186-8.
- Calliauw, G.; Gibon, V.; De Greyt, W.; Plees, L.; Foubert, I.; Dewettinck, K. Phase Composition during Palm Olein Fractionation and Its Effect on Soft PMF and Superolein Quality. JAOCS, Journal of the American Oil Chemists' Society 2007, 84(9), 885–891. DOI:10.1007/s11746-007-1118-5.
- Chaleepa, K.; Szepes, A.; Ulrich, J. Effect of Additives on Isothermal Crystallization Kinetics and Physical Characteristics of Coconut Oil. Chemistry and Physics of Lipids 2010, 163(4), 390–396. DOI:10.1016/j.chemphyslip.2010.03.005.
- Campos, R.; Narine, -S.-S.; Marangoni, A. G. Effect of Cooling Rate on the Structure and Mechanical Properties of Milk Fat and Lard. Food Research International 2002, 35(10), 971–981. DOI:10.1016/S0963-9969(02)00159-X.
- Da Silva, -T.-L.-T.; Domingues, M.-A.-F.; Chiu, M.-C.; Goncalves, L.-A.-G. Templating Effects of Dipalmitin on Soft Palm Mid Fraction Crystals. International Journal of Food Properties 2017, 20(1), 935–947. DOI:10.1080/10942912.2017.1318290.
- Puppo, M.-C.; Martini, S.; Hartel, R.-W.; Herrera, M.-L. Effects of Sucrose Esters on Isothermal Crystallization and Rheological Behavior of Blends of Milk‐Fat Fraction Sunflower Oil. The Journal of Food Science 2002, 67(9), 3419–3426. DOI:10.1111/j.1365-2621.2002.tb09600.x.
- Basso, R.-C.; Ribeiro, A.-P.-B.; Masuchi, M.-H.; Gioielli, L.-A.; Gonçalves, L.-A.-G.; Dos Santos, A.-O.; Cardoso, L.-P.; Grimaldi, R. Tripalmitin and Monoacylglycerols as Modifiers in the Crystallisation of Palm Oil. Food Chemistry 2010, 122(4), 1185–1192. DOI:10.1016/j.foodchem.2010.03.113.
- Błaszczak, W.; Fornal, J. Lipids in Food Structure. In Chemical, Biological, and Functional Aspects of Food Lipids; Sikorski, Z. Z., Kolakowska, A., Eds.; CRC Press: New York, 2011; pp. 81–96.
- Chen, C.-W.; Lai, O.-M.; Ghazali, H.-M.; Chong, C. L. Isothermal Crystallization Kinetics of Refined Palm Oil. JAOCS, Journal of Lipid Science and Technology . 2002, 79(4), 403–410. DOI:10.1007/s11746-002-0496-4.
- Saadi, S.; Ariffin, -A.-A.; Ghazali, H.-M.; Miskandar, M. S.; Boo, H.-C.; Abdulkarim, S.-M. Application of Differential Scanning Calorimetry (DSC), HPLC and pNMR for Interpretation Primary Crystallisation Caused by Combined Low and High Melting TAGs. Food Chemistry 2012, 132(1), 603–612. DOI:10.1016/j.foodchem.2011.10.095.
- Miskandar, M.-S.; Che Man, Y.-B.; Abdul Rahman, R.; Nor Aini, I.; Yusoff, M.-S.-A. Effects of Emulsifiers on Crystal Behavior of Palm Oil Blends on Slow Crystallization. J. Food Lip. 2007, 14(1), 1–18. DOI:10.1111/j.1745-4522.2006.
- Braipson‐Danthine, S.; Gibon, V. Comparative Analysis of Triacylglycerol Composition, Melting Properties and Polymorphic Behavior of Palm Oil and Fractions. European Journal of Lipid Science and Technology 2007, 109(4), 359–372. DOI:10.1002/ejlt.200600289.
- Garti, N.;. Effects of Surfactants on Crystallization and Polymorphic Transformation of Fats and Fatty Acids. In Crystallization and Polymorphism of Fats and Fatty Acids; Garti, N., Sato, K., Eds.; Marcel and Dekker: New York, 1988; pp. 267–300.
- Krog, N.; Larsson, K. Crystallization at Interfaces in Food Emulsions–A General Phenomenon. European Journal of Lipid Science and Technology 1992, 94(2), 55–57. DOI:10.1002/lipi.19920940205.
- Rousseau, D.; Hodge, S.-M.; Nickerson, M.-T.; Paulson, A.-T. Regulating the β′→ β Polymorphic Transition in Food Fats. JAOCS, Journal of the American Oil Chemists' Society 2005, 82(1), 7–12. DOI:10.1007/s11746-005-1035-z.
- Aronhime, J. S.; Sarig, S.; Garti, N. Dynamic Control of Polymorphic Transformation in Triglycerides by Surfactants: The Button Syndrome. JAOCS, Journal of the American Oil Chemists' Society. 1988, 65(7), 1144–1150. DOI:10.1007/BF02660571.
- Zhang, X.; Li, L.; Xie, H.; Liang, Z.; Su, J.; Liu, G.; Li, B. Effect of Temperature on the Crystalline Form and Fat Crystal Network of Two Model Palm Oil-Based Shortenings during Storage. Food and Bioprocess Technology. 2014, 7(3), 887–900. DOI:10.1007/s11947-013-1078-8.
- Tran, T.; Green, N.-L.; Rousseau, D. Spheroidal Fat Crystals: Structure Modification via Use of Emulsifiers. Crystal Growth & Design. 2015, 15(11), 5406–5415. DOI:10.1021/acs.cgd.5b01033.
