?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This work aims at improving the textural and whipping properties of whipped cream by the addition of milk fat globule membrane protein. The determination of particle size distribution and average diameter of whipped cream showed that the small particle size was shifted to a larger range after milk fat globule membrane protein was added. The average particle size (d3,2) of whipped cream reached a maximum value of 5.05 µm at 1% milk fat globule membrane protein, while slowly decreased with increasing milk fat globule membrane protein levels from 2% to 5%. In addition, the partial coalescence of fat increased with the increase of milk fat globule membrane protein levels, and the correlation between the whipping time and the overrun of whipped cream was positive. The addition of milk fat globule membrane protein also altered the rheological behaviour of whipped cream, resulting in the increase of modulus G′ and the loss modulus G″. The results also indicated that higher milk fat globule membrane protein level decreased the serum loss of whipped cream while improved its stability. While milk fat globule membrane protein levels had no significant effect on viscosity, its increasing levels effectively improved the hardness, consistency, and viscosity of whipped cream.
Introduction
Whipped cream is a popular dairy product that is widely used in different food products, such as cakes, desserts, ice creams, creamy coffees, and pastries. Whipped cream is an example of oil-in-water (O/W) emulsion with high fat content (typically 30–40%). It is processed by whipping dairy cream, to which large quality of gas bubbles is incorporated. During the whipping process, fat globules in the cream are absorbed by serum protein and milk fat globule membrane (MFGM) fractions at the air/water interface.[1,2] A partial crystal network is formed and then the air bubbles are coated; this phenomenon is called the surface-mediated partial coalescence.[Citation3] In addition to the surface-mediated partial coalescence, churning partial coalescence can also take place between the remaining fat globules in serum.[Citation4] The partial coalescence plays an important role in stabilizing the structure of whipped cream, resulting in its desirable texture.[Citation5]
An emulsion system is thermodynamically unstable due to flocculation, creaming, coalescence, phase inversion, and Ostwald ripening.[Citation6,Citation7] Therefore, some food additives, such as emulsifiers, proteins, or polysaccharides, are usually used to improve the process properties as well as the quality of dairy products. Emulsifiers play an important role in improving the stability of aerated food emulsions by absorbing at the air/water interface, thereby lowering the interfacial tension.[Citation8] Most milk proteins are excellent emulsifiers, which can prevent fat droplets in whipped cream from re-coalescence during emulsification by rapid adsorption at the oil/water interface and the formation of viscoelastic interfacial membranes.[Citation9–Citation11] Milk fat globule membrane protein (MFGMP), which mainly consists of mucin 1 (MUC), xanthine oxidase (XO), butyrophilin (BTN), and periodic acid-schiff 6 and 7 (PAS6 and PAS7), is a food ingredient with excellent nutritional value and emulsifying property.[Citation12] As an emulsifier, MFGMP can not only lead to desirable processing properties, texture, and stability, but also lead to excellent nutritional value of whipped cream products. Like whey protein and casein, MFGMP can absorb at the interface, whereby reduces interfacial tension, forms emulsions and foams, and stabilizes fat droplets and bubbles.[Citation11]
According to the literature, the majority of studies focused on utilizing whey proteins and sodium caseinate to improve the physicochemical and sensory properties of whipped cream, whereas the application of MFGMP in whipped cream has not been reported. Thus, the aim of this study is to investigate the influence of MFGMP levels on physical characteristics and whipping organoleptic properties of whipped cream. The average particle size, partial coalescence of fat, rheological behaviour, the overrun, serum loss, and texture of whipped cream were determined and compared.
Materials and methods
Materials
Raw cream with a range of fat contents (35.5–48.4) was obtained from the dairy after a hot separation (55°C). Oil Red O was purchased from the BIOTOPPED (Beijing, China).
Isolation of MFGMP
The isolation of MFGMP was carried out based on the procedure reported by Lu et al.[Citation13] Four hundred litres of fresh bovine milk was first fractionated by a 9NDS-50A cream separator (Qinghai Kangping, China). The cream was then washed three times with phosphate buffer (0.1 M PBS, pH 6.8), and thereafter centrifuged at 1500 × g for 10 min (the supernatant was discarded). The washed cream was mixed with 0.4% SDS at a 1:1(v:v) ratio, and then sonicated for 1 min. After centrifugation at 1500 × g for 10 min (to remove fat), the bottom fraction containing MFGMP was dialyzed against deionized water and then freeze-dried. The MFGMP isolated from fresh bovine milk contained about 85% protein and 12% lipid.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) of MFGMP
The MFGMP samples were mixed with 0.5 mL of reducing buffer (6% Tris-0.5 M, 10% glycerol, 5% β-mercaptoethanol, 2% SDS, and 0.05% bromophenol blue), then heated in boiling water for 5 min. After centrifugation at 2500 × g for 30 min to remove fat, the supernatants (10 mL) were loaded onto 12% SDS-polyacrylamide gels, which were run at 200 V and molecular mass markers ranging from 14.4 to 116 kDa (TransGen Biotech, Co., Ltd., China) were used. The gels were stained with a solution containing Coomassie Brilliant Blue R-250, and destained with a solution containing methanol and glacial acetic acid at concentrations of 160 and 10 mL/L, respectively.
Production of whipped cream
Whipped cream was produced according to the method reported by Sajedi.[Citation14] Fat content of whipped cream samples was first adjusted to 25% by addition of raw skimmed milk, and various MFGMP (0, 1, 2, 3, 4, and 5wt%) levels were then added. After that, the samples were pasteurized at 85°C for 5 min in a water bath. After the samples were cooled down to 50°C, they were homogenized at 3000 rpm for 3 min using a high-speed homogenizer (High-speed homogenizer, AD200-H, Angli Corporation, Shanghai, China). The resulting cream samples were subsequently stored at 4°C for 24 h to promote the crystallization of fat and the formation of foam during whipping. The whipped cream was then subjected to aeration: (i) a portion of whipped cream sample was whipped using a classic stand mixer for various times (to identify the maximum overrun); and (ii) each remaining sample was then whipped using the optimum time obtained in (i). The physical properties of samples were then examined, and all measurements were performed in triplicate.
Measurement of particle size distribution
The particle size distribution and the average diameter of samples were determined by laser scattering using a Malvern Mastersizer 2000 (Malvern Instruments Ltd, Worcestershire, UK). The samples were dispersed in water prior to the measurement of particle size distribution, and the volume-weighted mean diameter d3,2 (µm) was used to monitor the changes in the droplet-size distribution. The refractive indices used were 1.458 and 1.460 for milk fat at 633 and 466 nm, respectively, and the refractive index was 1.33 for water.[Citation15] The measurements were carried out in triplicate, from which an average value was calculated. The mean particle size d3,2 (μm) was calculated using Eq. (1):
where ni is the number of particles with the same diameter and di is the particle size.
Measurement of overrun
The measurement of overrun was carried out according to the method described by Scurlock.[Citation16] It was performed by filling a tub to a set volume with the whipped cream. The overrun was related to the weight of this volume and the density of the cream before whipping. It was determined according to the following equation:
where M1 is the weight of unwhipped cream with the set volume (g) and M2 is the weight of whipped cream with the same volume (g).
Measurement of partial coalescence of fat
The amount of free fat presented in the cream was determined according to the method described by Palanuwech et al.[Citation17] Oil Red O (0.1 mg/g) was stirred overnight in soybean oil using a magnetic stirrer (Meiyingpu Instrument Manufacturing Co., Shanghai, China). The solution’s absorbance at 520 nm was measured using a UV-visible spectrophotometer (Shanghai Jingke Industrial Co. Ltd), in which soybean oil was used as a blank. The principle of dilution technique was adopted: a dye solution in a non-polar solvent was poured onto the surface of the cream, and then gently mixed. After gentle centrifugation, the coloured oil was allowed to float on the cream surface and free fat presented in the cream is dissolved in the coloured oil, while other fat is remained in the droplets. The diluted dye solution fraction was transferred from the surface and its absorbance was then measured. The change in absorbance, which indicates the mass fraction that is not emulsified into fat (φd), was calculated by Eq. (3):
where φd is the mass fraction of fat in the cream, m0 is the weight of the added Oil Red O, me is the weight of the cream, ɑ is the ratio of absorbances of Oil Red O before and after centrifugation, and ϕ is the mass fraction of oil in the cream.
Measurement of rheological behaviour of whipped cream
The rheological measurements were conducted using a Kinexues rheometer (Malvern Instruments, England), equipped with a serrated parallel plate system (PP25-SN23755; d = 25 mm and gap size of 1 mm) and duplicated measurement. Prior to the tests, all samples’ temperatures were equilibrated to 25°C for about 10 min (to ensure a uniform temperature). Frequency sweep measurements were then carried out at a frequency range of 1.0–100 Hz, with 0.5% strain being selected for tests. All measurements were conducted at 25°C, using a 40 mm diameter parallel plate geometry with a 1 mm gap, from which the elastic (G″) and viscous (G″) moduli of whipped cream were obtained.
Determination of serum loss
Thirty milligrams of whipped cream was filtered through a glass filter, which was placed above a 100 mL Erlenmayer flask. After 20 day storage, the amount of serum drained from the whipped cream was gravimetrically determined (in triplicate), and the serum loss was calculated by Eq. (4):
Analysis of textural properties
The hardness, consistency, and viscosity of whipped cream were measured at 10°C by a TA-XT2i texture analyser (Stable Microsystems, Surry, UK) using an aluminium cylinder probe (35 mm diameter). The probe was inserted into the sample at a depth of 25 mm and a speed of 2.0 mm/s, and the force (associated with hardness, consistency, and viscosity) exerted onto the probe was then automatically recorded. Three aliquots containing150 mL of sample were used.
Statistical analysis
The statistical difference between mean values was analysed by the one-factor analysis of the variance and the least significant difference (LSD), in which a significant level was set at 0.05. The LSD values were calculated using Tukey’s post hoc test, and all calculations were performed using the Statistical Package for the Social Sciences v. 10.0 (SPSS, Chicago, IL, USA).
Results and discussion
Composition of MFGMP
The MFGMP isolated from fresh bovine milk was analysed by SDS-PAGE (), which showed that it was composed of MUC, XO, BTN, and periodic acid-schiff 6 and 7 (PAS6 and PAS7); the result is in agreement with previous studies.[Citation1,Citation18,Citation19] Additionally, MFGMP sample contained about 85% protein and 12% lipid, as well as small amounts of casein and whey proteins.
Figure1. SDS-polyacrylamide gel of: lane (1), protein molecular marker; and lane (2), MFGMP. The protein components of MFGMP were labelled according to Mather.[Citation17].
![Figure1. SDS-polyacrylamide gel of: lane (1), protein molecular marker; and lane (2), MFGMP. The protein components of MFGMP were labelled according to Mather.[Citation17].](/cms/asset/20cfa3f5-d460-430e-be48-d2a9c7dcd21d/ljfp_a_1460755_f0001_b.gif)
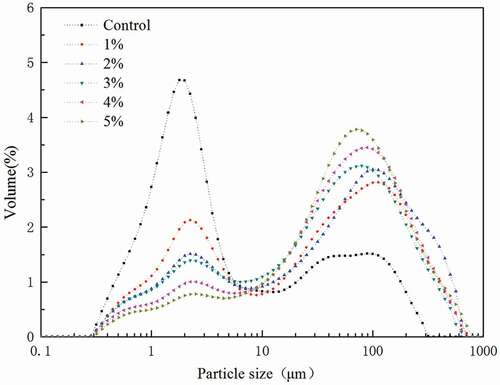
Particle size distribution of whipped cream
Particle size distribution, which indicates particle size and its volume percentage, can strongly influence the stability and property of whipped cream. shows the effect of MFGMP levels on the particle size distribution of whipped cream after 20 min whipping. The particle size distribution curve showed two distinguishable peaks at approximately 5 and 80 µm at all MFGMP levels. In addition, the particle size of whipped cream increased with increasing MFGMP levels, for both large and small particle sizes. It is possible that the interaction between mechanical shear and air incorporation during whipping causes the particle size to increase. Moreover, the addition of MFGMP may cause the protein-covered fat droplets to develop into an aggregated network, which can entrap air and increase structural integrity of foam, thus resulting in further increase of particle size.[Citation9,Citation20] However, because fat dispersion can lead to the formation of thin lamellae between bubbles, which may ultimately destabilize the film and break the air bubble, the dispersion of liquid fat at the air/water interface should be limited.[Citation21]
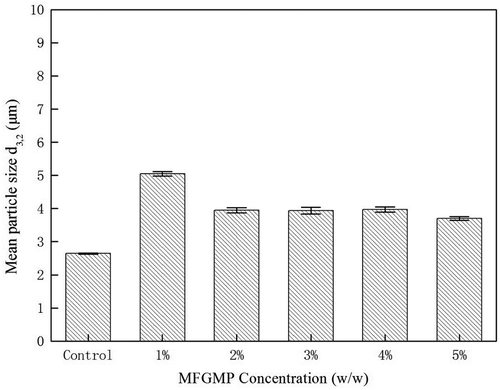
shows the average particle size d3,2 of whipped cream containing six different MFGMP levels. The data showed that MFGMP levels had a significant influence (p < 0.05) on the average particle size of whipped cream. Additionally, the average particle size d3,2 of whipped cream was significantly greater (p < 0.05) than that of control. The MFGMP can contribute to a network of partially coalesced droplets in whipped cream, connecting the fat clumps in the bulk and the droplets adhere to the air bubble surface, thus giving structural integrity to the foam and further increasing d3,2.[Citation20] Moreover, the interaction between MFGMP and air bubbles during aeration may also cause d3,2 to increase.[Citation9] The maximum average particle size d3,2 of 5.05 µm was obtained when 1% MFGMP was added, while smaller d3,2 of 3.70 µm was obtained when 5% MFGMP was added (). The average particle size d3,2 of whipped cream decreased with the increase of MFGMP levels; such a decrease was, however, not significant at a MFGMP level range of 2–5%. Although the effect of MFGMP levels on the average particle size d3,2 of whipped cream has not been studied previously, Zhao et al.[Citation22] have reported that high concentration of sodium caseinate or whey protein decreased the average particle size of cream. With the increase of MFGMP levels, MFGMP molecules may be competitively adsorbed onto the surface of fat droplets, thereby changing its surface tension and decreasing its particle size. On the other hand, the increase of MFGMP levels can cause the emulsion capacity to increase, which may thus limit the increase of average particle size d3,2.
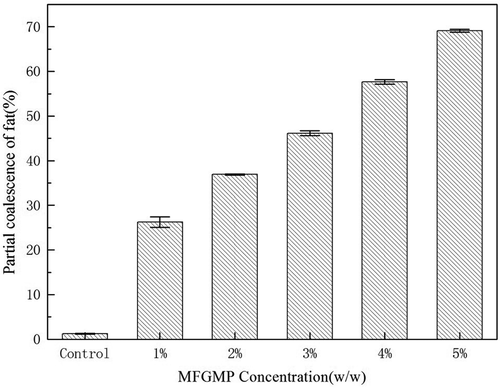
Effect of MFGMP on partial coalescence of fat
The partial coalescence of fat was determined by examining free fat in whipped cream. As shown in , the effect of MFGMP levels on the partial coalescence of fat in whipped cream was dose dependent; the partial coalescence of fat gradually increased with increasing MFGMP levels. The correlation between MFGMP levels and partial coalescence of fat was positive. The partial coalescence of fat in whipped cream was distinctly improved with increasing MFGMP levels. After 20 min whipping, the partial coalescence of fat in whipped cream containing 1% MFGMP was 25.15%, while that in whipped cream containing 5% MFGMP was 68.76%. This result indicated that MFGMP could effectively alter the partial coalescence of fat in whipped cream.
The partial coalescence of fat plays an important role in controlling the stability and in turn the desirable quality of whipped cream.[Citation23,Citation24] The higher the partial coalescence of fat in whipped cream, the higher the stability of whipped cream. Therefore, controlling the stability of whipped cream through partial coalescence of fat can be critical for the quality of whipped cream. The coalescence usually involves the combination of two or more droplets that leads to the formation of foam, as well as the protein-covered fat droplets, which promote the incorporation of air into whipped cream. When fat globules enter into an air bubble surface, the oil from a fat crystal network is spread over the bubble surface; two adjacent fat globules on the bubble surface can easily form a junction; thus, a partial coalescence is established and the bubble size becomes larger.[Citation3,Citation25] Furthermore, if protein molecules are unfolded during the whipping process, the foaming property of whipped cream can also be improved.[Citation26] This is in alignment with our results, in which the particle size of whipped cream was increased with the increase of MFGMP levels. An increase in partial coalescence of fat can be beneficial to the stability of whipped cream; however, an increase that occurs too quickly may result in destabilising.[Citation27] Therefore, a moderate coalescence of fat that results in desirable whipped cream is favourable.
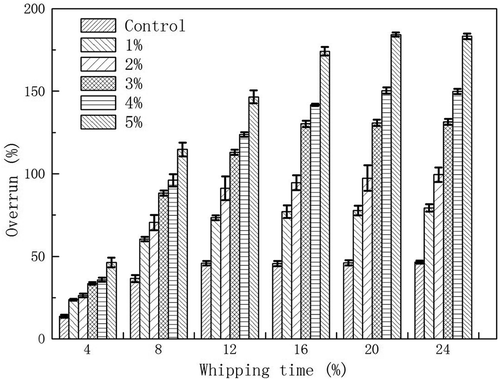
Effect of MFGMP on the overrun
The overrun is an indicator of gas holdup or the percentage of gas in whipped cream.[Citation28] A maximum overrun indicates maximum foam stability and stiffness.[Citation29] The effect of MFGMP levels and whipping time on the overrun of whipped cream is shown in , which showed that the correlation between whipping time and overrun of whipped cream was positive. The overrun increased with the increase of whipping time, from 0 to 24 min, and such increasing trend became insignificant after 16 min whipping time. The addition of MFGMP into the cream may contribute to the whipping process by incorporating a large quantity of air. With extended whipping time, larger quantity of air may be incorporated into the cream being whipped, which can result in higher overrun. All air bubbles, in this case, are encapsulated by coalesced fat droplets distributed around the air/slurry interface. Therefore, a moderate MFGMP level can provide more opportunity for air to incorporate into whipped cream, thus reinforcing the stability of already incorporated air.[Citation9]
Effect of MFGMP on rheological behaviour of whipped cream
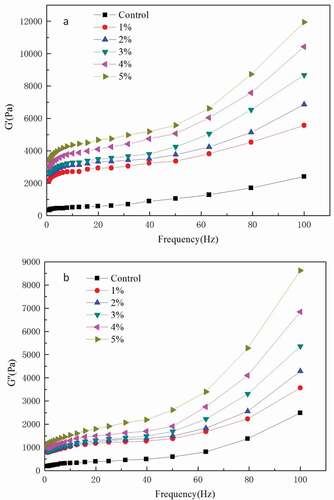
Analysis of rheological properties can provide information on the effect of MFGMP on rheological behaviour of whipped cream. shows the frequency sweeps of whipped cream containing different MFGMP levels. While both were low frequency dependent, the storage modulus G′ values were higher than the loss modulus G″ values, which indicate that the whipped cream has a behaviour of a typical weak gel system.[Citation30] Moreover, both the storage modulus G′ and the loss modulus G″ values increased with increasing MFGMP levels, indicating that MFGMP increases the rigidity of whipped cream, causing it to have a solid-like behaviour (G′ > G″). It also appears that MFGMP was adsorbed at the oil/water interface, and a pseudo-gel network was then formed; and as a result, G′ and G″ were increased. The findings showed that the addition of MFGMP into whipped cream caused higher G′ and G″, which in turn caused stronger molecular protein network.
Effect of MFGMP on serum loss of whipped cream
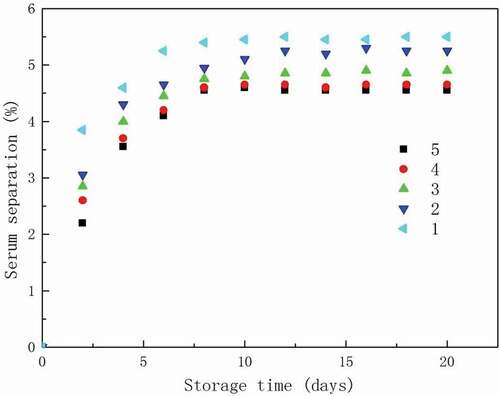
The serum loss of whipped cream during storage can provide an insight into the relationship between the partial coalescence of fat and the stability of product. The effect of MFGMP levels on the serum loss of whipped cream () illustrated that the serum loss of whipped cream increased with the increasing storage time of up to 10 days, while presented a stable trend after 10 days. In addition, higher MFGMP level caused lower serum loss. These can be explained as follows: (i) higher level of MFGMP may better arrange fat crystals at the O/W interface, causing a more orderly arranged structure of whipped cream, resulting in unlimited water leakage during storage; and (ii) the presence of higher amounts of protein components of MFGMP, such as XO and BTN, which have good emulsion ability, may lead to higher water-holding capacity and in turn reduce the serum loss. [Citation31]
Effect of MFGMP on texture of whipped cream
Obtaining desirable textural characteristics is one of the goals in food production.[Citation32] shows the effect of MFGMP levels on the hardness, consistency, and viscosity of whipped cream. The hardness and the consistency, which reflect whipped cream’s gel strength, were similar in all MFGMP levels; the hardness and consistency increased with the increase of MFGMP levels. The increase of such properties during storage may be resulted from late crystallization in whipped cream. During storage, late crystallization may take place, in which partial coalescence between fat globules in O/W emulsion is induced. Fat globules are merged and then formed bigger butter granules, which lead to the release of serum and rearrangement of whipped cream structure. As a result, the hardness and the consistency of whipped cream were increased.
Figure 8. Effect of MFGMP levels on hardness (a), consistency (b), and viscosity (c) of whipped cream.
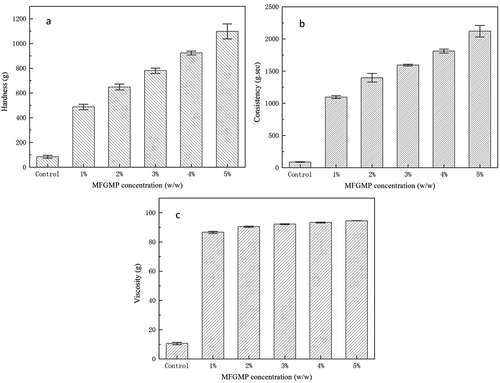
Viscosity is another important property, which indicates the organoleptic quality and acceptability of whipped cream. It can be expressed as flow rate per unit force.[Citation33] Viscosity plays an important role in the properties of whipped cream, including overall mouth feeling, fat/water interface, air/water interface, and texture, which are highly linked to consumers’ preferences. The viscosity of whipped cream was substantially improved after MFGMP was added. Whipped cream containing MFGMP had higher viscosity than control. Although the viscosity of whipped cream containing 1% MFGMP was slightly higher than that of whipped cream containing 5% MFGMP, the difference was not significant (p > 0.05). While MFGMP may be used as a stabilizer, it may also be used as an emulsifier. Because MFGMP can bind water and thus reduce water flow in the matrix, the whipped cream containing MFGMP had higher viscosity. In addition, MFGMP may act as a foam stabilizer; it can be adsorbed at the air/water interface, forming viscoelastic layers, resulting in a high viscosity protein network.[Citation34] These possible functions of MFGMP may explain its involvements in the improved textural properties of whipped cream. The MFGMP at the fat/water and air/water interfaces may be incorporated into whipped cream to provide a more desirable physical structure of the final product.[Citation35] These findings demonstrated that MFGMP could effectively improve the texture of whipped cream by increasing its hardness, consistency, and viscosity.
Conclusion
In summary, MFGMP could effectively improve the rheological behaviour and textural and whipping properties of whipped cream. The results showed that the particle size of whipped cream and the partial coalescence of fat in whipped cream were increased with the increase of MFGMP levels. In addition, both whipping time and MFGMP levels had positive effects on the overrun of whipped cream, and whipped cream containing MFGMP had reduced serum loss while improved stability. Moreover, MFGMP altered the rheological behaviour and increased hardness, consistency, and viscosity of whipped cream; however, MFGMP levels had no significant effect on viscosity. In future work, the effect of MFGMP on microstructure of whipped cream will be investigated. The study can be useful for better understanding the effects of MFGMP so that it can be better used.
Additional information
Funding
References
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T. T.; Messens, K.; Camp, J. V. Nutritional and Technological Aspects of Milk Fat Globule Membrane Material. International Dairy Journal 2008, 18, 436–457. DOI: 10.1016/j.idairyj.2007.10.014.
- Hasenhuettl, G. L.; Hartel, R. Food Emulsifiers and Their Applications, Second ed. Springer: New York. 2008, 419.
- Hotrum, N. E.; Cohen Stuart, M. A.; Van Vliet, T.; Avino, S. F.; Van Aken, G. A. Elucidating the Relationship between the Spreading Coefficient, Surface-Mediated Partial Coalescence and Whipping Time of Artificial Cream. Colloids and Surfaces 2005, 206, 71–78. DOI: 10.1016/j.colsurfa.2005.03.004.
- Walstra, P.; Wouters, J. T. M.; Geurts, T. G. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, 2006; pp 782.
- Nguyen, V.; Duong, C. T. M.; Vu, V. Effect of Thermal Treatment on Physical Properties and Stability of Whipping and Whipped Cream. Journal of Food Engineering 2015, 163, 32–36. DOI: 10.1016/j.jfoodeng.2015.04.026.
- Zhou, X. L.; Chen, L. T. X.; Han, J.; Shi, M. X.; Wang, Y. N.; Zhang, L. B.; Li, Y.; Wu, W. Stability and Physical Properties of Recombined Dairy Cream: Effects of Soybean Lecithin. International Journal of Food Properties 2017, 20, 10, 2223–2233. DOI: 10.1080/10942912.2016.1233434.
- Thanasukarn, P.; Pongsawatmanit, R.; Mc Clements, D. J. Influence of Emulsifier Type on Freeze-Thaw Stability of Hydrogenated Palm Oil-In-Water Emulsions. Food Hydrocolloids 2004, 18, 1033–1043. DOI: 10.1016/j.foodhyd.2004.04.010.
- Wollenweber, C.; Makievski, A. V.; Miller, R.; Daniels, R. Adsorption of Hydroxypropyl Methylcellulose at the Liquid/Liquid Interface and the Effect on Emulsion Stability. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2000, 172, 91–101.
- Allen, K. E.; Dickinson, E.; Murray, B. Acidified Sodium Caseinate Emulsion Foams Containing Liquid Fat: A Comparison with Whipped Cream. LWT-Food Science and Technology 2006, 39, 225–234. DOI: 10.1016/j.lwt.2005.02.004.
- Firebaugh, J. D.; Daubert, C. R. Emulsifying and Foaming Properties of a Derivatized Whey Protein Ingredient. International Journal of Food Properties 2005, 8, 2, 243–253. DOI: 10.1081/JFP-200060245.
- Needs, E. C.; Huitson, A. The Contribution of Milk Serum Proteins to the Development of Whipped Cream Structure. Food Structures 1991, 10, 353–360.
- He, S. H.; Tang, H. S.; Yi, H. X.; Xu, W. L.; Ma, Y.; Wang, R. C. Properties of Emulsions from Milk Fat Globule Membrane and Its Components. International Journal of Food Properties 2017, 1, 343–348.
- Lu, J.; Wang, X. Y.; Zhang, W. Q.; Liu, L.; Pang, X. Y.; Zhang, S. W.; Lv, J. P. Comparative Proteomics of Milk Fat Globule Membrane in Different Species Reveals Variations in Lactation and Nutrition. Food Chemistry 2015, 196, 665–672. DOI: 10.1016/j.foodchem.2015.10.005.
- Sajedi, M.; Nasirpour, A.; Keramat, J.; Desobry, S. Effect of Modified Whey Protein Concentrate on Physical Properties and Stability of Whipped Cream. Food Hydrocolloids 2014, 36, 5, 93–101. DOI: 10.1016/j.foodhyd.2013.09.007.
- Michalski, M.; Briard, V.; Michel, F. Apparent Zeta-Potential as a Tool to Assess Mechanical Damages to the Milk Fat Globule Membrane. Lait 2001, 81, 787–796. DOI: 10.1051/lait:2001105.
- Scurlock, P.;. Production of Cream from Ultrafiltered Milk. Journal of Dairy Research 1986, 53, 431. DOI: 10.1017/S0022029900025048.
- Palanuwech, J.; Potineni, R.; Roberts, R. F.; Coupland, J. N. A Method to Determine Free Fat in Emulsions. Food Hydrocolloids 2003, 17, 55–62. DOI: 10.1016/S0268-005X(02)00035-8.
- Mather, I. H.;. A Review and Proposed Nomenclature for Major Proteins of the Milk-Fat Globule Membrane. Journal of Dairy Science 2000, 83, 203–247. DOI: 10.3168/jds.S0022-0302(00)74870-3.
- Ye, A.; Singh, H.; Taylor, M. W.; Anema, S. Characterization of Protein Components of Natural and Heat-Treated Milk Fat Globule Membranes. International Dairy Journal 2002, 12, 393–402. DOI: 10.1016/S0958-6946(02)00034-1.
- Vanapalli, S. A.; Coupland, J. N. Emulsion Under Shear –The Formation and Properties of Partially Coalesced Lipid Structures. Food Hydrocolloids 2001, 15, 507–512. DOI: 10.1016/S0268-005X(01)00057-1.
- Hotrum, N. E.; Stuart, M. A. C.; Van Vliet, T.; Van Aken, G. A. Spreading of Partially Crystallized Oil Droplets on an Air/Water Interface. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2004, 240, 83–92. DOI: 10.1016/j.colsurfa.2004.03.015.
- Zhao, Q. Z.; Zhao, M. M.; Wang, J. S.; Wang, C. H.; Yang, B. Effects of Sodium Caseinate and Whey Proteins on Whipping Properties and Texture Characteristics of Whipped Cream. Journal of Food Process Engineering 2008, 31, 671–683. DOI: 10.1111/jfpe.2008.31.issue-5.
- Goff, H. D. Colloidal Aspects of Ice Cream – A Review. International Dairy Journal 1997, 7, 363–373. DOI: 10.1016/S0958-6946(97)00040-X.
- Leser, M.; Michel, M. Aerated Milk Protein Emulsions – New Microstructural Aspects. Current Opinion in Colloid and Interface Science 1999, 4, 239–244. DOI: 10.1016/S1359-0294(99)00037-0.
- Sofjan, R. P.; Hartel, R. W. Effects of Overrun on Structural and Physical Characteristics of Ice Cream. International Dairy Journal 2004, 14, 255–262. DOI: 10.1016/j.idairyj.2003.08.005.
- Dickinson, E.; Golding, M. Depletion flocculation of Emulsions Containing Unadsorbed Sodium Caseinates. Food Hydrocolloids 1997, 11, 13–18. DOI: 10.1016/S0268-005X(97)80005-7.
- Zhao, Q. Z.; Zhao, M. M.; Li, J. R.; Yang, B.; Su, G. W.; Cui, C.; Jiang, Y. M. Effect of Hydroxypropyl Methylcellulose on the Textural and Whipping Properties of Whipped Cream. Food Hydrocolloids 2009, 23, 2168–2173. DOI: 10.1016/j.foodhyd.2009.04.007.
- Jakubczyk, E.; Niranjan, K. Transient Development of Whipped Cream Properties. Journal of Food Engineering 2006, 77, 79–83. DOI: 10.1016/j.jfoodeng.2005.06.046.
- Birkett, R. J. Role of Interfacial Processes in the Whipping of Dairy Cream. In Proceedings of Sixth International Congress of Food Science and Technology; Mc Loughlin, J. V., McKenna, B. M., Ed(s).; Boole Press: Dublin, 1983; Vol. 2, pp 149–150.
- Speroni, F.; Beaumal, V.; De Lamballerie, M.; Anton, M.; Anon, M. C.; Puppo, M. C. Gelation of Soybean Proteins Induced by Sequential High-Pressure and Thermal Treatments. Food Hydrocolloids 2009, 23, 1433–1442. DOI: 10.1016/j.foodhyd.2008.11.008.
- Kneifel, W.; Paquin, P.; Abert, T.; Richard, J. P. Water-Holding Capacity of Proteins with Special Regard to Milk-Proteins and Methodological Aspects – A Review. Journal of Dairy Science 1991, 74, 2027–2041. DOI: 10.3168/jds.S0022-0302(91)78373-2.
- Stanley, D. W.; Goff, H. D.; Smith, A. K. Texture-Structure Relationships in Foamed Dairy Emulsions. Food Research International 1996, 29, 1–13. DOI: 10.1016/0963-9969(95)00063-1.
- Szczesniak, A. S.;. Texture Is a Sensory Property. Food Quality and Preference 2002, 13, 215–225. DOI: 10.1016/S0950-3293(01)00039-8.
- Rullier, B.; Axelos, M. A. V.; Langevin, D.; Novales, B. ß-Lactoglobulin Aggregates in Foam Films: Effect of the Concentration and Size of the Protein Aggregates. Journal of Colloid and Interface Science 2010, 343, 330–337. DOI: 10.1016/j.jcis.2009.11.015.
- Lal, S. N. D.; O’Connor, C. J.; Eyres, L. Application of Emulsifiers/Stabilizers in Dairy Products of High Rheology. Advances in Colloid and Interface Science 2006, 123, 433–437. DOI: 10.1016/j.cis.2006.05.009.