ABSTRACT
The reports on the volatile compounds of a dawadawa-type African food condiment produced from the alkaline fermentation of bambara groundnut (Vigna subterranea (L.) Verdc.) using Bacillus starter cultures are limited. Volatile compounds were isolated from dawadawa-type condiments using headspace solid phase microextraction and analysed by comprehensive gas chromatography coupled to time of flight mass spectrometry. Acids, aldehydes and alcohols accounted for over 70% of the volatile compounds produced in the Bacillus fermented samples. B. subtilis subsp. subtilis SFBA3 produced the highest content of acids (4969.60 µg kg−1), while the highest content of aldehydes (2811.90 µg kg−1) and alcohols (1247.60 µg kg−1) was detected with Bacillus cereus PALB7 and Bacillus licheniformis OALB2, respectively. Sulphur-containing compounds concentration (85.80 µg kg−1) was highest for Bacillus amyloliquefaciens SFBA2. Maximum 2-methyl butanoic acid and 3-methyl butanoic acid concentrations, indicative of typical dawadawa aroma, were produced by B. subtilis subsp. subtilis SFBA3.
Introduction
Dawadawa is a traditional condiment manufactured by fermenting the African locust bean (Parkia biglobosa). The condiment is a culinary product used to impart flavour and enhance meatiness in soups, sauces, and other prepared dishes.[Citation1] In the western and central African savannah regions, dawadawa is a delicacy considered as one of the most important food condiments. Dawadawa is generally produced from the spontaneous alkaline fermentation of the African locust bean with Bacillus species as the dominant microorganisms. However, there has been occasional production of dawadawa from soybeans (Glycine max) to manufacture soy-dawadawa or to a lesser extent use of bambara groundnuts (Vigna subterranean (L.) Verdc.). Bambara groundnut is an underutilized African legume with potential for production of dawadawa-type condiments.[Citation2–Citation5] The major role of Bacillus species in such condiments involves hydrolyzing proteins to peptides, amino acids and releasing ammonia thereby creating an alkaline pH, which aids the inhibition of spoilage microorganisms.[Citation6] The pungent ammoniacal flavour characteristic of these condiments is produced from proteolytic activities of microorganisms on legume proteins and utilization of free amino acids during fermentation; consequently, ammonia is formed.[Citation7–Citation10]
The profiling of volatile compounds production during controlled microbial alkaline fermentation of legumes is one of the steps which could give information for the future selection of starter cultures.[Citation11] Pyrazines, aldehydes, ketones, esters, alcohols, acids, alkanes, alkenes, benzenes, phenols, sulphurs, and furans groups were identified in dawadawa produced from African locust bean and soybeans.[Citation8,Citation12] However, volatile compounds that characterize dawadawa-type African food condiment produced from the controlled alkaline fermentation of bambara groundnuts have not been reported previously.
The methods that have been employed to isolate volatile compounds from fermented African locust bean (dawadawa) were microscale steam distillation low-density solvent extraction, mainly the Likens–Nikerson method[Citation8,Citation11,Citation12] and purge-and-trap onto Tenax[Citation10] extraction for fermented soybean (soy-dawadawa), followed by gas chromatography–mass spectrometry (GC–MS) analysis. The distillation method is employed for volatile compounds extraction, especially when heat-labile compounds need to be extracted. However, the main drawback is the possible formation of artefacts due to the long-term influence of high temperature on heat-labile compounds. Solid phase microextraction (SPME) is widely used for the solvent free extraction of food and beverages.[Citation13–Citation17] Solvent free analyte enrichment provides aroma extracts that are more representative of food aroma when compared to those obtained by solvent extraction.[Citation18] Headspace SPME involves sampling of the vapour that is directly above the food matrix and by concentrating the headspace with an adsorbent. This method has several advantages over classical distillation techniques; it is generally quicker, highly reproducible, and yields “true” aroma profiles, as artefact formation is minimised.[Citation19]
The purpose of this study was to determine and compare the profiles of volatile compounds produced during alkaline fermentation of bambara groundnut into dawadawa-type condiments by four Bacillus starter cultures using headspace SPME and comprehensive GC with time-of-flight MS (GC × GC–TOFMS). To the best of our knowledge, the characterization of volatile compounds of dawadawa-type condiments from alkaline fermented bambara groundnuts are reported here for the first time.
Materials and methods
Bacillus starter culture
Starter cultures B. subtilis subsp. subtilis SFBA3, Bacillus amyloliquefaciens subsp. plantarum SFBA2, Bacillus cereus PALB7, and Bacillus licheniformis OALB2 isolated and characterized from previous work in our research group[Citation20] were used to inoculate bambara groundnuts sourced from Zimbabwe, Southern Africa, by Triotrade (Silverton, South Africa).
Fermentation of bambara groundnut
Dawadawa-type condiments produced from bambara groundnut was prepared according to the process described by Barimalaa et al.[Citation4] with slight modifications. Forty grams of seeds were steeped in 100 ml distilled water at 24°C for 24 h, after which the seeds were dehulled manually using a mortar and pestle. Cotyledons recovered were boiled in distilled water at 100°C for 15 min. The cooked cotyledons were then drained with a sieve (1 mm pore size). Fifty grams of the cooked cotyledons were placed in 50 mm × 70 mm zip lock perforated polythene bags (Apak Packaging, Johannesburg, South Africa) before inoculation with a starter culture. The inoculum was thoroughly mixed with the substrate using a sterile spatula. The samples were incubated at 30°C for 120 h.
Headspace sampling with SPME
Five grams each of the alkaline fermented bambara groundnut dawadawa-type condiments were placed in 24 ml glass vials. The vials were sealed with tin foil and a screw cap with a centre hole of 3.2 mm radius lined with a Teflon® septum (Separations, Randburg, South Africa). Samples were vortexed for 50 s before immersing them in a water bath at 50°C for 15 min to equilibrate. The extractions were done with a SPME device fitted with a 2–50/30 µm DVB/Carboxen/PDMS StableFlex fibre (Supelco, Sigma-Aldrich (Pty) Ltd., Kempton Park, South Africa). The fibre was exposed to the headspace above the sample for 20 min. After extraction the SPME device was removed from the vial and desorbed in the injection port of a GC × GC-TOFMS as described below. The fibre was conditioned between extractions by heating it in a GC injection port (split flow mode 50:1) for 20 min at 250°C.
Chemical standards
Five grams of dawadawa were spiked with 6.25 µg 1,8-cineole (eucalyptol) analytical reference standard (Sigma-Aldrich (Pty) Ltd., Kempton Park, South Africa). The headspace sampling of the spiked sample was extracted as described above and analysed with GC × GC–TOFMS. For linear retention index determination n-alkanes (C8–C28) were used (Merck, Pretoria, South Africa).
Comprehensive gas chromatography–time of flight mass spectrometry (GC × GC–TOFMS)
Compound separation was done using a LECO Pegasus 4D GC × GC–TOFMS with an Agilent 7890 GC (LECO Africa (Pty) Ltd., Kempton Park, South Africa). The system included a secondary oven and a dual stage modulator. Nitrogen gas was used for the hot jets and nitrogen gas cooled with liquid nitrogen was used for the cold jets. The carrier gas, helium, was of ultra-high purity grade (Afrox, Gauteng, South Africa) and was set at a flow rate of 1.4 ml min−1 in the constant flow mode. The capillary column set consisted of an apolar Rxi-5SilMS 30 m × 0.25 mm ID × 0.25 µm df (Restek, Bellefonte, PA, USA) as the primary column and a high temperature midpolar Rxi-17Sil MS 0.97 m × 0.25 mm ID × 0.25 µm df (Restek, Bellefonte, PA, USA) as the secondary column. The SPME fibre was desorbed for 5 min in a SPME inlet liner (Supelco, Sigma-Aldrich (Pty) Ltd., Kempton Park, South Africa) of a GC inlet at 230°C. The GC inlet was operated in the splitless mode with a splitless time of 54 s. The GC primary oven temperature programme was 35°C (3 min) at 8°C min−1 to 280°C (5 min). The secondary oven was programmed identical to the primary oven, but offset by +5°C. The modulator temperature offset was 20°C. The modulation period was 2 s with a hot pulse time of 0.4 s. The MS transfer line temperature was set at 280°C and the ion source temperature was set at 200°C. The electron energy was 70 eV in the electron impact ionization mode (EI+), the data acquisition rate was 100 spectra s−1, the mass acquisition range was 35–500 Da, and the detector voltage was set at 1815 V. Identification of compounds in the samples was done by comparison of mass spectra to that of the National Institute of Standards and Technology (NIST14) library and by experimental linear retention indices (RIexp). Compounds reported had a spectral match quality of ≥80%. Semi-quantification of the compounds was performed by using the internal standard method of quantification.
Statistical analysis
Statistical analysis was performed using XLSTAT 2014 software (AddinSoft™ SARL, Paris, France). The interaction effects of different Bacillus species starter cultures and volatile compounds formed were determined using analysis of variance (ANOVA) by the modelling data option. Significant differences between mean were determined using Fisher least significant difference test at 5% probability level (p < 0.05). Principal component analysis (PCA) was conducted to show a visual interpretation of differences among Bacillus species starter cultures and volatile compounds formed using a vector distance plot.
Results and discussion
The microbial characteristics of the controlled fermented condiments showed that Bacillus starter cultures reached from about 4 × 104 cfu g−1 in the inoculated sterile cooked cotyledons to about 6 × 108 cfu g−1 at 120 h in cotyledons with B. cereus PALB7, B. amyloliquefaciens subsp. plantarum SFBA2, B. subtilis subsp. subtilis SFBA3, or B. licheniformis OALB2. The spontaneously fermented Bambara groundnut control had an initial Bacillus count of 2 × 103 cfu g−1 to attain a final count of 3 × 108 cfu g−1. The control produced a condiment with a mixed microbial population comprising of Bacillus species and pathogenic microbes such as Staphylococcus, Enterobacteriacae, yeasts, and moulds, which contaminated the final product (data not shown). In this study, as inocula of pure cultures of Bacillus have been used during controlled fermentation, no contaminated microbial population was seen. However, due to the level of microbial contamination in the control sample, it was not further analyzed for volatile compounds. Both B. cereus PALB7 and the control had rapid growth throughout the fermentation as indicated by an increase in spore-forming bacteria counts with a concomitant pH increase from 6.90 to 8.20 for B. cereus PALB7 at 48 h. B. amyloliquefaciens subsp. plantarum SFBA2 and B. licheniformis OALB2 had the highest growth at 72 h while B. subtilis subsp. subtilis SFBA3 at 96 h. The highest alkaline pH recorded for all of the Bacillus species was pH 8.5 at 96 h. The final pH values in all the condiments are in the range of the pH 8–9, which generally characterize alkaline fermented products.
A total of 125 volatile compounds were identified in the four dawadawa-type samples (), with 87, 54, 55, and 66 compounds in samples inoculated with Bacillus species SFBA3, PALB7, OALB2, and SFBA2, respectively (). These volatile compounds included aldehydes, acids, ketones, esters, pyrazines, alcohols, nitrogen-containing compounds, sulphur-containing compounds, and furans. Additionally, alkanes (59), alkenes (7), and aromatic compounds (30) were also detected (Table S1 of Supplementary Materials). Quantitatively, the most abundant volatile compounds were the organic acids (maximum total concentration 4969.60 µg kg−1 in B. subtilis subsp. subtilis SFBA3 sample) followed by the aldehydes (maximum total concentration of 2811.90 µg kg−1 in B. cereus PALB7 sample); alcohols and ketones (maximum total concentration 1247.60 µg kg−1 and 888.80 µg kg−1, respectively, in B. licheniformis OALB2). Comparatively, B. amyloliquefaciens SFBA2 had the highest concentration of sulphur-containing compounds (85.80 µg kg−1). Benzaldehyde and hexanal were prominent compounds produced by all the Bacillus strains. Likewise ethanol, benzyl alcohol and 1-octen-3-ol were produced by all the Bacillus strains. 1-Octen-3-ol is described as an important contributor to aroma with mushroom and fermented-like odour, the compound is considered to be a product of the oxidation of linoleic acid or other polyunsaturated fatty acid.[Citation21]
Table 1. Volatile compounds in the headspace of dawadawa-type condiment produced from Bacillus fermented bambara groundnut using GC × GC–TOFMS.
In B. subtilis subsp. subtilis SFBA3 inoculated samples, 3-methyl butanoic acid (1653 µg kg−1) and acetic acid (658 µg kg−1) were the organic acids produced at the highest levels. Acetic acid imparts a sour, vinegar note,[Citation16,Citation22] while 3-methyl butanoic acid is described as acidic, sour, pungent, fruity, stinky, ripe fatty, and fruity notes.[Citation23] This strain produced the highest concentration of esters. Esters have been known to constitute a major volatile compound in African fermented condiments. The esters are presumably the consequence of chemical reactions between microbial acidic and alcoholic metabolites.[Citation9] The profile of the condiment from B. cereus PALB7 was dominated by aldehydes, in particular benzaldehyde and hexanal, at 2711 µg kg−1 and 54 µg kg−1, respectively (). Benzaldehyde is generally responsible for a pleasant, sweet, aromatic note, while hexanal gives a green odour.[Citation14,Citation16,Citation22] B. cereus PALB7 produced the highest level of nitrogen-containing compounds. The condiment fermented with B. licheniformis OALB2 had the highest level of ketones at 888.80 µg kg−1 and was dominated by acetoin (875 µg kg−1), usually characterized as having a butter-like aroma.[Citation10] However, all Bacillus strains produced acetophenone, which is characterized by sweet and floral odours.[Citation24] The condiment fermented with B. licheniformis OALB2 had a profile characterized with overall high levels of ketones, pyrazines, and alcohols. Tetramethylpyrazine, trimethylpyrazine, and 2,5-dimethylpyrazine were produced in relatively high concentrations by all of the Bacillus starter cultures. Pyrazines are typical aroma components of heated food to which they give a characteristic roasted or nutty flavour. Metabolic activities of microorganisms generally generate various precursors such as amino acids, monosaccharides, and ammonia needed for the formation of pyrazines, while pyrazines are formed by accompanied non-enzymatic reaction such as heating.[Citation10] In terms of alcohol, 2,3-butanediol was produced at the highest level by B. licheniformis OALB2, with an odour description of fruity, creamy, and buttery.[Citation22] B. amyloliquefaciens SFBA2 produced the highest concentration of sulphur containing compounds. Sulphur-containing compounds, such as dimethyl disulphide, are reported to have strong pungent odours in dawadawa and they have a great influence on overall product aroma. B. amyloliquefaciens SFBA2 produced the highest level of dimethyl disulphide (41 µg kg−1). Dimethyl disulphide is described as onion, sulphurous, pungent[Citation25], sulphury, and cabbage-like.[Citation14] Compounds such as hexanal, 2-pentylfuran, 1-hexanol, and 1-octen-3-ol, produced by all the Bacillus strains, are recognized as contributing to the green and beany aroma of cooked beans.[Citation26]
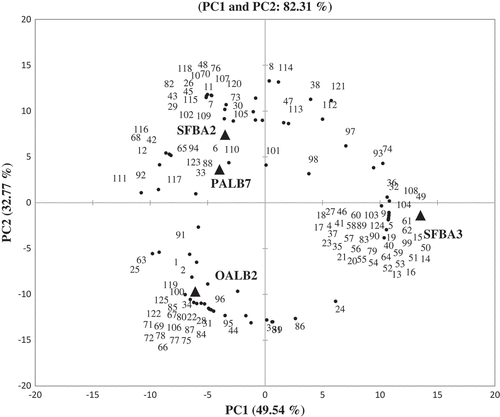
Despite some similarities, the aroma profiles of the different samples of dawadawa-type condiments were qualitatively and quantitatively different (). PCA on data from the aroma analysis of fermented samples showed that 82% of the variation could be explained by two principal components (P1 vs P2). A bi-plot showing scores and loadings shows the distinct compounds that differentiated each Bacillus group (). PC1 explaining 50% of variation, separated the condiment fermented with B. subtilis subsp. subtilis SFBA3 in the fourth quadrant on the right from the other three condiments on the left. The condiment fermented with SFBA3 was characterized by higher concentrations of 2-methyl-butanoic acid, 3-methyl-butanoic acid, 2-methyl-propanoic acid, acetic acid, 3-methyl-butanoic acid ethyl ester, 2-methyl-propanoic acid ethyl ester, 3-methyl-1-butanol, and benzyl alcohol ( and ). The B. licheniformis OALB2 condiment was associated with higher concentrations of acetoin, pyrazine, methylpyrazine, trimethylpyrazine, tetramethylpyrazine, and 2,3-butanediol. PC2 explained the additional 33% variation separating the condiment with B. amyloliquefaciens SFBA2 or B. cereus PALB7 in the second quadrant from the condiments fermented with B. licheniformis OALB2 in the third quadrant of the plot. Condiments fermented with the two strains, B. cereus PALB7 and B. amyloliquefaciens SFBA2, grouped together. The condiment fermented with B. cereus PALB7 was characterized with higher concentrations of benzaldehyde and hexanal, while B. amyloliquefaciens SFBA2 had higher concentrations of 2-methylbutanamine and indole.
Figure 1. Principal component loadings and scores of concentrations of volatile compounds in dawadawa-type condiments produced from alkaline fermentation of bambara groundnut. PALB7: with B. cereus PALB7; SFBA2: with B. amyloliquefaciens subp. plantarum SFBA2; OALB2: with B. licheniformis OALB2; and SFBA3: with B. subtilis subsp. subtilis SFBA3. Compounds 1–125, see .
The major volatile compound groups reported for traditional dawadawa made from African locust bean were pyrazines, aldehydes, alkenes, ketones, alcohols, esters, and benzene derivatives. Pyrazines have been found in highest concentrations in dawadawa from African locust bean.[Citation8,Citation11,Citation12,Citation27] In contrast, aldehydes, acids, and alcohols dominated dawadawa-type condiments from bambara groundnuts. Pyrazine levels in dawadawa-type condiments from bambara groundnut (339 µg kg−1) are considerably lower than that of African locust bean dawadawa (>448,000 µg kg−1).[Citation8,Citation11,Citation12] The low levels of pyrazines found in this study were probably due to the mild conditions of the headspace SPME method used. Other authors used the Likens–Nickerson simultaneous distillation–extraction method that could possibly influence pyrazine formation due to the higher heating step requirements. Heating protein-rich food matrices has been reported to influence the formation of pyrazines.[Citation10]
There are no studies reporting on the interrelationship of volatile compounds and dawadawa aroma/flavour in the literature. Beaumont[Citation1] reported on studies conducted in support of a US patent for flavourant composition prepared by fermentation[Citation28] suggesting a correlation between dawadawa aroma and the presence of 2-methyl butanoic acid and 3-methyl butanoic acid in the finished fermented protein base. Both 2,3-butanediol and 2-methylbutanoic acid are produced from branched-chained amino acids such as valine, leucine, and isoleucine during fermentation via catabolism processes, including oxidation and transamination.[Citation23] It is noteworthy that 2-methylbutanoic acid and 3-methylbutanoic acid with their accompanying esters, typically characteristic of dawadawa aroma, were produced by all the Bacillus starter cultures.
The odour activity values (OAVs) are presented in . OAVs give an indication of the odour potency of a single odourant in a food itself, based on its odour threshold in the respective food matrix.[Citation23,Citation29] The OAVs for prominent volatile compounds in dawadawa-type condiments were calculated by dividing the measured concentrations with odour thresholds obtained from the literature.[Citation29] Of all the volatile compounds detected, only those displaying OAVs greater than one, which are hexanal, benzaldehyde, benzeneacetaldehyde, nonanal, heptanal, decanal, 3-methylbutanoic acid, 2-methylbutanoic acid, acetoin, and dimethyl disulphide were deemed to contribute to overall dawadawa aroma. The OAVs for 3-methyl butanoic acid and 2-methyl butanoic acid, the compounds reported as the main indication of typical dawadawa aroma[Citation1] were highest for B. subtilis subsp. subtilis SFBA3 with values of 14 and 16, respectively (). However, the OAVs for these compounds were less than one for the other condiments, except for B. licheniformis OALB2 which had an OAV of two for 3-methyl butanoic acid. The aldehydes, hexanal, benzaldehyde, nonanal, and decanal, characterised the Bacillus fermented condiments with OAVs greater than one recorded for all. Dimethyl disulphide was not detected in OALB2, but high OAVs were recorded for the other three condiments.
Table 2. Odour thresholds and odour activity values (OAVs) of volatile compounds in dawadawa-type condiments.
Dawadawa aroma from Bacillus fermented legumes has not been fully characterised, yet the volatile compounds reported here is only an indication of several compounds that may be contributing to the perceived pungent, ammoniacal, and putrid aroma; however, more work is required in identifying correlations of specific volatile compounds that classify dawadawa aroma in particular. In addition, the suitability of the Bacillus starter cultures for dawadawa production needs to be ascertained by sensory evaluation and testing of consumer acceptability of the condiments produced.
Conclusion
In this study, the effect of different Bacillus starter cultures on the volatile flavour compounds of dawadawa-type condiment from bambara groundnut was investigated. Headspace SPME and GC × GC–TOFMS analysis of the volatile profiles indicate that distinct chemical profiles were observed for each of the four Bacillus strains. Differences in the levels of volatile compounds in condiments produced by Bacillus starter cultures were observed. Both the concentrations of components and also the proportions of compounds in the condiments from different starter cultures differed significantly. Aldehydes, acids, and ketones were identified as key volatile compounds. All Bacillus strains were able to produce 2- and 3-methylbutanoic acid in considerable amounts, B. subtilis subsp. subtilis SFBA3 fermentation was indicative of dawadawa aroma with the production of high levels of 2-methyl butanoic acid and 3-methyl butanoic acid in the finished fermented but low levels were observed in the other strains. This may suggest that this Bacillus strain has potential as a commercial starter culture for alkaline fermentation and production of dawadawa from bambara groundnuts.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Beaumont, M. Flavouring Composition Prepared by Fermentation with Bacillus spp. International Journal of Food Microbiology 2002, 75(3), 189–196. DOI: 10.1016/S0168-1605(01)00706-1.
- Omafuvbe, B. O.; Abiose, S. H.; Shonukan, O. O. Fermentation of Soybean (Glycine max) for Soy-Daddawa Production by Starter Cultures of Bacillus. Food Microbiology 2002, 19, 561–566. DOI: 10.1006/fmic.2002.0513.
- Amadi, E. N.; Barimalaa, I. S.; Omosigho, J. Influence of Temperature on the Fermentation of Bambara Groundnut (Vigna Subterranea) to Produce a Dawadawa-Type Product. Plant Foods for Human Nutrition 1999, 54(1), 13–20. DOI: 10.1023/A:1008003118374.
- Barimalaa, I. S.; Achinewhu, S. C.; Yibatama, I.; Amadi, E. N. Studies on the Solid Substrate Fermentation of Bambara Groundnut (Vigna subterranea (L) Verde). Journal of the Science of Food and Agriculture 1994, 66(4), 443–446. DOI: 10.1002/(ISSN)1097-0010.
- Fadahunsi, I. F.; Olubunmi, P. D. Microbiological and Enzymatic Studies during the Development of an ‘Iru’ (A Local Nigerian Indigenous Fermented Condiment) like Condiment from Bambara Nut [Voandzeia subterranea (L) Thours]. Malaysian Journal of Microbiology 2010, 6(2), 123–126.
- Parkouda, C.; Nielsen, D. S.; Azokpota, P.; Ouoba, L. I. I.; Amoa-Awua, W. K.; Thorsen, L.; Hounhouigan, J. D.; Jensen, J. S.; Tano-Debrah, K.; Diawara, B. The Microbiology of Alkaline-Fermentation of Indigenous Seeds Used as Food Condiments in Africa and Asia. Critical Review of Microbiology 2009, 35(2), 139–156. DOI: 10.1080/10408410902793056.
- Allagheny, N.; Obanu, Z. A.; Campbell-Platt, G.; Owens, J. D. Control of Ammonia Formation during Bacillus subtilis Fermentation of Legumes. International Journal of Food Microbiology 1996, 29(2–3), 321–333. DOI: 10.1016/0168-1605(95)00069-0.
- Azokpota, P.; Hounhouigan, J. D.; Annan, N. T.; Nago, M. C.; Jakobsen, M. Diversity of Volatile Compounds of Afitin, Iru and Sonru, Three Fermented Food Condiments from Benin. World Journal of Microbiology and Biotechnology 2008, 24(6), 879–885. DOI: 10.1007/s11274-007-9542-0.
- Leejeerajumnean, A.; Duckham, S. C.; Owens, J. D.; Ames, J. M. Volatile Compounds in Bacillus-Fermented Soybeans. Journal of the Science of Food and Agriculture 2001, 81(5), 525–529. DOI: 10.1002/(ISSN)1097-0010.
- Owens, J. D.; Allagheny, N.; Kipping, G.; Ames, J. M. Formation of Volatile Compounds during Bacillus subtilis Fermentation of Soya Beans. Journal of the Science of Food and Agriculture 1997, 74(1), 132–140. DOI: 10.1002/(ISSN)1097-0010.
- Azokpota, P.; Hounhouigan, J. D.; Annan, N. T.; Odjo, T.; Nagoa, M. C.; Jakobsen, M. Volatile Compounds Profile and Sensory Evaluation of Beninese Condiments Produced by Inocula of Bacillus subtilis. Journal of the Science of Food and Agriculture 2010, 90(3), 438–444.
- Ouoba, L. I. I.; Diawara, B.; Annan, N. T.; Poll, L.; Jakobsen, M. Volatile Compounds of Soumbala, a Fermented African Locust Bean (Parkia biglobosa) Food Condiment. Journal of Applied Bacteriology 2005, 99(6), 1413–1421. DOI: 10.1111/jam.2005.99.issue-6.
- Junior, S. B.; De Melo, A. D. M. T.; Zini, C. A.; Godoy, H. T. Optimization of the Extraction Conditions of the Volatile Compounds from Chili Peppers by Headspace Solid Phase Micro-Extraction. Journal of Chromatography 2011, 1218(21), 3345–3350. DOI: 10.1016/j.chroma.2010.12.060.
- Mahattanatawee, K.; Rouseff, R. L.Comparison of Aroma Active and Sulfur Volatiles in Three Fragrant Rice Cultivars Using GC–Olfactometry and GC–PFPD. Food Chemistry 2014, 154, 1–6. DOI: 10.1016/j.foodchem.2013.12.105.
- Xu, Y.; Limwachiranon, J.; Li, L.; Ru, Q.; Luo, Z. Characterisation of Volatile Compounds of Farmed Soft-Shelled Turtle (Pelodiscus sinensis) by Solid-Phase Microextraction and the Influence of Matrix pH on the Release of Volatiles. International Journal of Food Science & Technology 2017, 52(1), 275–281. DOI: 10.1111/ijfs.13279.
- Zhang, Y.; Fraatz, M. A.; Horlamus, F.; Quitmann, H.; Zorn, H. Identification of Potent Odorants in a Novel Nonalcoholic Beverage Produced by Fermentation of Wort with Shiitake (Lentinula edodes). Journal of Agricultural and Food Chemistry 2014, 62(18), 4195–4203. DOI: 10.1021/jf5005463.
- Gao, P.; Wang, W.; Jiang, Q.; Xu, Y.; Xia, W. Effect of Autochthonous Starter Cultures on the Volatile Flavor Compounds of Chinese Traditional Fermented Fish (Suan Yu). International Journal of Food Science & Technology 2016, 51, 1630–1637. DOI: 10.1111/ijfs.13134.
- Naudé, Y.; Rohwer, E. R. Investigating the Coffee Flavour in South African Pinotage Wine Using Novel Offline Olfactometry and Comprehensive Gas Chromatography with Time of Flight Mass Spectrometry. Journal of Chromatography 2013, 1271(1), 176–180. DOI: 10.1016/j.chroma.2012.11.019.
- Kataoka, H.; Lord, H.; Pawliszyn, J. Application of Solid-Phase Microextraction in Food Analysis: Review. Journal of Chromatography 2000, 880(1–2), 35–62. DOI: 10.1016/S0021-9673(00)00309-5.
- Akanni, G. B.; Naudé, Y.; De Kock, H. L.; Buys, E. M. Diversity and Functionality of Bacillus Species Associated with Alkaline Fermentation of Bambara Groundnut (Vigna subterranean L. Verdc) into Dawadawa-Type African Condiment. European Food Research Technology 2018, DOI: 10.1007/s00217-017-3024-x.
- Pham, A. J.; Schilling, M. W.; Mikel, W. B.; Williams, J. B.; Martin, J. M.; Coggins, P. C. Relationships between Sensory Descriptors, Consumer Acceptability and Volatile Flavour Compounds of American Dry-Cured Ham. Meat Science 2008, 80(3), 728–737. DOI: 10.1016/j.meatsci.2008.03.015.
- Zhao, J.; Dai, X.; Liu, X.; Zhang, H.; Tang, J.; Chen, W. Comparison of Aroma Compounds in Naturally Fermented and Inoculated Chinese Soybean Pastes by GC–MS and GC–Olfactometry Analysis. Food Control 2011, 22(6), 1008–1013. DOI: 10.1016/j.foodcont.2010.11.023.
- Park, H.-J.; Lee, S. M.; Song, S. H.; Kim, Y.-S. Characterization of Volatile Components in Makgeolli, a Traditional Korean Rice Wine, With or Without Pasteurization, During Storage. Molecules 2013, 18(5), 5317–5325. DOI: 10.3390/molecules18055317.
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace Leaf Samples Using GC, GC–MS, and Olfactometry. Journal of Agricultural and Food Chemistry 2002, 50(16), 4643–4646. DOI: 10.1021/jf020129n.
- Chin, S.-T.; Eyres, G. T.; Marriott, P. J. Identification of Potent Odourants in Wine and Brewed Coffee Using Gas Chromatography–Olfactometry and Comprehensive Two-Dimensional Gas Chromatography. Journal of Chromatography 2011, 1218(42), 7487–7498. DOI: 10.1016/j.chroma.2011.06.039.
- Sugawara, E.; Ito, T.; Odagiri, S.; Kubota, K.; Kobayashi, A. Comparison of Compositions of Odor Components of Natto and Cooked Soybeans. Agricultural and Biological Chemistry 1985, 49(2), 311–317.
- Onyenekwe, P. C.; Odeh, C.; Nweze, C. C. Volatile Constituents of Ogiri, Soybean Daddawa and Locust Bean Daddawa Three Fermented Nigerian Food Flavour Enhancers. Electronic Journal of Environmental, Agricultural and Food Chemistry 2012, 11, 115–221.
- Heyland, S.; Dac, T. H.; Hose, H.; Wood, R. D. Flavorant Composition Prepared by Fermentation. U.S. Patent 5,476,773, 1995.
- Van Gemert, L. J.; Netternbreijer, A. H. Compilation of Odour Threshold Values in Air and Water; TNO Central Institute for Nutrition and Food Research: Zeist, The Netherlands, 1977.
