 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Physicochemical and functional properties of freeze-dried egg powders (egg white, egg yolk, and whole egg) from Japanese quail and white Leghorn chicken were studied comparatively. All egg powders had protein content in the range of 91.13–97.03 g/100 g powder. The quail egg powder had higher mineral and essential amino acid contents, but lower fat content as compared to chicken egg powders (P < 0.05). Moreover, egg white powder from both quail and chicken presented higher total amino acids content than corresponding whole egg and egg yolk powders, respectively. Fourier transform infrared spectroscopic study revealed that β-sheet is the major secondary structure of all egg powders. Based on differential scanning calorimetry analysis, quail egg powders showed slightly lower denaturation temperatures than corresponding chicken egg powders (P < 0.05). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis study showed the slight difference in protein patterns of corresponding quail and chicken egg powders. The quail egg powders presented higher protein solubility than corresponding chicken egg powders at all pH tested. Furthermore, quail egg powders exhibited higher emulsion activity index and emulsion stability index with higher foam expansion and stability than the corresponding chicken egg powders. Therefore, Japanese quail egg powders could be used as an alternative to white Leghorn chicken egg in the preparation of foods and diets that require high protein content with positive health benefits.
Introduction
The eggs have been documented as one of the best sources of high-quality protein which contains all essential amino acids for human diet throughout the world.[Citation1,Citation2] The high protein content of egg makes it an excellent potential protein source for food industry applications. In addition to high-quality proteins, the egg is a good source of valuable vitamins, minerals, and growth factors.[Citation1,Citation3] Apart from nutritional importance, egg proteins possess multiple functional properties including emulsification, foaming, and gelation.[Citation4–Citation6] Protein solubility is considered as a prerequisite for the major functional properties including gelation, emulsification, and foam formation.[Citation6] Generally, egg white and the egg yolk were sources of dietary protein.[Citation1,Citation3] Egg white (albumen) accounts about 56.9% of the entire egg mass and has a protein content of 10.6%.[Citation7,Citation8] Egg white contains mainly ovalbumin, ovotransferrin, ovomucoid, globulins, and lysozyme.[Citation9] Besides, egg yolk represents about 32.8% of the total egg weight, of which 80% are the water-soluble plasma fraction and 20% insoluble granules.[Citation7,Citation8,Citation10] Yolk plasma is composed of 85% low-density lipoproteins (LDL) and 15% globular glycoproteins recognized as α-, β-, and γ-livetins.[Citation11,Citation12] LDL apoproteins were termed as lipovitellins, which are the main constituent of egg yolk and represent about 68% of its total dry matter.[Citation7,Citation8] Mine[13Citation13] demonstrated that the LDL is a prevailing factor responsible for emulsifying properties of egg yolk. On the other hand, the granule fraction of yolk consists of 70% high-density lipoproteins (HDL), 16% phosvitins, and 12% LDL.[Citation12] In general, egg yolk proteins are considered as more important egg proteins in the food industry due to the excellent functional properties of its lipoproteins, and hence it is widely used in many applications ranging from a bakery to the production of cold sauces and salad dressings.[Citation14,Citation15] Due to its application in the food system, chicken egg proteins have been very well investigated for physicochemical, nutritional, and functional properties. However, such studies were limited to other poultry eggs. Despite its wide applications in food, chicken eggs are traditionally associated with adverse factors on human health, mainly due to their high cholesterol content. Therefore, there has been growing interest in alternative bird species to meet the growing demand for high-value protein egg with reasonable cost as well as health conscious.
Japanese quail is one of the most promising sources of livelihood to accomplish the demand for high protein egg with positive health benefits. Japanese quail, Coturnix japonica belongs to the order Galliforms and family Phasianidae. The species have gained value as a food animal due to the unique flavor of its eggs and meat. It has been claimed that the eggs from Japanese quail are rich sources of protein with low fat and cholesterol.[Citation16] Apart from the high nutritional value, some experts have been appealed that the quail eggs help in the treatment of tuberculosis, bronchial asthma, and diabetes.[Citation17] Regardless of wide nutritional importance, the studies on physiochemical and functional properties of Japanese quail egg proteins are still limited concerning chicken egg proteins. Ironically, none has assessed the comparative studies on physiochemical, nutritional, and functional properties of Japanese quail egg proteins with white Leghorn chicken eggs. Moreover, the egg production from quail is negligible in India when compared to chicken mainly due to less awareness of quail egg productions and lack of studies on nutritional and functional qualities. Currently, the utilization of egg proteins in the form of dried egg powder has gained increasing attention due to convenient in the storage and transport at room temperature.[Citation2] Furthermore, dried egg powders possessed quite a stability and long shelf life at room temperature.[Citation2] However, drying of egg liquid might influence the functional properties of egg proteins. Therefore, the objective of this study was to compare the physicochemical and functional properties of freeze-dried egg powders prepared from Japanese quail and white Leghorn chicken.
Materials and methods
Chemicals
Sodium chloride, sodium hydroxide, hydrochloric acid, and hexane were purchased from Merck (Darmstadt, Germany). Bovine serum albumin, wide-range molecular weight protein markers were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Sodium dodecyl sulfate, Coomassie Blue R-250, and N, N, N′, N′-tetramethyl ethylene diamine were procured from Bio-Rad Laboratories (Hercules, CA, USA). All chemicals were of analytical grade.
Preparation of egg powders from Japanese quail eggs and chicken eggs
Eggs (1–2 days old) from Japanese quail (300 eggs) and white Leghorn chicken (100 eggs) were obtained from the College of Veterinary Science, Rajendranagar, Hyderabad, India, and Supermarket, Mysore, India, respectively. These eggs were kept at 4°C until use, but not longer than 15 days. Before powder preparation, eggs from both Japanese quail and white Leghorn chicken were checked for their freshness using the candling method. Good quality fresh eggs were cleaned by dusting and washing, and allowed to dry. These cleaned eggs were carefully deshelled and separated as egg white liquid, egg yolk liquid, and whole egg liquid manually. Resulting egg liquids from both quail and chicken eggs were homogenized for 4–5 min with a metal whisk to form a uniform solution. To prevent the browning and contamination of Salmonella, hydrogen peroxide (1–2 drop) was added during homogenization of egg liquids. Further, all these egg liquid samples were freeze-dried using a freeze dryer (ScanVac Model CoolSafe 55–4, Lynge, Denmark). Dried whole egg and yolk powders from both Japanese quail and white Leghorn chicken eggs were defatted by mixing hexane at a ratio of 1:5 (w/v) at room temperature for 30 min. Thereafter, the mixtures were subjected to centrifugation at 12,000 x g at 25°C for 20 min. The resulting pellet was air-dried at room temperature until dry and free of solvent odor. All egg powders were placed in polyethylene bags, sealed, and stored at −20°C until analyses.
Proximate composition
All egg powder samples from both Japanese quail and white Leghorn chicken were subjected to proximate analyses, including moisture, protein, fat, and ash content following the method of Association of Official Analytical Chemists[Citation18] with the analytical no. 925.09, 954.01, 920.39, and 923.03, respectively.
Amino acid analysis
Amino acid compositions of all egg powders were determined using a commercial EZ:faast™ amino acid analysis kit (Phenomenex, Torrance, CA, USA) using gas chromatography system (Shimadzu GC 2014; M/s Shimadzu, Kyoto, Japan) furnished with a flame ionization detector for identifying individual amino acids. All samples were hydrolyzed under reduced pressure in 6 M hydrochloric acid containing 4% thioglycolic acid at 110°C for 24 h. The acid hydrolyzed samples were esterified according to EZ:faast™ amino acid analysis kit. Amino acid esters dissolved in chloroform were analyzed on a Column ZB-AAA-10 m (10 m × 0.25 mm × 0.25 m; Phenomenex, Torrance, CA, USA) with a split ratio of 1:15. The temperatures of injector, column, and detector were set at 300°C, 110°C, and 320°C at a gradient rate of 35°C min−1, respectively. The amino acids were identified by comparing them with authentic standards. Amino acid contents were expressed as g/100 g sample.
Fourier transform infrared (FTIR) spectra analysis
FTIR analysis of all egg powder samples was performed using Perkin Elmer FTIR Spectrophotometer (Perkin Elmer Spectrum Version 10.03.09, Spectrum Two). FTIR spectrophotometer furnished with an attenuated total reflectance (ATR) (Pike Miracle, Pike Technologies, Madison, USA), deuterated triglycine sulfate detector, mid-infrared source, and single reflection horizontal ATR accessory (PIKE instruments) having a diamond ATR crystal fixed at an incident angle of 45°. For spectral analysis, egg powder samples were placed in the crystal cell which was clamped into the mount of the FTIR spectrometer. The spectra in the range of 4000–600 cm−1 were ratioed and automatic signals gained were collected in 16 scans at a resolution of 4 cm−1 against a background spectrum recorded from the clean empty cell at room temperature (25°C).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Protein patterns of all egg powder samples were determined using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) according to the method of Laemmli.[Citation19] The egg powder samples were mixed with the sample buffer (0.5 M Tris HCl, pH 6.8, containing 4% SDS, 20% glycerol, and 10% βME) at the ratio of 1:1 (v/v) and boiled for 3 min. The samples (15 μg protein) were loaded onto the polyacrylamide gel made of 12% running gel and 4% stacking gel. Gel subjected to electrophoresis at a constant current of 25 mA per gel using a Mini-Protean III Bio-Rad unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, the gel was stained with 0.02% (w/v) Coomassie brilliant blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid, and destained with 50% methanol (v/v) and 7.5% (v/v) acetic acid, followed by 5% methanol (v/v) and 7.5% (v/v) acetic acid. Wide-range molecular weight markers (Sigma Chemical Co., St. Louis, MO, USA) were used to estimate the molecular weight (MW) of proteins.
Differential scanning calorimetry
Thermal properties of egg powder samples were examined using a differential scanning calorimeter (DSC) (Perkin Elmer, Model DSC-7, Norwalk, CT, USA) as per the method of Segura-Campos et al.[Citation5] with some modifications. Egg powders (25%, w/v) were dispersed in water (pH 7.0). The mixtures were allowed to equilibrate for 1 h at room temperature. The samples (5 mg) were accurately weighed into aluminum pans. The pans were hermetically sealed and heated from 30°C to 120°C at a rate of 5°C min−1. A sealed empty pan (aluminum) was used as a reference. Denaturation temperature (Td) and enthalpy (ΔH) were computed from the thermograms.
Solubility study
Effect of pH (3–10) on the solubility of egg powders (1%, w/v) was determined according to the method of Machado et al.[Citation9] with some modification. Protein isolates (100 mg) were suspended in 10 ml of distilled water, and the pH of the suspensions was adjusted to different pHs (3–10) with either 1 M HCl or 1 M NaOH using a pH meter (model pH2700, Eutech Instrument, Pte, Ltd, Singapore). These suspensions were stirred magnetically for 1 h at room temperature. The pH was checked, readjusted to the tested pH and then centrifuged at 11000 x g for 20 min. The supernatants were collected separately. The protein content of all supernatants was determined by the method of Lowry et al.[Citation20] using BSA as a standard. Total protein content in egg powders was determined after complete solubilization of sample using 0.5 M NaOH as described by Kudre et al.[Citation21] Protein solubility was calculated as follows:
Determination of emulsifying properties
Emulsion activity index (EAI) and emulsion stability index (ESI) of egg powder samples were determined according to the method of Kudre and Benjakul[Citation22] with a slight modification. Soybean oil (2 ml) and egg powder suspension (1%, 2%, or 3% egg powder, 6 ml) were homogenized using a homogenizer at a speed of 20,000 x g for 2 min. Emulsion samples were pipetted out at 0 and 10 min and diluted with 0.1% SDS at 100 fold. The mixture was vortexed for 10 s to mix thoroughly. The resulting dispersion was measured at 500 nm using a spectrophotometer. EAI and ESI were determined by the following formulae:
where A is the absorbance measured at 500 nm, l is the path length of cuvette (m), DF is the dilution factor (100), ø is the oil volume fraction, and C is the protein concentration in aqueous phase (g/m3).
Here, A0 = absorbance at time of 0 min; A10 = absorbance at time of 10 min; and Δt = 10 min.
Determination of foaming properties
Foam expansion (FE) and foam stability (FS) of egg powder samples were determined as described by Kudre and Benjakul[Citation22] with a slight modification. Egg powder suspension (1%, 2%, or 3%) was transferred into 100-ml cylinders. The suspension was homogenized at a speed of 13,400 x g for 1 min at room temperature. The suspension was allowed to stand for 0 and 60 min. FE and FS were then calculated using the following equations:
where V0 = the original volume of suspension before whipping; VT = total volume of suspension after whipping; and Vt = total volume of suspension after leaving at room temperature for 60 min.
Statistical analysis
All experiments were run in triplicate, and a completely randomized design was used. Data were subjected to analysis of variance. Mean was compared by Duncan’s multiple range tests.[Citation23] The analysis was performed using an SPSS package (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Proximate compositions
Proximate compositions of egg powders from quail and chicken are shown in . In general, quail egg powders showed slightly lower in lipid content and higher in mineral content when compared with respective chicken egg powders. All egg powders exhibited the protein contents in the range of 91.13–97.03 g/100 g powder, which were higher than that of the Department of Agriculture database.[Citation24] Baniel et al.[Citation25] and Segura-Campos et al.[Citation5] reported that aspersion-dried chicken egg and air-dried quail egg white contained 90 g/100 g powder and 93.96 g/100 g powder protein, respectively. Among all egg powders, egg white showed slightly higher protein content, followed by whole egg powders and yolk powders, respectively, irrespective of egg source (P < 0.05). The relatively lower protein content of whole egg and yolk powders could be explained by its high lipid, moisture, and ash contents. It has been reported that generally egg white contained slightly higher protein than egg yolk when dried.[Citation3,Citation16] Furthermore, no significant difference in the protein contents of corresponding powders of quail and chicken egg was observed (P ˃ 0.05). This result suggested that quail and chicken eggs were quite similar in protein contents. The fat content of all egg powders was ranged from 0.10 to 3.45 g/100 g powder. The yolk powder from both quail and chicken had a higher fat content than that of whole egg and egg white powders, respectively (P < 0.05). In general, egg yolk possesses higher lipids than egg white. Further, the quail egg powders showed slightly lower fat contents compared to the corresponding chicken egg powders (P < 0.05). This result suggested that the quail eggs were low in lipid content. All egg powders showed moisture contents in the range of 0.64–1.29 g/100 g powder, indicated that all egg powders had above 95 g/100 g powder solid matter content. The solid matter contents of egg powders were in accordance with the criteria of the Department of Agriculture database [Citation24] in which solid matter of dried egg must be above 95 g/100 g powder. Yolk powders exhibited slightly higher moisture contents compared to a whole egg and egg white powders, respectively (P < 0.05). Quail egg powders had higher ash contents than that of corresponding chicken egg powders (P < 0.05). The results suggested that quail egg had a higher concentration of minerals compared to the chicken counterpart. Furthermore, yolk powder from quail and chicken had higher ash content followed by whole egg powders and egg white, respectively, regardless of egg source (P < 0.05). This result indicated that a yolk portion of quail and chicken egg contains a higher amount of minerals compared to the egg white. Ash content of yolk powder from quail and chicken was 4.08 g/100 g powder and 3.49 g/100 g powder, respectively. From the results, it is worth highlighting that egg powders from quail egg were richer in minerals and lower in lipid content as compared to chicken egg powders. Therefore, quail egg could be a good egg resource for consumers to choose.
Table 1. Proximate compositions of egg powders from Japanese quail and white Leghorn chicken.
Amino acid composition
Amino acid compositions of egg powders from quail and chicken are depicted in . Amino acid composition is one of the most important factors determining the quality of food protein. Egg powders from both quail and chicken possessed different amino acid compositions, more likely due to variation in protein composition, egg source, and diet. Egg white powder from both quail and chicken had a higher content of total (ΣNAA + ΣEAA) amino acids followed by the whole egg and yolk powder, respectively. The higher content of amino acids in egg white powders is more likely due to high protein contents (). All egg powders exhibited aspartic acid, alanine, glutamic acid, proline, glycine, and serine were the dominant non-essential amino acids, whereas leucine, phenylalanine, isoleucine, lysine, and valine were the dominant essential amino acids. It was interesting to note that all egg powders from quail showed higher in essential amino acids and lower in nonessential amino acids as compared to corresponding chicken egg powders. Among quail egg powders, egg white had a high content of total essential amino acids (49.72 g/100 g powder) than that of whole egg (46.92 g 100 g−1 powder) and yolk powders (45.65 g/100 g powder), respectively. Genchev[Citation17] also reported that egg white of quail exhibited the high content of essential amino acids as compared to yolk protein.
Table 2. Amino acid compositions of egg powders from Japanese quail and white Leghorn chicken.
FTIR spectra
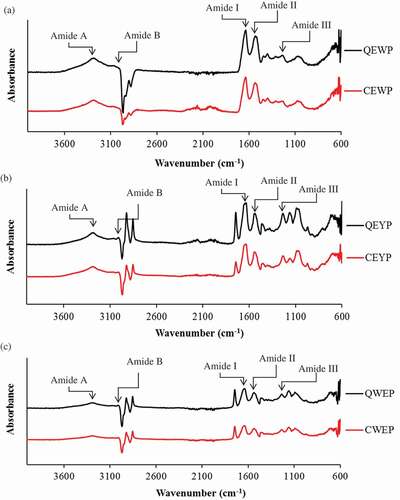
FTIR spectra of all egg powders from quail and chicken egg are depicted in . Several major bands were observed in the region of 4000–600 cm−1. All egg powders exhibited amide A band at a wavenumber of 3292 cm−1. This band is associated with free N–H stretching vibration and indicates the existence of hydrogen bonds, which occurs in the range of 3300–3440 cm−1.[Citation21] When the NH group of a peptide is involved in a hydrogen bond, the position is shifted to lower wavenumber. The amide B band was observed for the yolk and whole egg powders from both quail and chicken eggs at 2923 cm–1 while the egg white powders from quail and chicken egg showed at 2907 and 2903 cm–1, respectively. Amide B band is corresponding to the asymmetric stretch vibration of = C–H as well as –NH3+. All egg powders displayed amide I band at the wavenumber of 1633–1640 cm−1. The amide I region between the wave numbers 1700 and 1600 cm−1 is the most informative part of the FTIR spectrum of the secondary structure of proteins. The amide I vibration mode is associated with stretching vibration of C = O, which belongs to the amide groups weakly coupled with in-plane NH bending and CN stretching.[Citation21] Egg white, yolk, and whole egg powder from quail eggs had the amide I bands at the wavenumber of 1635, 1636, and 1640 cm−1, respectively, whereas egg white, yolk, and whole egg powder from chicken appeared at the wavenumbers 1636, 1633, and 1641 cm−1, respectively. The absorption peak at amide І (1623–1641 cm−1) was characteristic of the β-sheet structure of egg proteins.[Citation26] The results indicated that the predominant secondary structure of all egg powders from both quail and chicken were β-sheet. Moreover, some prominent peaks were observed at wavenumbers of 1744–1746 cm−1 for the yolk and whole egg powders from both quail and chicken, more likely corresponding to C = O stretching frequency of a protonated carboxylic group. For the amide II band, quail egg powders showed the peaks at a wavenumber of 1536 cm−1, whereas chicken egg powders exhibited the amide II peak at a wavenumber of 1537 cm−1. The amide II vibration modes are attributed to an out-of-phase combination of the NH in-plane bend and the CN stretching vibration with smaller contributions from the CO in-plane bend and the CC and NC stretching vibrations. Amide II arises from bending vibration of N–H groups and stretching vibrations of C–N groups. Furthermore, bands for amide III appeared at a wavenumber of 1235, 1244, and 1238 cm−1 for egg yolk, egg white, and whole egg powder of quail, and 1234, 1244, and 1235 cm−1 for egg yolk, egg white, and whole egg powder of chicken, respectively. Amide III bands were associated with the in-phase combination of the NH bending and the CN stretching vibration with small contributions from the CO in-plane bending and the CC stretching vibration.[Citation21] Also, some prominent peaks were observed at wavenumbers of 1155–1164 cm−1 for all egg powders, corresponding to C–O stretching modes resulting from ester bonds O = C–O and vibrations of OH, CC, CO, CCH, COH, and POC bonds, respectively.[Citation21] The spectral differences in amide bands of different egg powder samples were mainly attributed to a different conformation of polypeptide chains. Furthermore, whole egg and yolk powders from both quail and chicken egg were shown two peaks at 2854 and 2931 cm−1 more likely denoted to the fat or lipid.[Citation26] This result was in accordance with the higher content of lipid in whole egg and yolk powders (). It was noticed that quail egg powders showed slightly higher amplitude amide bands compared to corresponding chicken egg powders which indicated that chicken egg powders had slightly looser structure. This might be due to the unfolding of proteins to some extent. This was in agreement with the lower Td of quail egg powders (). The lower amplitude at amide bands was associated with the greater degree of molecular order due to the interaction of C = O with adjacent chains via hydrogen bonds.
Table 3. DSC thermogram values of egg powders from Japanese quail and white Leghorn chicken.
Figure 1. FTIR spectra of egg white powder (a), egg yolk powder (b), and whole egg powder (c) from Japanese quail and white Leghorn chicken. QEWP: quail egg white powder; QEYP: quail egg yolk powder; QWEP: quail whole egg powder; CEWP: chicken egg white powder; CEYP: chicken egg yolk powder; CWEP: chicken whole egg powder.
Protein patterns
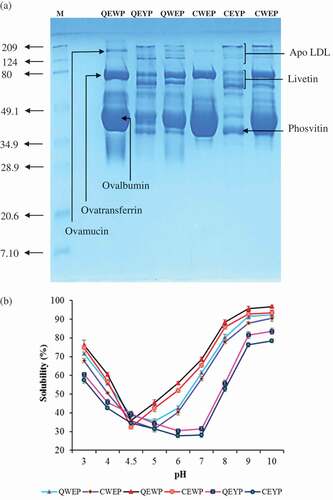
SDS–PAGE protein patterns of all egg powders from quail and chicken under reducing conditions are illustrated in . Slight difference in protein patterns of corresponding egg powders of quail and chicken were observed. It was noticed that all egg powders had several polypeptide bands. Egg white powder from both quail and chicken showed similar protein patterns, except slight differences in band intensity of proteins with an MW of 200 and ˃209 kDa. Three major protein bands were found in both quail and chicken egg white powders, which were attributed to ovalbumin (45.5 kDa), ovotransferrin (80.0 kDa), and ovomucin (200 kDa). The smear bands with MW ranged from 50 to 68 kDa were probably corresponded to G2 or G3 globulins and avidin.[Citation27] As per literature information, lysozyme is a relatively small protein with MW of ~14.3 kDa.[Citation5,Citation28] However, in the present study, such a lysozyme band did not appear in both egg white powders. During freeze-drying, lysozyme protein might have denatured, resulted in a loss of the lysozyme in both egg white powders. Electrophoretic study of yolk proteins () revealed that both yolk powders had several protein bands with an MW range of 37–209 kDa. However, the slight difference in protein patterns of quail and chicken yolk powders was noticed. The differences in protein compositions might result in the different properties and characteristics between the quail and chicken. In egg yolk proteins, the protein bands with an apparent MW of 194 and 136 kDa were most likely ascribed as LDL apoprotein. Different studies were reported in the literature that the MW for LDL apoproteins (18 polypeptides) is between 15 and 240 kDa.[Citation29] Furthermore, these bands with an MW of 194 and 136 kDa were appeared in both egg yolk powders, suggesting that both quail and chicken eggs had LDL apoproteins. Nevertheless, chicken egg yolk showed a low-intensity band of 136 kDa, compared to quail egg yolk powder. The protein bands with MW ranged from 60 to 83 kDa were attributed to α-livetin (72–83 kDa) and γ-livetin (60–70 kDa) proteins in the avian egg yolk.[Citation11,Citation29] However, some authors have proposed that some polypeptides ranged from 60 to 70 kDa belonged to LDL apoproteins.[Citation4,Citation11] Both egg yolk powders showed the similar four protein bands with an MW of 80, 72, 68, and 60 kDa. The results indicated that egg yolk powders contained α- and γ-livetin proteins. However, slight differences in protein band intensities or thickness were found between quail and chicken. The protein bands with an MW of 72 and 68 kDa were thicker and lighter in quail yolk compared to corresponding bands in chicken yolk protein. The thickness of the band corresponds to the amount of protein present; thus, result suggested that the proportions of livetin proteins varied between quail and chicken egg yolk powders. Furthermore, some protein bands found in the range of 43–48 kDa in both egg yolk powders mostly corresponded to yolk phosvitin. Phosvitin is a phosphoglycoprotein which forms the structure of yolk granules by making phosphocalcic bridges with HDL. Additionally, phosvitin has strong chelating properties and is a natural metal-binding biomolecule. It is composed of α- and β-phosvitin. The α-phosvitin is an aggregate of three or four subunits, and the β-phosvitin is an aggregate of four or five subunits. Quail yolk powder exhibited three bands with MW of 48, 46, and 43 kDa, while chicken egg powder had two bands with MW of 48 and 45 kDa. Additional protein with an MW of 43 kDa appeared in quail egg yolk powder more likely corresponded to subunit α-phosvitin or HDL apoproteins.[Citation11] The result suggested that quail egg yolk powder has a higher content of α-phosvitin or HDL as compared to chicken yolk powder. Since, yolk phosvitin has a very strong affinity to bivalent metals such as calcium, magnesium, and iron, and it carries a high number of minerals. Thus, the result was in agreement with the higher ash content (mineral) in quail egg yolk powder (). The main protein components of the egg whites and yolks of quail and chicken egg appeared in respective whole egg powders. Furthermore, diffused smear faint bands with MW ranged from 30 to 40 kDa were observed in quail whole egg and yolk powder. These bands most likely contributed to HDL apoproteins.[Citation11]
Figure 2. Protein patterns (a) and effects of different pHs on solubility (b) of egg powders from Japanese quail and white Leghorn chicken. M: marker; QEWP: quail egg white powder; QEYP: quail egg yolk powder; QWEP: quail whole egg powder; CEWP: chicken egg white powder; CEYP: chicken egg yolk powder; CWEP: chicken whole egg powder. Bars represent the standard deviation (n = 3).
Thermal transition
DSC thermograms of egg powders from quail and chicken eggs are expressed as denaturation temperature (Td) and ΔH as shown in . Egg white and whole egg powders from both quail and chicken showed two major endothermic peaks. However, yolk powders had single endothermic peaks. The result suggested that all egg powders had variance in thermostability more likely due to the difference in proteins configuration and conformation. For egg white powders, peak 1 and peak 2 could be attributed to Td of the ovotransferrin and ovalbumin, respectively.[Citation2,Citation6] Several researchers stated that egg white proteins, such as ovotransferrin, were less heat stable which could denature between 55°C and 65°C, whereas ovalbumin is more heat stable protein and denature around 84–86°C.[Citation6,Citation30] Lai et al.[Citation30] found that an endothermic peak at 72.8°C was attributed to lysozyme. In the present study, lack of this endothermic peak was more likely to denaturation of lysozyme during freeze-drying. This result was in accordance with the disappearance of lysozyme band in protein pattern (). In case of yolk powders, single major endothermic peak resembled to HDL/phosvitin. It has been postulated that phosvitin/HDL were more resistant to heat denaturation proteins due to their compact structure, while livetin and LDL proteins were mostly heat sensitive proteins.[Citation30,Citation31] Huang et al.[Citation31] proposed that the lack of endotherms for LDL might be due to the random coil protein moieties of LDL. However, in whole egg powders, peak 1 and peak 2 might be due to ovotransferrin and ovalbumin or phosvitin from egg white and yolk proteins, respectively. Td values of egg white and whole egg powder from quail egg were at 62.37°C and 63.23°C for endothermic peak I, and 84.33°C and 85.59°C for peak II, respectively, while the egg white and whole egg powder from chicken egg had Td at 65.43°C and 68.43°C for endothermic peak I, and 86.43°C and 88.57°C for peak II, respectively. Yolk powders from both quail and chicken exhibited Td at 87.23°C and 89.63°C, respectively. The present results indicated that quail egg powders showed slightly lower denaturation temperatures than corresponding chicken powders possibly due to the varying protein structures, amino acid sequence, and bonding among proteins. Furthermore, higher denaturation temperature in yolk powders is more likely due to the higher thermostability of egg yolk than egg white proteins. For ∆H, it is correlated with the extent of ordered structure of protein.[Citation29,Citation30] Egg white and whole egg powder from quail had ΔH values of 1.22 and 1.31 J g−1 for peak I, and 2.19 and 2.63 J g−1 for peak II, respectively (). For chicken egg powder, egg white and whole egg powder exhibited ΔH values of 1.59 and 1.48 J g−1 for peak I and 2.22 and 1.98 J g−1 for peak II, respectively. ΔH values of yolk powder from quail and chicken were 2.34 and 2.73 J g−1, respectively. Varying ΔH among egg powder samples was plausibly governed by the different extents of denaturation, especially during freeze-drying. Denaturation of native proteins might take place during freeze-drying. Regardless of egg source, yolk powders showed the higher ΔH than egg white and whole egg powders. This was more likely due to the difference in protein type and structure, which might be denatured to heat at different degrees. Varying thermal stability of egg powders might determine their functional properties.
Solubility
Effect of different pHs on the protein solubility of egg powders from quail and chicken is presented in . In general, quail egg proteins were more soluble than chicken egg proteins. The protein solubility is an important property governing the functional behavior of proteins and their potential application to food processing.[Citation9,Citation21] Egg white, whole egg, and egg yolk powders from both quail and chicken showed lowest solubility around pH 4.5, 5.0, and 6.5, respectively. The results indicated that pH 4.5, 5.0, and 6.0 were the isoelectric point (pI) of egg white, whole egg, and egg yolk proteins, respectively. At these pH values, the protein molecules gain net zero charges leading to decrease in water–protein electrostatic forces and increase the protein–protein interactions, resulting in a reduction in protein solubility. Similar results were observed by Machado et al.[Citation9] and Sousa et al.[Citation32] who reported the minimum solubility of ovalbumin and egg yolk proteins around pH 4.5 and 6.5, respectively. The minimum solubility of whole egg powders around pH 5.0 is more likely due to egg white and yolk proteins whose lowest solubility is between pH 4.5 and 6.5. Furthermore, egg white, whole egg, and egg yolk powders from quail and chicken displayed increased solubility on either side of isoelectric pHs. At pH away from the pI, protein molecules became charged, inducing repulsion among protein molecules which led to the increased solubility. Generally, the dependency of the solubility on pH has been attributed to the change in the net charges carried by the egg proteins as the pH changes.[Citation9,Citation32] The protein solubility profile closely resembles those already reported for egg albumin and yolk protein by Machado et al.[Citation9] and Sousa et al.[Citation32], respectively. Protein solubility of all egg powders in both acid and alkaline pH regions may serve as a useful indicator for the performance of egg proteins in the food formulation. Additionally, good protein solubility is believed to be a prerequisite for many functional properties, including gelation and emulsification.[Citation4,Citation5] All egg powders exhibited higher solubility at alkaline pH than at acidic pH, suggesting that all egg powders had the larger number of negatively charged ions at pH > pI than the number of positively charged ions at pH < pI.[Citation9] Furthermore, all egg powders showed the highest solubility at pH 9–10. Among the egg powders, egg white powders showed higher solubility followed by whole egg and egg yolk powders, respectively, regardless of egg source (P < 0.05). The low solubility of yolk powders might be due to the presence of lipoproteins. All quail egg powders exhibited slightly higher solubility than the corresponding chicken egg powders (P < 0.05). The high solubility of quail egg powders might be due to differences in protein types, degrees of association or dissociation of protein molecules, and amino acid compositions. The protein solubility of quail egg powders from the whole egg, egg white and egg yolk at pH 9 were 92.3%, 96.8%, and 83.5%, respectively. Therefore, quail egg powders could be used as a valuable ingredient in many food applications.
Emulsifying property
EAI and ESI of egg powders from quail and chicken egg are presented in . Overall, quail egg powders were good in emulsion potential than chicken egg powders. EAI of all egg powder samples decreased as the level increased from 1% to 3% (P < 0.05). At higher levels (3%), the activation energy barrier does not allow protein migration to take place in a diffusion-dependent manner.[Citation4,Citation5] This leads to accumulation of proteins in the aqueous phase and decreased EAI of egg powders. Conversely, low protein concentrations and protein adsorption at the oil–water interface are diffusion-controlled. Thus, proteins were available to unfold and localized at the oil–water interface. The result indicated that 1% egg powders from quail and chicken egg were appropriate to migrate to interface and allocate surrounding oil droplets. Yolk powders from both quail and chicken egg exhibited higher EAI, followed by whole egg and egg white, respectively (P < 0.05). The higher EAI in yolk powders was explained by the presence of proteins including LDL, HDL livetin, and phosvitin which act as emulsifiers.[Citation4,Citation30] These emulsifier proteins were amphiphilic in nature which have some amino acids that repel water and some amino acids that attract water. Thus, in the emulsion solution, one part of the protein could stick to the water, and another part could stick to the oil. All quail egg powders showed higher EAI than that of corresponding chicken powders (P < 0.05). The result suggested that the protein of quail egg powders had superior emulsifying properties than chicken egg proteins. This was more likely related to the higher solubility of quail egg powders compared to corresponding chicken egg powders. Therefore, high-soluble proteins could unfold rapidly at the interface and form a film around an oil droplet effectively. ESI of all egg powder samples increased with increasing level of egg powders from 1% to 3% (P < 0.05). Protein at higher concentration facilitated more protein adsorption at interfaces, in which thicker and stronger films could be formed.[Citation5,Citation11] Similarly, yolk powder from quail and chicken eggs displayed higher ESI than those powders prepared from the whole egg and egg white of quail and chicken eggs. Yolk powder formed a highly viscous film in the interphase due to the amphiphilic nature of the proteins which concentrated there and conferred resistance to emulsion particle coalescence. Furthermore, all quail egg powders showed higher ESI compared to corresponding chicken egg powders (P < 0.05). Formation of a stable emulsion due to rapid adsorption of the quail egg proteins into the interphase depends on the distribution of hydrophobic and hydrophilic zones on the surface. When hydrophobic zones are numerous and distributed with enough energy to interact, the probability of adsorption toward the interphase is much greater.[Citation5,Citation11] Partial protein denaturation through drying improves emulsifying properties because it increases molecular flexibility and superficial hydrophobicity, thus favoring formation of viscoelastic films in the oil–water interphase.
Table 4. Emulsifying and foaming properties of egg powders from Japanese quail and white Leghorn chicken.
Foaming property
FE and FS of egg powders from quail and chicken egg are presented in . Generally, quail egg powders had superiority for foaming properties as compared to chicken egg powders. FE and FS of all egg powders from quail and chicken eggs increased as the level of egg powders increased (P < 0.05). Foams with higher levels of proteins might be denser and more stable, owing to an increase in thickness of interfacial films.[Citation25,Citation27] Egg white powder from quail and chicken eggs had higher FE and FS as compared to whole egg powder and yolk powder of both quail and chicken egg, respectively (P < 0.05). This result indicated that the egg white proteins possess excellent foaming properties than yolk proteins of both quail and chicken eggs. It has been postulated that globulins from egg white facilitate foam formation, while the ovomucin confers stability to the foam.[Citation25,Citation27] Generally, the foaming ability of proteins is associated with their film-forming ability at the air–water interface. Egg powders from quail egg white, egg yolk, and whole egg showed higher FE and FS as compared to corresponding chicken egg powders (P < 0.05). The result suggested that the powders from the quail egg possess excellent foaming properties than chicken egg powders. The ideal foam-forming and foam-stabilizing proteins are characterized by low MW, high surface hydrophobicity, good solubility, and easy to denature.[Citation5,Citation25] The result was in agreement with the slightly higher solubility () and looser structures () of quail egg powders, compared with corresponding chicken egg powders. Therefore, powder from quail eggs could be used as an alternative to chicken eggs with excellent emulsifying and foaming property.
Conclusion
From the investigations of the present work, it can be concluded that egg powders (egg white, egg yolk, and whole egg) prepared from quail and chicken eggs had protein content higher than 91%. Further, all egg powders differed in amino acid composition, molecular structure, thermal stability, and functional properties. The quail egg powders showed higher mineral (ash) and essential amino acid contents, but lower fat contents compared to corresponding chicken egg powders. However, quail egg powders displayed slightly lower thermal stability with less ordered molecular structure than corresponding chicken egg powders. Egg white, whole egg, and egg yolk powders from both quail and chicken had minimum protein solubility at pH 4.5, 5.0, and 6.5, respectively. Furthermore, egg white powders possessed higher foaming property, whereas yolk powders exhibited higher emulsifying property. Despite higher nutritional values, the quail egg powders exhibited higher functional properties, such as solubility, emulsifying, and foaming capacities, than that of corresponding chicken powders. Therefore, quail egg powders could be used as an alternative to chicken egg in the preparation of foods and diets that require high protein content with positive health benefits.
Acknowledgments
The authors would like to express their sincere thanks to the Director, CSIR-Central Food
Technological Research Institute, Mysore for the financial support, encouragement, and permission to publish this work.
Additional information
Funding
References
- Zaheer, K. An Updated Review on Chicken Eggs: Production, Consumption, Management Aspects and Nutritional Benefits to Human Health. Food and Nutrition Sciences 2015, 6, 1208–1220. DOI: 10.4236/fns.2015.613127.
- Rao, Q.; Labuza, T. P. Effect of Moisture Content on Selected Physicochemical Properties of Two Commercial Hen Egg White Powders. Food Chemistry 2012, 132, 373–384. DOI: 10.1016/j.foodchem.2011.10.107.
- Bashir, L.; Ossai, P. C.; Shittu, O. K.; Abubakar, A. N.; Caleb, T. Comparison of the Nutritional Value of Egg Yolk and Egg Albumin from Domestic Chicken, Guinea Fowl and Hybrid Chicken. American Journal of Experimental Agriculture 2015, 6, 310–316. DOI: 10.9734/AJEA.
- Le Denmat, M.; Anton, M.; Beaumal, V. Characterization of Emulsion Properties and of Interface Composition in O/W Emulsions Prepared with Hen Egg Yolk, Plasma and Granules. Food Hydrocolloids 2000, 14, 539–549. DOI: 10.1016/S0268-005X(00)00034-5.
- Segura-Campos, M.; Pérez-Hernández, R.; Chel-Guerrero, L.; Castellanos-Ruelas, A.; Gallegos-Tintoré, S.; Betancur-Ancona, B. Physicochemical and Functional Properties of Dehydrated Japanese Quail (Coturnix japonica) Egg White. Food and Nutrition Sciences 2013, 4, 289. DOI: 10.4236/fns.2013.43039.
- Talansier, E.; Loisel, C.; Dellavalle, D.; Desrumaux, A.; Lechevalier, V.; Legrand, J. Optimization of Dry Heat Treatment of Egg White in Relation to Foam and Interfacial Properties. LWT Food Science and Technology 2009, 42, 496–503. DOI: 10.1016/j.lwt.2008.09.013.
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Eggs. In Food Chemistry, 4th; Springer Berlin Heidelber: Berlin, Heidelberg, 2009; pp 546–562.
- Ahn, D.; Egg Components. Animal Science Department, Iowa State University; 2014. http://www.public.iastate.edu/
- Machado, F. F.; Coimbra, J. S. R.; Garcia Rojas, E. E.; Minim, L. A.; Oliveira, F. C.; Sousa, R. D. C. S. Solubility and Density of Egg White Proteins: Effect of pH and Saline Concentration. LWT Food Science and Technology 2007, 40, 1304–1307. DOI: 10.1016/j.lwt.2006.08.020.
- Strixner, T.; Sterr, J.; Kulozik, U.; Gebhardt, R. Structural Study on Hen-Egg Yolk High Density Lipoprotein (HDL) Granules. Food Biophysics 2014, 9, 314–321. DOI: 10.1007/s11483-014-9359-y.
- Laca, A.; Paredes, B.; Rendueles, M.; Díaz, M. Egg Yolk Plasma: Separation, Characteristics and Future Prospects. LWT Food Science and Technology 2015, 62, 7–10. DOI: 10.1016/j.lwt.2015.01.048.
- McCully, K. A.; Mok, C. C.; Common, R. H. Paper Electrophoretic Characterization of Proteins and Lipoproteins of Hen’s Egg Yolk. Canadian Journal of Biochemistry and Physiology 1962, 40, 937–952. DOI: 10.1139/y62-105.
- Mine, Y. Adsorption Behavior of Egg Yolk Low-Density Lipoproteins in Oil-In-Water Emulsions. Journal of Agricultural and Food Chemistry 1998, 46, 36–41. DOI: 10.1021/jf970306y.
- Lechevalier, V.; Croguennec, T.; Anton, M.; Nau, F. Processed Egg Products. In Improving the Safety and Quality of Eggs and Egg Products, Filip, V. I., Yves, N., Maureen, B., Eds; Woodhead Publishing: Cambridge, UK: 2011; pp 538-58.
- Strixner, T.; Kulozik, U. Egg Proteins. In Handbook of Food Proteins, Phillips, G. O., Williams, P. A., Eds; Woodhead Publishing: Cambridge, UK: 2011; pp 150–209.
- Genchev, A. Quality and Composition of Japanese Quail Eggs (Coturnix japonica). Trakia J. Sci.2012, 10, 91–101.
- Tunsaringkarn, T.; Tungjaroenchai, W.; Siriwong, W. Nutrient Benefits of Quail (Cortunix cortunix japonica) Eggs. International Journal of Environmental Research and Public Health 2013, 3, 1–8.
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; (4th revision); Association of Official Analytical Communities; Gaithersburg MD, 1998.
- Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. DOI: 10.1038/227680a0.
- Lowry, O. H.; Rosebrough, N. J.; Farr, A. L.; Randall, R. J. Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry 1951, 193, 265–275.
- Kudre, T. G.; Benjakul, S.; Kishimura, H. Comparative Study on Chemical Compositions and Properties of Protein Isolates from Mung Bean, Black Bean and Bambara Groundnut. Journal of the Science of Food and Agriculture 2013, 93, 2429–2436. DOI: 10.1002/jsfa.2013.93.issue-10.
- Kudre, T. G.; Benjakul, S. Physicochemical and Functional Properties of Beany Flavour-Free Bambara Groundnut Protein Isolate. Journal of the Science of Food and Agriculture 2014, 94, 1238–1247. DOI: 10.1002/jsfa.2014.94.issue-6.
- Steel, R. G. D.; Torrie, J. H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: New York, 1980; pp 20–90.
- USDA. Agricultural Research Service. USDA National Nutrient Database for Standard Reference; Release 28; 28, Revised; 2016. http://www.ars.usda.gov/ba/bhnrc/ndl.
- Baniel, A.; Fains, A.; Popineau, Y. Foaming Properties of Egg Albumen with a Bubbling Apparatus Compared with Whipping. Journal of Food Science 1997, 62, 377–381. DOI: 10.1111/jfds.1997.62.issue-2.
- Blume, K.; Dietrich, K.; Lilienthal, S.; Ternes, W.; Drotleff, A. M. Exploring the Relationship between Protein Secondary Structures, Temperature-Dependent Viscosities, and Technological Treatments in Egg Yolk and LDL by FTIR and Rheology. Food Chemistry 2015, 173, 584–593. DOI: 10.1016/j.foodchem.2014.10.084.
- Stadelman, W. I.; Cotterill, O. J. Foaming. In Egg Science and Technology, Stadelman, W. I., Cotterill, O. J., Eds; Food Product Press; Haworth Press Inc: Binghamton, 1994; pp 418–434.
- Chang, C.; Niu, F.; Su, Y.; Qiu, Y.; Gu, L.; Yang, Y. Characteristics and Emulsifying Properties of Acid and Acid-Heat Induced Egg White Protein. Food Hydrocolloids 2016, 54, 342–350. DOI: 10.1016/j.foodhyd.2015.09.026.
- Guilmineau, F.; Krause, I.; Kulozik, U. Efficient Analysis of Egg Yolk Proteins and Their Thermal Sensitivity Using Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis under Reducing and Nonreducing Conditions. Journal of Agricultural and Food Chemistry 2005, 53, 9329–9336. DOI: 10.1021/jf050475f.
- Lai, K. M.; Chuang, Y. S.; Chou, Y. C.; Hsu, Y. C.; Cheng, Y. C.; Shi, C. Y.; Chi, H. Y.; Hsu, K. C. Changes in Physicochemical Properties of Egg White and Yolk Proteins from Duck Shell Eggs Due to Hydrostatic Pressure Treatment. Poultry Science 2010, 89, 729–737. DOI: 10.3382/ps.2009-00244.
- Huang, S.; Herald, T.; Mueller, D. Effect of Electron Beam Irradiation on Physical, Physiochemical, and Functional Properties of Liquid Egg Yolk during Frozen Storage. Poultry Science 1997, 76, 1607–1615. DOI: 10.1093/ps/76.11.1607.
- Sousa, R. D. C. S.; Coimbra, J. S. R.; Garcia Rojas, E. E.; Minim, L. A.; Oliveira, F. C.; Minim, V. P. R. Effect of pH and Salt Concentration on the Solubility and Density of Egg Yolk and Plasma Egg Yolk. LWT Food Science and Technology 2007, 40, 1253–1258. DOI: 10.1016/j.lwt.2006.08.001.
