ABSTRACT
Collagen is the major connective tissue (CT) protein and one of the main constituents of the jumbo squid (Dosidicus gigas). Therefore, physicochemical changes of pepsin-solubilized collagen (PSC) and insoluble collagen (IC) were studied after cooking (100°C/30 min) of muscle (mantle, fins, and arms). Different pyridinoline (Pyr) contents (the major cross-linking molecule in collagen fibers) were found in the fresh muscle of the three anatomical regions. After the cooking process, a decrease from 10 to 30% in the thermal resistance of collagen was observed, depending on the anatomical region and fraction evaluated. Furthermore, the electrophoretic profile, Fourier transform infrared (FTIR) spectroscopy, and the amino-acid profile revealed that structural changes occurred in the two different collagen fractions caused by the thermal process, and the changes were greater in the mantle. Under the conditions applied in this study, collagen fractions from the squid arms showed more stability during the cooking process due to the high cross-linking degree of their fibers.
Introduction
The jumbo squid (Dosidicus gigas) is a cephalopod distributed from the coasts of California to Chile, with a relatively short life cycle and high growth sizes.[Citation1,Citation2] The functional and nutritional characteristics of its muscle, such as white meat, low fat, and high protein content, make the jumbo squid a more desirable product compared to other marine species. Hence, fishery of this cephalopod has undergone considerable changes during the last few years, generating the interest of consumers and researchers since its muscle properties make it a valuable study model for diverse investigations.[Citation3–Citation5] One of the major components of both the muscle and the skin of jumbo squid is connective tissue (CT). The CT plays an important role on the postcapture handling of the species and on its commercialization (fresh frozen or cooked frozen).[Citation5,Citation6] In the CT, collagen is the main component controlling the heat stability of the muscle fibers due to its structure and cross-linking degree, a feature not found in fish.[Citation7,Citation8] Moreover, it has been reported that jumbo squid presents differences in its collagen and muscle behavior depending on the anatomical region evaluated during postcapture storage. These differences were mainly associated to the heat stability of collagen, and were shown to affect the sensory, chemical, and textural properties of the final sea product.[Citation5,Citation9,Citation10]
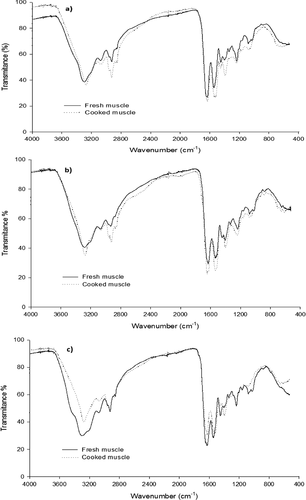
Figure 3. FTIR spectrum analysis of PSC extracted from fresh and cooked jumbo squid (Dosidicus gigas) muscle. (a) Fins; (b) arms; and (c) mantle.
The low solubility and cross-linking behavior in collagen fibers of jumbo squid muscle are attributed to the presence of pyridinoline (Pyr), which has the capacity to cross-link up to three collagen molecules through its covalent interaction with lysine and hydroxylisine residues, located either in helicoidal or in central regions of the triple helix of collagen.[Citation7,Citation11] Pyr is produced by an enzymatic mechanism, started by lysyl oxidase (LOX) during the maturation of collagen fibers [Citation11]; this mechanism has been widely studied in terrestrial organisms. However, in marine organisms, this information is still scarce. Some studies carried out in fresh and iced jumbo squid showed that some of the cross-linking mechanisms of collagen fibers involve the presence of LOX and Pyr.[Citation5,Citation12,Citation13] Moreover, Ando et al.[Citation7] found high resistance of collagen fibers during the incubation of squid (Todarodes pacificus and Loligo pealeii) CT in boiling water, suggesting differences in the cross-linking degree of collagen fibers from the evaluated squids. In the present article, changes in the physicochemical and structural characteristics of pepsin-solubilized collagen (PSC) and insoluble collagen (IC) extracted from raw and cooked jumbo squid (Dosidicus gigas) muscle were evaluated.
Materials and methods
Jumbo squid (12 organisms of 25–30 cm and 3–3.5 kg.) was obtained frozen from a local market and transported to the Seafood Laboratory at the University of Sonora, Mexico. The mantles, fins, and arms were thawed, skinned, and separated into three batches (n = 3). A pool of four specimens integrated each batch. Later, each pool was separated, packed in 2 bags, and cooked in boiling water for 0 (raw muscle) and 30 min (cooked muscle). All determinations were carried out in triplicate.
Collagen extraction
PSC and IC fractions were prepared at 4°C as described by Osuna-Amarillas et al.[Citation5] with slight modifications. The CT was extracted from the mantle, fins, and arms of the jumbo squid by precipitation. The raw and cooked muscle were homogenized for 2 min with 10 volumes (v/w) of 0.1N NaOH, stirred overnight, and centrifuged at 8000 × g (Ramírez-Guerra et al., 2015). Then, the insoluble residues were extracted with pepsin (10 mg/g tissue in acetic acid, PSC), and the final precipitate was the IC. The extracted fractions were dialyzed thoroughly with distilled water and lyophilized for later analysis.
Protein concentration
The protein concentration in the lyophilized samples of PSC and IC were determined according to the micro-Kjeldahl method.[Citation14]
Electrophoretic analysis
The molecular mass of the PSC extracted from raw and cooked muscle was estimated by SDS-PAGE (sodium dodecyl sulfate 7.5% polyacrylamide gel electrophoresis) under reducing conditions,[Citation15] using a broad-range standard molecular marker mixture (Sigma, SDS-6H), which included myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), and carbonic anhydrase (29 kDa). The gel was stained with Coomassie R-250 and the images of the samples on the electrophoresis gels were captured using an image densitometer (Model GS-800; Bio-Rad Laboratory Chemicals, Hercules, CA, USA).
Off-gel-electrophoresis fractionation
The proteins and peptides in the PSC extracted from raw and cooked muscle were separated by its isoelectric point using a 3100 OffGel Fractionator (Agilent Technologies) equipped with a 24-well frame and 24-cm immobilized pH gradient (IPG) strips (GE Health-care) suitable for determinations at 4–7 pH range. The IPG strips were rehydrated during 15 min with 40 μL of rehydration solution. Then, the sample was solubilized in 3.6 mL of the same solution, added to the frame (150 μL in each well), and focused during 18 h at 64 kVh (Franchin et al., 2014). Finally, the protein presence found in each well was identified by measuring absorbance at 220 nm and analyzed by SDS-PAGE (described previously).
Amino-acid profile
The amino-acid composition was evaluated in PSC and IC by reversed-phase high-performance liquid chromatography (HPLC) according to Vázquez-Ortiz et al.[Citation16] using a Hewlett-Packard 1200 series HPLC system (Hewlett Packard Co., Waldbronn, Germany). Briefly, the samples were hydrolyzed using 6 M HCl in an evaporator for 6 h in sealed tubes at 150°C. The hydrolysates were diluted with 0.4 M sodium borate (Na2B4O7) buffer, derivatized with O-phthalaldehyde (OPA) for determination of the primary amino acids, and derivatized with 9-fluorenylmethyl chloroformate for determination of secondary amino acids. Chromatographic separation was carried out using a C18 column (4.6 mm ID × 100 mm; Agilent Technologies, Inc., Palo Alto, CA, USA), and the integrations were calculated using ChemStation software. Fluorescence emission was continuously monitored at 330 and 418 nm.
Pyridinoline content
The Pyr content found in the CT from fresh and cooked muscle was calculated according to Ramirez-Guerra et al.[Citation17] The CT was hydrolyzed for 6 h with 6 M HCl at 150°C. The hydrolysates were pre-fractionated using a Chromabond® cross-links column and diluted with an equal volume of 90% acetic acid and 2.5 mL of acetonitrile. Then, the pyridinium cross-links were eluted with 1% heptafluorobutyric acid and separated on a Series 1200 HPLC system (Hewlett Packard Co., Waldbrom, Germany) coupled to a fluorescence detector (λex = 297 nm and λem = 395 nm), using an ODS C18 Microsorb-MV column (100 C18, 4.6 mm ID × 250 mm, Microsorb, Rainin, CA, USA). The Pyr content was estimated as moles per mole of collagen.
Differential scanning calorimetry (DSC)
The thermal profiles of the collagenous extracts were obtained according to Torres-Arreola et al.,[Citation10] using a heating rate of 10°C min−1 from 15 to 150°C. A total of 20 to 25 mg of lyophilized PSC and IC were placed in hermetically sealed stainless steel capsules, and enthalpy (J/g) and maximal temperature of the transition (°C) were determined using a Perkin-Elmer DSC-8000 calorimeter (Norwalk, CT, USA). All measurements were performed in triplicate, and the reported data include the transition temperature and enthalpy values.
Fourier transform infrared spectroscopy (FTIR)
The functional groups of the lyophilized PSC extracted from fins, arms and mantle were determined directly by FTIR spectroscopy (Thermo Scientific, model Nicolet iS50 FTIR), with an average of 16 scans in a spectral range of 4000–400 cm−1.
Data analysis
The statistical design of this work was planned to decrease the variation within the replicates. The data were analyzed as a randomized design in a 3 × 2 factorial arrangement (three anatomical regions × raw and cooked muscle). Three repetitions of all analyses were performed. The solubility and calorimetry data analysis of the CT fractions are based on the average of three experiments, using analysis of variance. Mean differences were established employing the Tukey test with a variation by replication of <5%. The data were analyzed using the JMP program ver. 5.0 statistical software program (StatSoft, Tulsa, OK, USA).
Results and discussion
Changes in collagen solubility (CS)
The solubility changes detected in the CT and collagen (PSC and IC) extracted from raw and cooked jumbo squid muscle are shown in . The highest yield of CT obtained from fresh muscle was from the arms. This observation is in agreement with other studies, where it has been reported that the greatest amount of collagen in jumbo squid is located in this anatomical region, which is also highly cross-linked.[Citation5,Citation10] The differences between anatomical regions could be related to their specific biological functions: the mantle has more protein replacement (including collagen). Furthermore, the collagen content in the marine organism has been established within a wide range, varying from 3 to 18% in different squid species.[Citation10,Citation18–Citation21] Since there is a close relationship between the extraction yield (EY) and CS from jumbo squid muscle, this variability also could be observed in the cross-linking degree of the collagen fibers.[Citation5] In jumbo squid muscle, Sarabia-Sainz et al.[Citation19] found a lower solubility in collagen extracted from arms compared to the collagen extracted from fins and mantle. The differences in solubility were attributed to cross-linkage degree of the collagen strands.
Table 1. Changes in collagen solubility (mg/100 g muscle) of fresh muscle (mantle, fins and arms) of jumbo squid (Dosidicus gigas) subjected to a cooking process.
After the cooking process, the EYs of CT, PSC, and IC varied in all the anatomical regions evaluated. More CT was extracted from the fins after 30 min in boiling water compared to the raw muscle (p < 0.05), whereas this EY decreased significantly in mantle and arms. These findings could be attributed to structural modifications occurring in the muscle that promote changes in the solubility of CT proteins during the postcapture handling of the jumbo squid.[Citation5] The CS of the three anatomical regions studied was also altered by the thermal process applied. Only the PSC extracted from fins and mantle did not show any changes in their EY. Conversely, the PSC extracted from arms and the IC extracted from all anatomical regions underwent a significant decrease (p < 0.05) in their EY. These results may be associated to the breakdown of some collagen-fiber regions into gelatin caused by the cooking of the jumbo squid muscle. Gelatin is soluble in 0.1 M NaOH, the solvent used in the first extraction step.[Citation22] CS is affected by its intra- and intermolecular interactions, such as hydrogen bonds, hydrophobic and electrostatic interactions, as well as the covalent bonds associated to Pyr, and all these interactions could be modified when heat is applied (i.e., cooking process).[Citation6,Citation7,Citation23]
Estimation of molecular mass
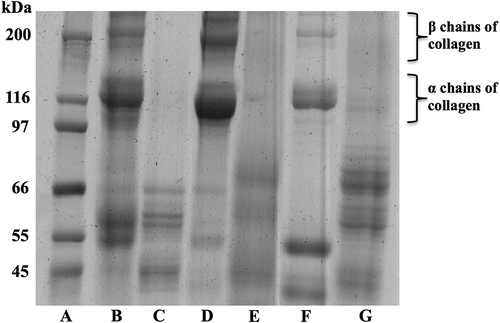
PSC extracts from fresh and cooked muscle (mantle, fins, and arms) were analyzed by SDS-PAGE in order to detect potential changes in their protein profile. shows bands corresponding to α1, α2, and β chains as the principal constituents of the collagen derived from the fresh muscle. This finding is in agreement with other studies where these three chains have been detected in different anatomical regions of the jumbo squid. The ratio of α:β chains depends on the cross-linking degree of the collagen fibers and the anatomical region evaluated (higher cross-linkage produces more β chains in the pepsin-solubilized extracts).[Citation10,Citation13,Citation24] Recently, a greater proportion of β chains has been reported in the mantle, fins, and arms of jumbo squid, indicating the presence of lysine-derived cross-links, where the Pyr predominates.[Citation23] After the cooking process, fading of band intensity is observed for all the anatomical regions evaluated, probably as a result of the partial hydrolysis of the collagen chains, which in turn leads to new bands appearing at molecular weights of less than 100 kDa. The smaller new bands are likely the products of the conversion of collagen into gelatin, a phenomenon that could occur when high temperatures are applied to collagen matrices in the presence of water. Moreover, the reported molecular weights for gelatin bands vary from 45 to 100 kDa, supporting gelatization of collagen as one of the main processes occurring within the protein extracts.[Citation25,Citation26] Raman and Mathew [Citation27] observed in squid muscle (Loligo duvauceli) that with cooking temperatures above 65°C, the α and β chains of collagen would undergo denaturation and protein gelatinization could occur at approximately 80°C.[Citation28] In the present study, although protein denaturation was detected on SDS-PAGE, it was not possible to determine differences in the behavior of the PSC extracted from the different body parts studied of jumbo squid (mantle, fins, and arms) after the cooking process. However, recent reports show that collagen-fiber denaturation could be associated with a decrease in the firmness of the jumbo squid muscle after cooking at 100°C.[Citation29]
Figure 1. SDS–polyacrylamide gel electrophoresis of PSC extracted from fresh and cooked jumbo squid (Dosidicus gigas) muscle. (A) Molecular weight markers, kDa; (B) PSC from fresh fins; (C) PSC from cooked fins; (D) PSC from fresh mantle; (E) PSC from cooked mantle; (F) PSC from fresh arms; and (G) PSC from cooked arms.
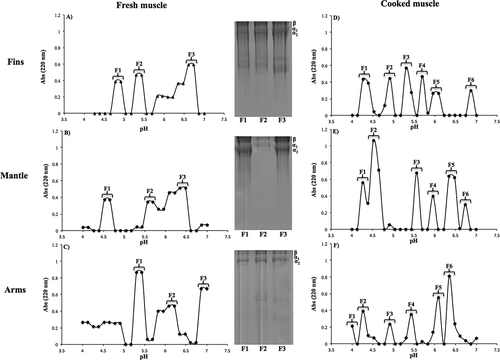
Isoelectric point
PSC was fractionated by its isoelectric point using an Ofgel-Electrophoresis in order to elucidate the differences between α and β chains of the extracted collagen (). In fresh muscle, various protein fractions were separated within the tested pH range (4 to 7), and these fractions presented different profiles compared to the isoelectric point reported for collagen extracted with acidic solutions (pH 6 to 7).[Citation30] These differences could be attributed to the presence of different collagen types depending on the anatomical region from which the extraction was carried out. Since the electrophoretic profile observed in the SDS-PAGE also revealed differences between the protein bands detected in each analyzed fraction (), two α chains of collagen may have small differences in their molecular weights, causing changes on their isoelectric point.[Citation18] In different squid species, a type I collagen has been reported, with higher proportion of β chain (cross-linked component) compared to α chains.[Citation20,Citation23,Citation30] Type I collagen is the most common collagen, and is formed by three polypeptide chains: two of the strands (denominated α1) have an identical amino-acid sequence, whereas the third one (denominated α2) has a different amino-acid sequence.[Citation31] Another type of collagen found in the muscle is Type III. This collagen plays an important role on texture and is composed by three identical α chains (α1) .[Citation31] Based on the latter information, except for fraction 1 of the collagen extracted from the squid arms, all samples derived from fresh muscle appear to be type I collagen (–) since two bands at approximately 120–110 kDa (α1 and α2 chains) and one band at around 200 kDa (β chain) are observed. On the other hand, in fraction 1 obtained from the squid arms (), the presence of a single band at approximately 120–110 kDa is observed, indicating that the collagen strands are composed by three identical units (i.e., three identical α chains), matching the reported characteristics for collagen type III.[Citation31] The latter may be related to the amino-acid composition of the different PSC fractions since it has been reported that the composition and distribution of amino acids can vary among different collagen types, consequently causing changes in their isoelectric point.[Citation32] Kittiphattanabawon et al.[Citation30] reported differences between the isoelectric points of acid-soluble collagen and PSC extracted from shark skin, which was attributed to the amino-acid composition of each sample since the PSC in the telopeptidic region was removed.
Amino-acid composition
and presents the amino-acid profile along with the concentration of each residue measured in PSC, and IC extracted from fresh and cooked muscle. In fresh muscle, a typical collagen profile can be observed in all the evaluated anatomical regions and no differences between their amino-acid profiles were detected (p ≥ 0.05). Even though these results are in agreement with the results reported for different squid species,[Citation5,Citation7,Citation13] each extract (PSC, and IC) contained a different concentration of the most common amino acids found in CT proteins, such as glycine, proline, hydroxyproline, cysteine, and lysine.
Table 2a. Amino-acid profile (residues/1000 amino-acid residues) of PSC extracted from fresh and cooked muscle of jumbo squid (Dosidicus gigas).
Table 2b. Amino-acid profile (residues/1000 amino-acid residues) of IC extracted from fresh and cooked muscle of jumbo squid (Dosidicus gigas).
Different amino-acid composition in CT extracts have been previously observed by Osuna-Amarillas et al.,[Citation5] who reported differences between the amino-acid concentrations of salt-soluble collagen (SSC) and IC from jumbo squid muscle, even though the amino-acid content was basically the same in all the anatomical regions. Cysteine detection (≈30–50 residues/1000 residues) in each fraction extracted from fresh muscle suggests the presence of elastin as a component of the squid’s CT. Cysteine is not commonly found in type I collagen because it is not part of its structure; however, this amino acid is characteristic of the elastin’s amino-acid sequence.[Citation20,Citation33] On the other hand, it also has been reported that type III collagen possesses intramolecular disulfide bonds, attributed to the presence of sulfur amino acids, such as cysteine.[Citation31] Hence, the presence of more than one type of collagen in jumbo squid muscle must be considered. Moreover, the glycine content measured is lower than those reported by others for different squid species (≈300–350 residues/1,000 residues). This observation is consistent with the electrophoretic profile obtained where several protein bands are observed in the PSC, indicating that the extracts are most likely formed by collagen and elastin as major components.[Citation18]
After the cooking process of the jumbo squid muscle, a significant decrease (p < 0.05) of up to 80% was observed in the glycine, proline, and hydroxyproline contents of PSC extracted from the three anatomical regions. The decrease observed is related to collagen denaturation since the solubility and EY of the PSC fractions was also affected. The heat applied to the squid muscle also reduced the cysteine content found in most of the extracts. However, under the applied conditions, this behavior is not likely related to elastin denaturation because, unlike with collagen denaturation, it was not possible to clearly appreciate these changes in the electrophoretic profile, reinforcing the hypothesis that the decrease of cysteine in the extracts is due to the presence of more than one type of collagen. Finally, the lysine content increased considerably (p < 0.05) in all the extracts (mainly in PSC) obtained from the three anatomical regions after the cooking process, which may be related to the cross-linking degree of the collagen fibers since lysine and hydroxylysine are the primary substrates for such cross-linking process by Pyr formation.[Citation33,Citation34] Although these amino acids are not abundant in collagen fibers (found mainly in the telopeptide region),[Citation33] the increase in their concentration within the PSC extracts is probably associated to changes in the cross-linking degree of the collagen fibers since during the cooking process, a rupture in these covalent bonds could occur.
Cross-linking behavior
Pyr concentration as cross-linking index was evaluated in the CT after the cooking process of jumbo squid and was compared to fresh muscle (). The lowest Pyr content in fresh muscle was found in the mantle (p < 0.05), which agrees with other literature reports where poor collagen cross-linking has been attributed to this anatomical region in jumbo squid.[Citation5,Citation10,Citation13] Moreover, no significant differences (p ≥ 0.05) were found between fins and arms, suggesting that both anatomical regions present a high degree of collagen-fiber cross-linking. The results obtained in the present study are higher than those reported by Ramírez-Guerra et al.,[Citation13] who monitored the Pyr content in jumbo squid mantle during ice storage, finding a maximal Pyr value of 1.32 mmol/mol after 20 days of storage, while no Pyr was detected in fresh muscle. In another study, also conducted by Ramírez-Guerra et al.,[Citation17] the authors found a Pyr content of 4.6 mmol/mol of collagen in jumbo squid muscle, indicating fluctuations in the Pyr concentration that could be found in jumbo squid muscle, although the presence of highly cross-linked collagen has been reported recently in jumbo squid muscle.[Citation20] Moreover, in other marine species, such as Pagrus major and Seriola quinqueradiata, Pyr values ranging from 3.44 to 8.80 mmol/mol of collagen have been reported.[Citation35] These differences may be due to the species studied and to the postcapture handling applied to the squid bodies prior to performing the analytical determinations: in the present study, frozen muscle (obtained from a local fish market) and larger organisms (measuring 50 cm and weighing 6 kg) were utilized compared to the ones used by Ramírez-Guerra et al.[Citation13] It has been widely reported that age, size, and postcapture handling of the organisms can influence the collagen and Pyr content detected in their muscle.[Citation5,Citation13,Citation35]
Table 3. Pyridinoline content (mmol/mol collagen*) in connective tissue extracted from fresh and cooked muscle (fins, mantle, and arms) of jumbo squid (Dosidicus gigas).
A significant decrease (p < 0.05) of Pyr content was observed in all the evaluated anatomical regions after the cooking process. The values measured ranged from 0.68 to 3.95 mmol/mol (), indicating that a fracture in the collagen fibers could have occurred as a result of the thermal treatment, releasing the Pyr and causing an underestimation of its content within the protein fibers. These observations confirm the results discussed earlier regarding the electrophoretic profile and the solubility of the different fractions of collagen. Torres-Arreola et al.[Citation29] found fractures in the muscle structure and CT fibers of jumbo squid muscle after 30 min in boiling water. These fractures were greater in the arms and were attributed to the CT peculiar structural arrangement.
Thermal profile
The denaturation temperature of PSC and IC extracted from the fresh and cooked fins, mantle, and arms of jumbo squid was evaluated by DSC (). In fresh muscle, with the exception of PSC extracted from the squid arms, both PSC and IC from all the anatomical regions exhibited denaturation temperatures higher than 100°C; this behavior is consistent with the Pyr content, as previously discussed, suggesting a high cross-linking degree in the collagen fibers. This evidence is in agreement with previous literature reports, where denaturation temperatures higher than 100°C were measured in collagen from jumbo squid muscle.[Citation8,Citation10,Citation23,Citation36] Other denaturation temperatures have also been reported for collagen extracted from the squid muscle. For example, Osuna-Amarillas et al.[Citation5] reported a denaturation temperature between 45 and 60°C for SSC extracted from the same squid species. The differences in denaturation temperatures could be attributed to a low cross-linking degree present in SSC as salt solutions are commonly used for extractions of newly synthesized collagen.[Citation5,Citation37]
Table 4. Denaturation temperatures (DT) and enthalpies (ΔH) of PSC and IC extracted fresh and cooked muscle of jumbo squid (Dosidicus gigas).
The stability of PSC and IC was affected after 30 min in boiling water (), regardless of the anatomical region evaluated. All the extracts exhibited a significant decrease in their transition temperature (p < 0.05), possibly due to protein denaturation. Such transition was observed at approximately 75–92°C. These observations may be related to the fracture of the collagen fibers and to the decrease in Pyr content after the cooking process, explaining why the protein is susceptible to denaturation at lower temperatures compared to fresh muscle. A decrease in the protein transition temperature after heat treatment agrees with other transition temperatures reported by different authors for cooked muscle from terrestrial animals, such as rabbit and pork: these authors have measured denaturation temperatures of collagen fibers at approximately 65–80°C.[Citation38,Citation39] In jumbo squid muscle, Torres-Arreola et al.[Citation29] reported a breakdown of the collagen network in fins, mantle, and arms after treating the muscle for 30 min in boiling water. The treatment applied caused muscle softening, which could be explained by the protein denaturation observed in this work.
FTIR spectroscopy
According to the FTIR spectra measured, structural changes happened in the PSC extracted from fins, mantle, and arms of jumbo squid subjected to a cooking process (). In fins and arms, the main functional groups of collagen (, ) were observed: the stretching vibration of the C–O groups corresponding to the amide I around 1650 cm−1, the N-H (amide II) at 1543–1531 cm−1, and the C-N (amide III) at 1235–1237 cm−1. In addition, a band at around 1630 cm−1 was observed, indicating the absorption of collagen triple helix; another band was observed at 1452–1446 cm−1 representing the C-N stretching of proline, and another band was observed at around 1400–1200 belonging to the CH2 of glycine and proline side chains.[Citation40–Citation42] The observed bands at 1083 and 1029 cm−1 are related to a stretching vibration of carbohydrate residues linked to collagen.[Citation40,Citation43] The bands seen at around 3270, 2960 and 2930 cm−1 are related to N–H stretching.[Citation44] It has been reported that the preservation degree of the triple hélix can be measured by the ratio between the amide III N-H absorbance and the C–N stretching of the proline ring (A1235/A1452), where a value of 1 indicates that the helix is preserved, while a decrease in this value is related to protein denaturation.[Citation42,Citation44,Citation45] Therefore, based on the above evidence, the cooking process of fins and arms did not have an effect on the integrity of the collagen triple helix. However, displacements in the amide I and II bands at 1649–1645 and 1533–1526 cm−1, respectively, and a displacement along with a decrease in the absorbance of the characteristic band of amide III (≈1240 cm−1) are shown in the spectra measured on the fractions extracted from the cooked muscle. These observations indicate structural modifications occurring in the triple helix,[Citation42,Citation45] which agrees with the results shown in the electrophoretic profile (). Other modifications observed in the spectra of the cooked fractions were the following: the bands corresponding to the CH2 stretching at 1337 cm−1 disappeared; the bands at 1398–1394 cm−1 increased significantly; and the bands at 1235 cm−1 decreased. The spectral changes detected with FTIR are attributed to modifications in the main chain of the glycine and the side chain of the proline by effect of the thermal treatment.[Citation41,Citation42,Citation45] Finally, the bands at 1083–1029 cm−1 decreased, indicating carbohydrate–collagen separation.[Citation43]
In PSC from fresh mantle (Fig. 3c), the same characteristic bands for collagen were detected (Fig. 3b), highlighting the bands at 1649, 1543, and 1237 cm−1, corresponding to amide I, II, and III respectively. According to the calculated ratio on the A1235/A1452, the thermal treatment applied to the muscle did not have an effect over the triple-helix integrity. However, evident changes in the intensity and position of the bands corresponding to amides I, II, and III and the triple helix were observed at 1649, 1527, 1237, and 1626 cm−1, respectively, indicating structural modifications of the extracted collagen.[Citation42,Citation45] Two important observations are the decrease in the intensity of the amide III band at 1237 cm−1 and the vibration of the proline ring band at 1450 cm−1, showing a different behavior than those observed in PSC extracted from fins and arms. It should also be noted that the characteristic band of the CH2 vibration in the proline side chain at 1336 cm−1 disappeared, while the band intensity of CH2 in glycine and proline (1395 cm−1) increased. These changes indicate greater modifications of the collagen triple-helix structure and hydrogen bond breakage in jumbo squid mantle compared to the other anatomical regions evaluated, mainly shown by the glycine and proline alterations, which in turn could explain the greater softening of the mantle during cooking.[Citation29] This behavior is consistent with the results reported by Sarabia-Sainz et al.,[Citation23] where the authors found a lower Pyr content in mantle compared to the Pyr content found in fins and arms, correlating this with the IR values and the muscle firmness.
Conclusion
The major EY of collagen was obtained from the squid arms; however, CS was affected by the cooking process in all the anatomical regions. Although no differences were found in the thermal resistance of the collagen extracted from fins, mantle, and arms after the cooking process, the collagen fibers of the arms presented the greatest stability based on the cross-linking degree and Pyr content. Therefore, in jumbo squid muscle, collagen fibers derived from arms undergo fewer structural changes compared to other anatomical regions after the cooking process, which results in a greater muscle stability during its postcapture handling.
Acknowledgments
The authors thank Consejo Nacional de Ciencia y Tecnología (CONACyT) for the funding through grant 180214.
Additional information
Funding
References
- Bakun, A.; Csirke, J. Environmental Processes and Recruitment Variability. FAO Fisheries Technical Paper 1998, 105–124.
- Nigmatullin, C.; Nesis, K.; Arkhipkin, A. A Review of the Biology of the Jumbo Squid Dosidicus Gigas (Cephalopoda: Ommastrephidae). Fisheries Research 2001;54(1):9–19.
- Gómez-Guillén, M.; Martínez-Alvarez, O.; Montero, P. Functional and Thermal Gelation Properties of Squid Mantle Proteins Affected by Chilled and Frozen Storage. Journal of Food Science 2003;68(6):1962–1967.
- Salinas, Z.; Sánchez, H.; Aragón, N.; Sánchez, V.; Soria, M.; Escoto, G.; Bazzino, F. Programa Maestro De La Pesquería De Calamar Gigante (Dosidicus gigas). Programa maestro de comité sistema producto de la pesquería de calamar gigante en el estado de sonora, 2005.
- Osuna-Amarillas, P.; Márquez-Ríos, E.; Rouzaud-Sandez, O.; Suarez-Jiménez, G.; Cota‐Arriola, O.; Ocaño-Higuera, V.; Arvizu-Flores, A.; Torres-Arreola, W. Physicochemical Changes of Connective Tissue Proteins in Jumbo Squid (Dosidicus gigas) Muscle during Ice Storage. Journal of Food Processing and Preservation 2017;41(1):1–9.
- Ando, M.;. Correspondence of Collagen to the Softening of Fish Meat during Refrigeration. Extracellular Matrix Fish Shellfish Trivandrum, India: Research Signpost. 1999, 69–79.
- Ando, M.; Makino, M.; Tsukamasa, Y.; Makinodan, Y.; Miyosh, M. Interdependence between Heat Solubility and Pyridinoline Contents of Squid Mantle Collagen. Journal of Food Science 2001;66(2):265–269.
- Valencia-Pérez, A.; García-Morales, M.; Cárdenas-López, J.; Herrera-Urbina, J.; Rouzaud-Sández, O.; Ezquerra-Brauer, J. Effect of Thermal Process on Connective Tissue from Jumbo Squid (Dosidicus gigas) Mantle. Food Chemistry 2008;107(4):1371–1378.
- Ramírez-Olivas, R.; Rouzaud-Sández, O.; Haard, N.; Pacheco-Aguilar, R.; Ezquerra-Brauer, J. Changes in Firmness and Thermal Behavior of Ice-Stored Muscle of Jumbo Squid (Dosidicus gigas). European Food Research and Technology 2004;219(4):312–315.
- Torres-Arreola, W.; Pacheco-Aguilar, R.; Sotelo-Mundo, R.; Rouzaud-Sández, O.; Ezquerra-Brauer, J. Partial Characterization of Collagen from Mantle, Fin, and Arms of Jumbo Squid (Dosidicus gigas). CYTA Journal of Food 2008;6(2):101–108.
- Palamakumbura, A.; Trackman, P. A Fluorometric Assay for Detection of Lysyl Oxidase Enzyme Activity in Biological Samples. Analytical Biochemistry 2002;300(2):245–251.
- Torres-Arreola, W.; Ezquerra-Brauer, J.; Pacheco-Aguilar, R.; Valenzuela-Soto, E.; Rouzaud-Sandez, O.; Lugo-Sanchez, M.; Ezquerra-Brauer, J. Lysyl Oxidase from Jumbo Squid (Dosidicus gigas) Muscle: Purification and Partial Characterization. International Journal of Food Science and Technology 2012;47(5):947–953.
- Ramirez-Guerra, H.; Fimbres-Romero, M.; Tapia-Vazquez, A.; Ezquerra-Brauer, J.; Márquez-Ríos, E.; Suarez-Jimenez, G.; Torres-Arreola, W. Relationship between Lysyl Oxidase Activity, Pyridinoline Content and Muscle Texture during Ice Storage of Jumbo Squid (Dosidicus gigas). International Journal of Food Science and Technology 2015;50(12):2700–2706.
- AOAC. Oficial Methods of Analysis, Association of Official Analytical Chemists, 18th ed.; Washington, DC, USA, 2005.
- Laemmli, U.;. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970;227(5259):680–685.
- Vazquez-Ortiz, F.; Pacheco-Aguilar, R.; Lugo-Sanchez, M.; Villegas-Ozuna, R. Application of the Freshness Quality Index (K Value) for Fresh Fish to Canned Sardines from Northwestern Mexico. Journal of Food Composition and Analysis 1997;10(2):158–165.
- Ramírez-Guerra, H.; Mazorra-Manzano, M.; Ezquerra-Brauer, M.; Carvajal-Millán, E.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.; Ramnírez-Suarez, J. Hydroxylysyl-Pyridinoline Occurrence and Chemical Characteristics of Collagen Present in Jumbo Squid (Dosidicus gigas) Tissues. Journal of Food Composition and Analysis 2015;44:10–17.
- Sikorski, Z.; Borderias, J. Collagen in the Muscles and Skin of Marine Animals. Seafood Proteins 1994;1:58–70.
- Sarabia-Sainz, H. M.; Ezquerra-Brauer, J. M.; Santacruz-Ortega, H. C.; Rouzaud-Sández, O.; Valenzuela-Soto, E. M.; Acosta-Elias, M.; Torres-Arreola, W. Muscle Lysyl Oxidase Activity and Structural/Thermal Properties of Highly Cross-Linked Collagen in Jumbo Squid (Dosidicus gigas) Mantle, Fins and Arms. Food Science and Biotechnology 2018, 27, 57–64.
- Morales, J.; Montero, P.; Moral, A. Isolation and Partial Characterization of Two Types of Muscle Collagen in Some Cephalopods. Journal of Agricultural and Food Chemistry 2000;48(6):2142–2148.
- Arias-Moscoso, J.; Soto-Valdez, H.; Plascencia-Jatomea, M.; Vidal-Quintanar, R.; Rouzaud-Sández, O.; Ezquerra-Brauer, J. Composites of Chitosan with Acid‐Soluble Collagen from Jumbo Squid (Dosidicus gigas) By‐Products. Polymer International 2011;60(6):924–931.
- Sánchez-Basurto, B.; Ramírez-Gilly, M.; Tecante, A.; Severiano-Pérez, P.; Wacher, C.; Valdivia-López, M. Effect of High Hydrostatic Pressure Treatment on the Preservation of Beef Meat. Industrial and Engineering Chemistry Research 2011;51(17):5932–5938.
- Sarabia-Sainz, H. M.; Torres-Arreola, W.; Márquez-Ríos, E.; Santacruz-Ortega, H. C.; Rouzaud-Sández, O.; Valenzuela-Soto, E. M.; Burgara-Estrella, A. J.; Ezquerra-Brauer, J. M. Interrelation of Collagen Chemical Structure and Nanostructure with Firmness of Three Body Regions of Jumbo Squid (Dosidicus gigas). Food Biophysics 2017, 12, 491–499.
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocolloid 2011, 25, 1813–1827.
- Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M. Antioxidant and Functional Properties of Gelatin Hydrolysates Obtained from Skin of Sole and Squid. Food Chemistry 2009;114(3):976–983.
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.; Montero, M.; Gómez-Guillén, M. Role of Lignosulphonate in Properties of Fish Gelatin Films. Food Hydrocolloid 2012;27(1):60–71.
- Raman, M.; Mathew, S. Physiochemical and Textural Alterations in Indian Squid (Loligo duvauceli) Mantle during Frozen Storage and Cooking. Journal of Aquatic Food Product Technology 2015;24(5):454–467.
- Di Luccia, A.; La Gatta, B.; Nicastro, A.; Petrella, G.; Lamacchia, C.; Picariello, G. Protein Modifications in Cooked Pork Products Investigated by a Proteomic Approach. Food Chemistry 2015;172:447–455.
- Torres-Arreola, W.; Ocaño-Higuera, V. M.; Ezquerra-Brauer, J. M.; López-Corona, B. E.; Rodríguez-Felix, F.; Castro-Longoria, R.; Ramírez-Guerra, H. E. Effect of Cooking on Physicochemical and Structural Properties of Jumbo Squid (Dosidicus gigas) Muscle. Journal of Food Processing and Preservation 2018;42(2):2.
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Characteristics of Collagen from the Skin of Clown Featherback (Chitala ornata). International Journal of Food Science and Technology 2015;50(9):1972–1978.
- Mahecha, H.; De Francisco, A.; Beirão, L.; Carrasco, S.; Rodríguez, M. Perdida De Textura Post Mortem De La Carne De Pescado Por Almacenamiento En Frio. Acta Biológica Colombiana 2007;12(1):3.
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of Collagens from the Swim Bladders of Yellowfin Tuna (Thunnus albacares). Food Chemistry 2014;155:264–270.
- Moreno, H.; Montero, M.; Gomez-Guillen, M.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen Characteristics of Farmed Atlantic Salmon with Firm and Soft Fillet Texture. Food Chemistry 2012;134(2):678–685.
- Eyre, D.; Wu, J. Collagen Cross-Links. In: Brinckmann J., Notbohm H., Müller P.K. (eds) Collagen. Topics in Current Chemistry, Springer, Berlin, Heidelberg. 2005;247:207–229.
- Ando, M.; Nakagishi, Y.; Yoshida, K.; Nakao, M.; Nakagawa, T.; Makinodan, Y.; Tsukamasa, Y.; Kawasaki, K. Pyridinoline Concentrations in Muscular and Skin Collagen of Fish and Relationship between Collagen Solubility and Pyridinoline Concentration in Fish Muscular Collagen. Fisheries Science 2006;72(5):1104–1108.
- Uriarte-Montoya, M.; Arias-Moscoso, J.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.; Rouzaud-Sández, O.; Cardenas-Lopez, J.; Ezquerra-Brauer, J. Jumbo Squid (Dosidicus gigas) Mantle Collagen: Extraction, Characterization, and Potential Application in the Preparation of Chitosan–Collagen Biofilms. Bioresource Technology 2010;101(11):4212–4219.
- Friess, W.;. Collagen–Biomaterial for Drug Delivery. European Journal of Pharmaceutics Biopharmaceutics 1998;45(2):113–136.
- Combes, S.; Lepetit, J.; Darche, B.; Lebas, F. Effect of Cooking Temperature and Cooking Time on Warner–Bratzler Tenderness Measurement and Collagen Content in Rabbit Meat. Meat Science 2004;66(1):91–96.
- Zielbauer, B.; Franz, J.; Viezens, B.; Vilgis, T. Physical Aspects of Meat Cooking: Time Dependent Thermal Protein Denaturation and Water Loss. Food Biophysics 2016;11(1):34.
- Petibois, C.; Gouspillou, G.; Wehbe, K.; Delage, J.; Déléris, G. Analysis of Type I and IV Collagens by FT-IR Spectroscopy and Imaging for a Molecular Investigation of Skeletal Muscle Connective Tissue. Analytical and Bioanalytical Chemistry 2006;386(7-8):1961–1966.
- Kanungo, I.; Fathima, N.; Rao, J.; Nair, B. Influence of PCL on the Material Properties of Collagen Based Biocomposites and in Vitro Evaluation of Drug Release. Materials Science and Engineering C 2013;33(8):4651–4659.
- Lafisco, M.; Foltran, I.; Sabbatini, S.; Tosi, G.; Roveri, N. Electrospun Nanostructured Fibers of Collagen-Biomimetic Apatite on Titanium Alloy. Bioinorganic Chemistry and Applications 2012, 1-8.
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen Types Analysis and Differentiation by FTIR Spectroscopy. Analytical and Bioanalytical Chemistry 2009;395(3):829–837.
- Qi, P.; Zhou, Y.; Wang, D.; He, Z.; Li, Z. A New Collagen Solution with High Concentration and Collagen Native Structure Perfectly Preserved. RSC Adv. 2015;105(6):87180–87186.
- Fernandes, L.; Resende, C.; Tavares, D.; Soares, G.; Castro, L.; Granjeiro, J. Cytocompatibility of Chitosan and Collagen-Chitosan Scaffolds for Tissue Engineering. Polimeros. 2011;21(1):1–6.