?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to investigate changes in flavor components in broth prepared by pork ribs that were aged for 1 d, 3 d, and 7 d. The contents of free amino acids (FAAs), nucleic acid-related compounds, organic acids, and volatile compounds in broth were measured. The overall taste and aroma profiles were evaluated by electronic tongue, electronic nose, and sensory panelists. The results showed that the FAAs and succinic acid contents increased, while the 5ʹ-guanosine monophosphate, 5ʹ-inosine monophosphate, and 5ʹ-adenosine monophosphate contents decreased as the aging time increased. These changes led to the taste characteristics of broth being more savory. However, the results of gas chromatography-mass spectrometry, electronic nose, and sensory test all showed that there were no significant differences in aroma among the samples, where the main volatile compounds of broth were aldehydes, including hexanal, nonanal, octanal, heptanal, (Z)-2-heptanal, and (E)-2-decenal. Hence, postmortem aging affected the taste rather than the aroma of pork rib broth, and extending aging time can improve the taste of broth.
Introduction
Pork is one of the most commonly consumed meats worldwide, and its consumption continues to increase.[1] In China, pork ribs are very popular and widely consumed[Citation2] though its price is higher than any other pork cuts. It is commonly used for preparing traditional dishes such as Wuxi sauce ribs and rib broth combined with various food ingredients. Pork rib broth is particularly popular for its palatable taste.
Flavor represents one of the most important quality attributes contributing to the widespread consumption of the broth.[Citation3] Previous studies have indicated that flavor components in meat were affected by chiller aging. Flavor precursors including free amino acids (FAAs), reducing sugars, 5ʹ-inosine monophosphate (5ʹ-IMP), and polyunsaturated fatty acids are all in a dynamic state of degradation during storage.[Citation4–Citation7] Such changes may affect the product flavor because flavor precursors can react with other degradation products for the formation of volatile components responsible for meat aroma or directly present meat taste when heating the meat. Gorraiz et al.[Citation8] reported that aging of beef yielded an increase in its characteristic flavor and aftertaste intensity. Ba et al.[Citation9] showed that the volatile compounds present in beef from Korean native cattle increased while important volatile compounds such as octanal and nonanal associated with pleasant flavor decreased with longer chiller aging period. Nishimura[Citation10] claimed that pork and chicken broth taste intensity increased with longer chilling time of raw meat. Daszkiewicz et al.[Citation11] observed that longer chiller aging time had a positive effect on the taste of beef samples. Sensory test has been widely utilized to judge food flavor, and recently more sensitive electronic tongue and electronic nose have been frequently applied in meat,[Citation12] fruits,[Citation13] and beverages[Citation14] for sample recognition, identification, or classification. In this study, the electronic tongue and electronic nose were used to distinguish the integral profile of nonvolatile and volatile compounds in the pork broth.
Although changes in the flavor components in meat during chiller aging have been widely studied, there are few studies that have simultaneously focused on the taste and aroma of pork rib broth which is prepared by aged ribs. Therefore, in the present study, the effect of postmortem aging (1, 3, and 7 days) on the volatile and nonvolatile components of pork rib broth was evaluated by using traditional chemical methods, electronic instruments, and a sensory panel.
Materials and methods
Chemicals
All reference compounds were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, Mo., U.S.A.). All other chemicals were of least analytical grade from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Sample preparation
The pork spare ribs were collected from Duroc × Landrace × Yorkshire crossbred pigs that had similar feeding conditions and body weights. Animals were slaughtered in a commercial meat company (Sushi, Jiangsu, China). After slaughtering, both sides of spare ribs were removed immediately from hot carcasses and cut into strips of 5 cm in length. The strips were divided into three portions, vacuum packed, and then chilled at 4°C. The ribs were chilled for 1, 3, and 7 days. At each time point, 500 g of ribs were cooked in 1000 mL of purified water (Wahaha, Hangzhou, China) with an addition of 7.5 g salt. The samples were cooked in an electric cooker (MD-WBGS401, Midea, China) for 240 min at a power of 280 W. Salt was added at the end of cooking. After cooking, broth and boned-in meat were separated by filtering through quantitative filter paper. The broth was stored at −80°C for further analysis.
Free amino acids
The FAAs in broth were profiled on an automatic analyzer (L-8900, Hitachi Co., Tokyo). The sample (4 mL) was sonicated with 3 mL of 5-sulfosalicylic acid (10 g/L) and 1 mL of ethylenediaminetetraacetic acid disodium salt (10 g/L) in a vial for 1 h and then the mixture was held overnight. The solution was transferred to a 25 mL colorimetrical cylinder, and 2 mL of the supernatant was then dried using a Nitrogen Evaporator (N-EVAPTM 112, organomation, USA). Then 2 mL of hydrochloric acid (0.02 mol/L) was applied to re-dissolve the amino acids. The resulting sample was filtered through a 0.22 μm filtration membrane.
Twenty microliters of the filtrate were subjected to the automatic analyzer equipped with a BioBasic SCX cation exchange column (4.6 mm × 60 mm, 5 μm) and a UV detector. The absorbance at 440 and 570 nm was measured after a postcolumn reaction with ninhydrin reagent at 135°C. Each FAA was identified and quantified by comparing the peak areas of the amino acids with those of the external standards.
Nucleic acid-related compounds
Nucleic acid-related compounds were quantified according to the method of Li and Wang[Citation15] with some modifications. Broth samples (4 mL) were mixed with 20 mL of 5% cold perchioric acid for 1 min and centrifuged at 12,000 g for 10 min at 4°C (Avanti J-26S XP centrifuge, Beckman Coulter, USA).A space character should be added here. The supernatant was adjusted to pH 4.5 by adding 1 M potassium hydroxide and then centrifuged again in the same conditions. Subsequently, the supernatant was diluted to 100 mL with Milli-Q water and filtered through a 0.45 μm filtration membrane before analysis.
Twenty microliters of the samples were injected in a high-performance liquid chromatography (HPLC) system (Alliance e2695, Waters, USA) equipped with an X Bridge C18 chromatographic column (5 µm, 4.6 × 250 mm) and a photodiode array detector (Waters 2998). Column temperature was set at 25°C and the wavelength of the detector was 254 nm. Potassium dihydrogen orthophosphate buffer (Eluent A, pH 4.5, 0.05 M) and methanol of HPLC grade (Eluent B) were used as eluents. A gradient elution program was conducted as follows: 98% eluent A for 14 min; 85% eluent A for 5 min; and 98% eluent A for 9 min. The flow rate was kept at 1 mL/min with a total run time of 30 min. Identification and quantification of the nucleotides were evaluated by comparing the retention times and peak areas of samples with those of nucleotide standards.
Organic acids
Oxalic, tartaric, lactic, citric, malic, and succinic acids were extracted as described by Chen and Zhang[Citation16] with some modifications. Briefly, 4 mL of the broth and 20 mL of Milli-Q water were mixed thoroughly. The diluted sample was then centrifuged at 12,000 g for 20 min at 4°C. The supernatant was filtered through a 0.45 μm filtration membrane.
The filtrate (25 µL) was then injected into an HPLC system equipped with Atlantis T3 column (5 µm, 4.6 × 150 mm) and monitored at 214 nm using a photodiode array detector at 30°C. A 5 g/L of ammonium phosphate buffer (pH 2.9) was used as the mobile phase at a flow rate of 0.8 mL/min and the total run time was 20 min. The organic acids in the broth were identified and quantified by comparing the retention times and peak areas to those of the standards.
Taste activity value (TAV)
TAV reflects the contribution of a single compound to the taste profile and is calculated as a ratio of a substance concentration in rib broth to its taste threshold value obtained from previous studies. The substance is recognized to contribute less to the taste when TAV is less than 1 and significantly contributes to the taste when TAV is greater than 1.[Citation17]
Equivalent umami concentration (EUC)
EUC is defined as the concentration of monosodium glutamate (MSG, g/100 mL) equivalent to the umami intensity imparted by the synergistic effect of umami amino acids (aspartic acid and glutamic acid) and nucleotides (5ʹ-IMP, 5ʹ-adenosine monophosphate (5ʹ-AMP), and 5ʹ-guanosine monophosphate (5ʹ-GMP)),[Citation18] and is calculated as follows: [Citation19]
where αi is the concentration (g/100 mL) of umami active amino acids (Asp or Glu), αj is the concentration (g/100 mL) of nucleotides (5ʹ-IMP, 5ʹ-GMP, or 5ʹ-AMP), βi is the relative umami concentration (RUC) for umami amino acids [Glu (1) and Asp (0.077)] to MSG, βj is the RUC of nucleotides [5ʹ-IMP (1), 5ʹ-GMP (2.3), and 5ʹ-AMP (0.18)] to MSG, and 1218 is a synergistic constant.
Electronic tongue
An electronic tongue system (ASTREE, Alpha MOS, France) was utilized to analyze the taste of broth samples.[Citation20]The tongue system consists of a conventional Ag/AgCl reference electrode, a 16 position auto-sampler, and an array of cross-selectivity sensors (ZZ, JB, GA, CA, and JE). Each sensor has different sensitivity when they respond to sweetness, umami, saltness, sourness, and bitterness. Prior to testing the broth samples, the electronic tongue sensors were activated, calibrated, and diagnosed for stability and reliability. A 50 mL broth sample was diluted by adding an equal volume of Milli-Q water and used for e-tongue test. The acquisition time was 120 s. After each test, the sensors were cleaned in Milli-Q water.
Gas chromatography-mass spectrometry
The volatile compounds were extracted from 5 mL of broth samples by the solid-phase micro-extraction technique and analyzed using a gas chromatography coupled to triple quadrupole mass spectrometry (TSQ Quantum XLS, Thermo Fisher Scientific, USA). The volatile compounds in the broth were collected for 30 min at 50°C in an solid-phase microextraction fiber (50/30 µm, divinylbenzene/carboxen on polydimethylsiloxane, Supelco, USA). The fiber was then inserted into the gas chromatography injector and a DB WAX capillary column (30 m length × 0.25 mm i.d. × 0.25 µm film thickness, Agilent, USA) was utilized to separate the volatile compounds. The oven temperature was programmed at 40°C for 3 min, followed by an increase at 5°C/min to 100°C, then further increased to 240°C at a rate of 10°C/min, and maintained at this temperature for 5 min. The carry gas was helium at a flow rate of 10 mL/min, and the split ratio was 10:1. The parameters of the mass spectra were set as follows: ion source temperature at 230°C, ionization energy at 70 eV, and data collection over the m/z range of 35–500 amu. The n-alkanes (C7–C30) were run under the same conditions as the samples. The volatile compounds were identified by comparing the mass spectra with data contained in the National Institute of Standards and Technology database and the linear retention index (LRI) values with data reported in the literature and authentic online databases (http://www.flavornet.org/flavornet.html). The volatile compound contents were quantified based on the semi-quantitative determinations using 2-octanol (100 ppm, 1 μL) as an internal standard. Each volatile compound was calculated by comparing the peak area of the compound with that of the internal standard.
Electronic nose
The electronic nose system (αFox 4000, Alpha MOS, Toulouse, France) equipped with 18 metal oxide sensors was used to analyze the headspace volatiles from the broth samples.[Citation21] For the analysis, 2 g of the broth samples were pipetted into 10 mL headspace vials and sealed with magnetic gold caps. Preliminary tests were made in order to find optimum analysis conditions that were acceptable for all of the samples. The preoptimized test parameters were set as follows: headspace temperature 60°C, headspace heating time 120 s, injection volume 600 μL, injection speed 500 μL/s, and acquisition time 120 s. Eight replicates were analyzed for each sample and the maximum resistance changes of each sensor were used for analysis.
Sensory test
A trained 10-member panel participated in the sensory test by using a 9-point hedonic scale (1 = very weak flavor intensity and 9 = very strong flavor intensity).[Citation22] For the evaluation, all panelists were seated in the sensory analysis laboratory. Broth samples were held in transparent plastic cups and provided to panelists with a single random three-digit code to reduce the effect of the order of presentation. Warm water (37°C) was served for gargling between sample evaluations. The evaluation characteristics of broth included aroma, clarity, and taste.
Statistical analysis
All analyses were performed in eight replicates. The taste components data were subjected to analysis of variance using the SAS program (version 9.1.3, SAS Institute Inc., Cary, NC, USA). The significant differences between time points were compared by the procedure of Duncan’s multiple-range test at the significance level of 0.05. Electronic tongue and nose data were analyzed using principal component analysis (PCA). A program in STATISTICA 7 (Analytical Software, St Paul, MN, USA) to manage PCA was developed.
Results and discussion
Free amino acids
FAAs are important for the formation of aroma volatiles as well as taste characteristics. Different amino acid profiles could result in the generation of different taste and flavor compounds.[Citation23] shows the contents, taste thresholds, and TAVs of FAAs broth for 1, 3, and 7 days. The total FAA content increased gradually (from 23.96 to 34.78 mg/100 mL). Although no pronounced differences were observed between day 1 and day 3 in alanine, lysine, proline, valine, tyrosine, phenylalanine, and cysteine, they increased significantly from day 3 to day 7 (p < 0.05). The concentrations of the other FAAs showed a significant increase from day 1 to day 7 (p < 0.05). This indicated that the postmortem aging process of raw meat resulted in the release of FAAs. Similar results were also reported by Nishimura et al.,[Citation24] who found that the levels of FAAs in heated meat broth increased after 6 days aging.
Table 1. Contents and TAVs of free amino acids (mg/100 mL) in broth.
This could be caused by a higher degree of proteolysis during meat storage.[Citation4] During postmortem aging, endogenous enzymes like amino-peptidases C, H, and P increase the release of FAAs.[Citation10] Thus, the FAA contents in raw material were increased as the aging time prolonged. Furthermore, the meat structure became progressively loose during aging which was probably due to the weakening of myofibrillar structures.[Citation25,Citation26] More FAAs may release from meat to broth during heating. This led to an increase in the concentration of FAAs in broth.[Citation27]
In addition, these amino acids were sorted into umami, sweet, and bitter by their flavor characteristics, and each type of amino acids was increased. The contents increased from 4.32 to 7.63 mg/100 mL for umami amino acids, from 12.01 to 17.21 mg/100 mL for sweet amino acids, and from 6.23 to 9.78 mg/100 mL for bitter amino acids. The concentration of sweet-taste active amino acids was far higher than umami-taste active amino acids, indicating that the sweet-taste amino acids were predominant in the pork rib broth. Among these amino acids, the concentrations of glutamic acid and alanine were higher than any other amino acids (), and they accounted for more than 40% of the total FAAs in pork rib broth, followed by glycine (12%), proline (8%), and threonine (5%). Similarly, Zhang et al.[Citation29] found that arginine, glutamic, and alanine acids were the predominant FAAs in crucian carp soup. However, the TAVs of all amino acids were lower than 1 (), indicating that they may not contribute directly to food taste, but they may make food savory through their synergistic effect.[Citation30] Kawai et al.[Citation31] found that sweet amino acids contribute a synergistic interaction with IMP of umami and sweetness that would increase in the presence of IMP.
Nucleic acid-related compounds
Nucleotides that exist in meat are of importance in meat flavor perception, as they can provide umami taste characteristics.[Citation5,Citation32] The levels, taste thresholds, and TAVs of nucleic acid-related compounds in broth samples are shown in . Postmortem aging had a strong impact on the concentrations of the nucleic acid-related compounds. During aging, the concentrations of 5ʹ-GMP, 5ʹ-IMP, 5ʹ-AMP, and inosine (In) decreased (p < 0.05), whereas hypoxanthine (Hx) increased (p < 0.05). Aging had no effect on 5ʹ-cytidine monophosphate (5ʹ-CMP). Similarly, Ngapo and Vachon[Citation33] tracked the concentrations of CMP, UMP, IMP, and GMP in raw chilled pork and found that they decreased with increasing aging time.
Table 2. Contents and TAVs of nucleic acid-related compounds (mg/100 mL) in broth.
The reduction of 5ʹ-AMP content in the broth samples can be explained by its degradation into 5ʹ-IMP under the action of AMP deaminase. Also, the deamination of AMP to IMP would cause the concentration of IMP to increase.[Citation23] In our study, the level of 5ʹ-IMP was reduced simultaneously, which could be because the degradation of 5ʹ-IMP was faster than its synthesis. 5ʹ-IMP can be further degraded into inosine, and then into Hx through 5ʹ-IMP phosphatase and nucleosidase during aging.[Citation32] A slight decline in inosine content could be an indication that 5ʹ-IMP is probably hydrolyzed to hypoxanthine and ribose 5-phosphate rather than directly dephosphorylated to inosine,[Citation4] and during heating, inosine may go into subsequent reactions with other constituents.
In taste perception, 5ʹ-GMP, 5ʹ-IMP, and 5ʹ-AMP are of great importance as they can provide umami taste characteristics.[Citation16] In this study, 5ʹ-IMP was the predominant nucleotide in the broth samples as its TAV was higher than any others (). Additionally, the synergistic interaction of sweet amino acids (e.g. serine, glycine, alanine, and proline) with 5ʹ-IMP has been shown to enhance strongly the umami taste.[Citation31] Although the TAVs of 5ʹ-GMP, 5ʹ-IMP, and 5ʹ-AMP in the broth were less than 1, there could be a synergistic effect between 5ʹ-AMP and 5ʹ-IMP to elicit the umami taste. 5ʹ-GMP is a flavor-enhancer that is stronger than MSG.[Citation34] Thus, the change of taste profile can be reflected by the synergistic interaction between umami active amino acids and nucleotides.
Organic acids
The concentrations, taste thresholds, and TAVs of organic acids in pork rib broth during postmortem aging are shown in . No significant differences were observed between any two aging time points for oxalic, tartaric, and lactic acids (p > 0.05). The malic acid content was increased from day 1 to day 3 (p < 0.05) and then remained constant (p > 0.05). The citric acid content decreased slightly as storage time increased (p < 0.05), while the succinic acid content increased from day 1 to day 7 (p < 0.05). Nishimura et al.[Citation24] observed that lactic acid in heated pork soup did not contribute to the improvement in meat taste during storage. In the present study, the content of lactic acid remained constant and its impact on broth taste may be negligible.
Table 3. Contents and TAVs of organic acids (mg/100 mL) in broth.
Citric, succinic, and malic acids are the intermediates of the tricarboxylic acid cycle. Thus, the reduction in citric acid, accompanied with an increment of malic and succinic acids, should be attributed to the conversion of citric acid to succinic and malic acids. In foods, succinic acid is one of the main taste-active components, and succinic acid together with lactic and citric acids contribute to a special sour and umami taste.[Citation32] In stewed beef juice, umami taste was reduced by the omission of succinic acid.[Citation37] In the present study, the content of succinic acid was higher than any other acids in pork rib broth, and its TAV was greater than 1. Thus, it can be concluded that succinic acid may contribute to the sour and umami taste of the broth. Generally, we were unable to taste the intense sour in the broth, which may be due to the existence of sourness-suppressing peptides.[Citation38]
Changes of the EUC in broth
EUC was utilized to investigate the difference in the umami intensity among different broth samples. A significant increase was observed during the first 3 days of storage while EUC decreased slightly between day 3 and day 7 of storage (). The results were in accordance with the taste sensory score of broth samples.
Electronic tongue
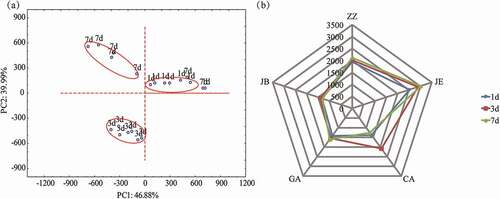
PCA and radar plots of the electronic tongue data of broth samples are shown in . There was a good separation among the three aging times. The first two principal components (PCs) explained more than 85% of the data variations, with PC1 and PC2 accounting for 46.88% and 39.99%, respectively. The variation resulting from PC1 accounted for the difference between day 1 and the other two aging periods, while PC2 mainly corresponded to the difference between day 3 and day 7. Overall, the samples at each of three aging time were well separated, indicating significant difference in taste characteristics. This indicates that the e-tongue can recognize the broth samples completely. Moreover, the radar plots displayed a clear pattern of variation between broth samples: higher relative signals for sensor CA of day 3, and lower for sensor JE of day 1.
Gas chromatography-mass spectrometry
The results of gas chromatograph-mass spectrometry are shown in . A total of 38 compounds were identified, with 28, 26, and 29 compounds corresponding to day 1, day 3, and day 7 of chiller aging, respectively. They were composed of aldehydes, alcohols, ketones, aliphatic hydrocarbons, heterocyclic compounds, esters, and other components, of which the aldehydes were at the highest amount, followed by alcohols. The contents of alcohols, ketones, aliphatic hydrocarbons, heterocyclic compounds, esters, and other volatile compounds were significantly different at different times (p < 0.05). However, no significant changes were observed in the concentration of aldehydes or total amount of volatile compounds (p > 0.05).
Table 4. Contents of volatile compounds (μg/L) in broth.
Aldehydes were the most abundant (85.30–96.40 μg/L) volatile compounds in the broth samples, it represented 72–77% of the total volatile compounds in broth samples. Although there was no significant difference in aldehyde concentration, the number of individual aldehydes increased during chiller aging, with 9, 11, and 13 aldehydes for 1, 3, and 7 days, respectively. Aldehydes might be the products of lipid oxidation and degradation and Strecker reactions of the amino acids,[Citation39,Citation40] while chiller aging may cause changes in fatty acid composition[Citation8] and amino acid composition.[Citation4] Thus, the increase in aldehydes may be related to storage time. Furthermore, aldehydes would contribute considerably to the aroma of rib broth due to their higher concentration and lower thresholds. Hexanal, heptanal, octanal, and nonanal were the major aldehydes that accounted for about 90% of the total aldehydes. With a grease, green grass, and apple flavor, hexanal derived from linoleic and arachidonic acids was predominant among the aldehydes.[Citation41]
Alcohols represented 6–9% of the total volatile compounds in broth samples. The content of alcohols decreased from 9.76% on day 3 to 5.94% on day 7. It may be related to the production of aldehydes and esters. Six aliphatic alcohols were identified, including 1-pentanol, 1-hexanol, 1-heptanol, 1-octen-3-ol, 2-ethyl-1-hexanol, and 1-undecanol. Of these alcohols, 1-octen-3-ol was the highest, which is originated from the oxidative breakdown of linoleic acid and being described as an important meat volatile. It is responsible for a sweet, earthy odor,[Citation42] and it has been found in black-pig pork broth[Citation43] and shiitake mushrooms.[Citation44]
Only one furan compound was found, representing 0.4–1.2% of total volatiles. 2-Pentyl furan, derived from linoleic and other n-6 fatty acids,[Citation41,Citation45] has been identified in black-pig meat broth[Citation43] and common white-pig meat broth.[Citation46] It has a green, earthy, beany flavor note,[Citation47] and it might be a major contributor to the odor of pork rib broth due to its low threshold. In the present study, the content of 2-pentyl furan increased during the first 3 days of chilling but decreased afterwards.
Three ketones, six aliphatic hydrocarbons, and four esters were found in pork rib broth, representing 5–9% of total volatiles. The contents of total ketones and total aliphatic hydrocarbons decreased from day 1 to day 7, while the content of esters increased. Ketones and aliphatic hydrocarbons were also generated from lipid oxidation and degradation reactions during heating,[Citation48] and the esters can be formed by the esterification of acids and alcohols. However, their contribution to the whole aroma profile was negligible due to their high odor thresholds.
In addition, four other compounds were identified, representing 0.7–3% of total volatiles. P-xylene and 1, 4-dichloro-benzene, which are benzene derivatives, were considered as volatile organic chemicals in foods.[Citation49] The antioxidant 2, 4-bis (1, 1-dimethylethyl)-phenol might originate from animal feeds[Citation43] and methoxy-phenyl-oxime was recognized as a contaminant.[Citation50]
Electronic nose
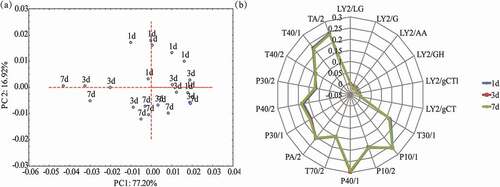
PCA and radar plots of electronic nose data are shown in . The first two PCs explained 94% of the total variation with 77.20% from PC1 and 16.92% from PC2. There was no visible separation between broth samples in PC1, indicating that the aroma of different samples cannot be well distinguished by electronic nose, although the 1-day broth samples were well separated from the other samples in PC2. This might be due to the different concentrations of the volatile compounds. The electronic nose results indicated that postmortem aging of raw pork had weaker effect on the aroma profiles of the pork broth, which was in agreement with the GC-MS data. In addition, the radar plots showed that all sensors of three broth samples were coincided with each other, which confirmed that the aroma of different samples were not significantly different.
Figure 2. (a) PCA plots and (b) radar plots of the data from the electronic nose for broth samples at different postmortem aging periods of raw pork (n = 8).
Notes: LY2/LG, LY2/G, LY2/AA, LY2/GH, LY2/gCT1, LY2/gCT, T30/1, P10/1, P10/2, P40/1, T70/2, PA/2, P30/1, P40/2, P30/2, T40/2, T40/1, and TA/2 are names of e-nose sensors.
Sensory test
The sensory scores are shown in . These indicated that the visual clarity and taste intensity of rib broth increased as postmortem aging time increased (p < 0.05). There was no obvious alteration in the aroma. Thus, in this case, postmortem aging of raw meat can affect the taste rather than the aroma of product. During 3–day aging, the content of 5ʹ-GMP, 5ʹ-IMP, and 5ʹ-AMP decreased slightly while the FAAs increased steadily, the synergistic interaction between nucleotides and FAAs would contribute a savory flavor to the broth, thus the EUC value and sensory scores escalated. Additionally, the levels of hypoxanthine increased significantly with extended storage time, which may lead to unpleasant taste in the broth, leading to reduced EUC value and sensory scores. The taste discrepancies led to the separation of broth samples with the e-tongue. The scores of aroma were in accordance with those provided by e-nose, combined with the GC-MS data. These results showed that the postmortem aging process had no influence on the aroma of rib broth.
Table 5. Sensory scores of different broth samples
Conclusion
Postmortem aging of raw pork ribs had significant effects on the taste-active compounds in the broth, which led to an increase in the levels of FAAs and succinic acid, and a decrease in the important umami-related compounds. The synergistic effects between FAAs and nucleotides or between succinic acid and nucleotides might give a positive effect on overall taste intensity of pork rib broth. Regarding aroma, the contents of volatile compounds were shown to increase slightly, but there was no apparent difference. The main volatile compounds of pork ribs were hexanal, nonanal, octanal, heptanal, (Z)-2-heptanal, and (E)-2-decenal. The present findings provide a reference for consumers on how postmortem aging of raw material affects the flavor of product. The next step will be the investigation of the changes in flavor components in broth prepared from fresh and frozen ribs, and why the differences exist will be explored.
Declaration of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Funding
References
- Xiong, C.; Liu, C.; Chen, F.; Zheng, L. Performance Assessment of Food Safety Management System in the Pork Slaughter Plants of China. Food Control.2017, 71, 264–272. DOI: 10.1016/j.foodcont.2016.07.006.
- Grunert, K. G.; Loose, S. M.; Zhou, Y.; Tinggaard, S. Extrinsic and Intrinsic Quality Cues in Chinese Consumers’ Purchase of Pork Ribs. Food Quality & Preference.2015, 42, 37–47. DOI: 10.1016/j.foodqual.2015.01.001.
- Jayasena, D. D.; Jung, S.; Kim, H. J.; Yong, H. I.; Nam, K. C.; Jo, C. Taste-Active Compound Levels in Korean Native Chicken Meat: The Effects of Bird Age and the Cooking Process. Poultry Science.2015, 94(8), 1964–1972. DOI: 10.3382/ps/pev154.
- Koutsidis, G.; Elmore, J. S.; Orunaconcha, M. J.; Campo, M. M.; Wood, J. D.; Mottram, D. S. Water-Soluble Precursors of Beef Flavour. Part II: Effect of Post-Mortem Conditioning. Meat Science.2008, 79(2), 270–277. DOI: 10.1016/j.meatsci.2007.09.010.
- Tikk, M.; Tikk, K.; Tørngren, M. A.; Meinert, L.; Aaslyng, M. D.; Karlsson, A. H.; Andersen, H. J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. Journal of Agricultural and Food Chemistry.2006, 54(20), 7769–7777. DOI: 10.1021/jf060145a.
- Meinert, L.; Tikk, K.; Tikk, M.; Brockhoff, P. B.; Bredie, W. L.; Bjergegaard, C.; Aaslyng, M. D. Flavour Development in Pork. Influence of Flavour Precursor Concentrations in Longissimus Dorsi from Pigs with Different Raw Meat Qualities. Meat Science.2009, 81(1), 255–262. DOI: 10.1016/j.meatsci.2008.07.031.
- Mungure, T. E.; Bekhit, A. E.-D. A.; Birch, E. J.; Stewart, I. Effect of Rigor Temperature, Ageing and Display Time on the Meat Quality and Lipid Oxidative Stability of Hot Boned Beef Semimembranosus Muscle. Meat Science.2016, 114, 146–153. DOI: 10.1016/j.meatsci.2015.12.015.
- Gorraiz, C.; Beriain, M. J.; Chasco, J.; Insausti, K. Effect of Aging Time on Volatile Compounds, Odor, and Flavor of Cooked Beef from Pirenaica and Friesian Bulls and Heifers. Journal of Food Science.2002, 67(3), 916–922. DOI: 10.1111/jfds.2002.67.issue-3.
- Ba, H. V.; Park, K.; Dashmaa, D.; Hwang, I. Effect of Muscle Type and Vacuum Chiller Ageing Period on the Chemical Compositions, Meat Quality, Sensory Attributes and Volatile Compounds of Korean Native Cattle Beef. Animal Science Journal.2014, 85(2), 164–173. DOI: 10.1111/asj.12100.
- Nishimura, T.; Mechanism Involved in the Improvement of Meat Taste during Postmortem Aging. Food Science and Technology International. 1998, 4, 241–249.
- Daszkiewicz, T.; Wajda, S.; Matusevi, P. Changing of Beef Quality in the Process of Storage. Veterinarija Ir Zootechnika. 2003, 21, 62–65.
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of Beef by Electronic Tongue System TS-5000Z: Flavor Assessment, Recognition and Chemical Compositions according to Its Correlation with Flavor. PLoS ONE.2015, 10(9), e0137807. DOI: 10.1371/journal.pone.0137807.
- Qiu, S.; Wang, J. Application of Sensory Evaluation, HS-SPME GC-MS, E-Nose, and E-Tongue for Quality Detection in Citrus Fruits. Journal of Food Science.2015, 80(10), s2296–s2304. DOI: 10.1111/1750-3841.13012.
- Buratti, S.; Casiraghi, A.; Minghetti, P.; Giovanelli, G. The Joint Use of Electronic Nose and Electronic Tongue for the Evaluation of the Sensorial Properties of Green and Bblack Tea Infusions as Related to Their Chemical Composition. Food & Nutrition Sciences. 2013, 4, 605–615.
- Li, J. S.; Wang, Y. M. Determination of Nucleotide’s Metabolites in Chicken by HPLC. Bulletin of Science & Technology (In Chinese). 2002, 18, 323–326.
- Chen, D.; Zhang, M. Non-Volatile Taste Active Compounds in the Meat of Chinese Mitten Crab (Eriocheir Sinensis). Food Chemistry.2007, 104(3), 1200–1205. DOI: 10.1016/j.foodchem.2007.01.042.
- Qi, J.; Liu, D. Y.; Zhou, G. H.; Xu, X. L. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. Journal of Food Science.2017, (5). DOI: 10.1111/1750-3841.13801.
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir Sinensis). Journal of Food Science.2016, 81(4), s968–s981. DOI: 10.1111/1750-3841.13229.
- Chiang, P. D.; Yen, C. T.; Mau, J. L. Non-Volatile Taste Components of Various Broth Cubes. Food Chemistry.2007, 101(3), 932–937. DOI: 10.1016/j.foodchem.2006.02.041.
- Raithore, S.; Bai, J. H.; Plotto, A.; Manthey, J.; Irey, M.; Baldwin, E. Electronic Tongue Response to Chemicals in Orangejuice that Change Concentration in Relation to Harvest Maturity and Citrus Greening or Huanglongbing (HLB) Disease. Sensors.2015, 15(12), 30062–30075. DOI: 10.3390/s151229787.
- Collier, W. A.; Baird, D. B.; Park-Ng, Z. A.; More, N.; Hart, A. L. Discrimination among Milks and Cultured Dairy Products Using Screen-Printed Electrochemical Arrays and an Electronic Nose. Sensors&Actuators BChemical. 2003, 92, 232–239.
- Li, Y.; Li, C.; Zhao, F.; Lin, X.; Bai, Y.; Zhou, G. The Effects of Long‐Duration Stewing Combined with Different Cooking and Heating Methods on the Quality of Pork Belly. Journal of Food Processing and Preservation.2016, 40(1), 94–102. DOI: 10.1111/jfpp.2016.40.issue-1.
- Williamson, J.; Ryland, D.; Suh, M.; Aliani, M. The Effect of Chilled Conditioning at 4°C on Selected Water and Lipid-Soluble Flavor Precursors in Bison Bison Longissimus Dorsi Muscle and Their Impact on Sensory Characteristics. Meat Science.2014, 96(1), 136–146. DOI: 10.1016/j.meatsci.2013.06.023.
- Nishimura, T.; Rhue, M. R.; Okitani, A.; Kato, H. Components Contributing to the Improvement of Meat Taste during Storage. Agricultural & Biological Chemistry. 1988, 52, 2323–2330.
- Abbott, M.; Pearson, A.; Price, J.; Hooper, G. Ultrastructural Changes during Autolysis of Red and White Porcine Muscle. Journal of Food Science.1977, 42(5), 1185–1188. DOI: 10.1111/jfds.1977.42.issue-5.
- Moczkowska, M.; Półtorak, A.; Montowska, M.; Pospiech, E.; Wierzbicka, A. The Effect of the Packaging System and Storage Time on Myofibrillar Protein Degradation and Oxidation Process in Relation to Beef Tenderness. Meat Science.2017, 130, 7. DOI: 10.1016/j.meatsci.2017.03.008.
- Sasaki, K.; Motoyama, M.; Mitsumoto, M. Changes in the Amounts of Water-Soluble Umami-Related Substances in Porcine Longissimus and Biceps Femoris Muscles during Moist Heat Cooking. Meat Science.2007, 77(2), 167–172. DOI: 10.1016/j.meatsci.2007.02.025.
- Lou, X. W.; Ye, Y. F.; Wang, Y.; Sun, Y. Y.; Pan, D. D.; Cao, J. X. Effect of High-Pressure Treatment on Taste and Metabolite Profiles of Ducks with Two Different Vinasse-Curing Processes. Food Research International.2018, 105, 703–712. DOI: 10.1016/j.foodres.2017.11.084.
- Zhang, J.; Yao, Y.; Ye, X.; Fang, Z.; Chen, J.; Wu, D.; Liu, D.; Hu, Y. Effect of Cooking Temperatures on Protein Hydrolysates and Sensory Quality in Crucian Carp (Carassius auratus) Soup. Journal of Food Science& Technology.2013, 50(3), 542–548. DOI: 10.1007/s13197-011-0376-2.
- Kato, H.; Rhue, M. R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste; ACS Symposium Series; American Chemical Society: Washington, DC, 1989. pp 158–174.
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements between Various Amino Acids and IMP. Chemical Senses.2002, 27(8), 739–745. DOI: 10.1093/chemse/27.8.739.
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of Specific Taste-Active Components on Meat Flavor as Affected by Intrinsic and Extrinsic Factors: An Overview. European Food Research and Technology.2015, 241(2), 157–171. DOI: 10.1007/s00217-015-2449-3.
- Ngapo, T. M.; Vachon, L. Umami and Related Components in “Chilled” Pork for the Japanese Market. Meat Science.2016, 121, 365–374. DOI: 10.1016/j.meatsci.2016.05.005.
- Litchfield, J. H.;. Morel Mushroom Mycelium as a Food-Flavoring Material. Biotechnology & Bioengineering.1967, 9(3), 289–304. DOI: 10.1002/(ISSN)1097-0290.
- Zhong, Y.; Huang, Z.; Wu, L. Identifying Critical Factors Influencing the Safety and Quality Related Behaviors of Pig Farmers in China. Food Control.2017, 73, 1532–1540. DOI: 10.1016/j.foodcont.2016.11.016.
- Weng, L. P.;, Research on Flavor of Breeding Large Yellow Croaker and Wild Large Yellow Croaker. Doctoral dissertation (in chinese) 2011.
- Schlichtherlecerny, H.; Grosch, W. Evaluation of Taste Compounds of Stewed Beef Juice. European Food Research and Technology. 1998, 207, 369–376.
- Okumura, T.; Yamada, R.; Nishimura, T. Sourness-Suppressing Peptides in Cooked Pork Loins. Bioscience, Biotechnology, & Biochemistry.2004, 68(8), 1657–1662. DOI: 10.1271/bbb.68.1657.
- Leroy, F.; Vasilopoulos, C.; Van Hemelryck, S.; Falony, G.; De Vuyst, L. Volatile Analysis of Spoiled, Artisan-Type, Modified-Atmosphere-Packaged Cooked Ham Stored under Different Temperatures. Food Microbiology.2009, 26(1), 94–102. DOI: 10.1016/j.fm.2008.08.005.
- Theron, L.; Tournayre, P.; Kondjoyan, N.; Abouelkaram, S.; Santelhoutellier, V.; Berdague, J. Analysis of the Volatile Profile and Identification of Odour-Active Compounds in Bayonne Ham. Meat Science.2010, 85(3), 453–460. DOI: 10.1016/j.meatsci.2010.02.015.
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D. D.; Sun, Y. Y.; Cao, J. X. Study on the Volatile Compounds Generated from Lipid Oxidation of Chinese Bacon (Unsmoked) during Processing. European Journal of Lipid Science and Technology.2017. DOI: 10.1002/ejlt.201600512.
- Hong, J. S.; Lee, K. R.; Kim, Y. H.; Kim, D. H.; Kim, M. K.; Kim, Y. S.; Yeo, K. Y. Volatile Flavor Compounds of Korean Shiitake mushroom(Lentinus edodes). Korean Journal of Food Science & Technology. 1988, 20, 606–612.
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile Flavor Constituents in the Pork Broth of Black-Pig. Food Chemistry.2017, 226, 51–60. DOI: 10.1016/j.foodchem.2017.01.011.
- Dermiki, M.; Phanphensophon, N.; Mottram, D. S.; Methven, L. Contributions of Non-Volatile and Volatile Compounds to the Umami Taste and Overall Flavour of Shiitake Mushroom Extracts and Their Application as Flavour Enhancers in Cooked Minced Meat. Food Chemistry.2013, 141(1), 77–83. DOI: 10.1016/j.foodchem.2013.03.018.
- Elmore, J. S.; Mottram, D. S.; Enser, M.; Wood, J. D. Effect of the Polyunsaturated Fatty Acid Composition of Beef Muscle on the Profile of Aroma Volatiles. Journal of Agricultural and Food Chemistry.1999, 47(4), 1619–1625. DOI: 10.1021/jf980718m.
- Wang, M.; Hou, L.; Cao, C. C.; Liang, J. J.; Zheng, F. P.; Sun, B. G. Characterization of the Aroma Compounds in Stewed Pork Broth. Food Science (In Chinese). 2015, 36, 105–111.
- Stetzer, A. J.; Cadwallader, K. R.; Singh, T. K.; Mckeith, F. K.; Brewer, M. S. Effect of Enhancement and Ageing on Flavor and Volatile Compounds in Various Beef Muscles. Meat Science.2008, 79(1), 13–19. DOI: 10.1016/j.meatsci.2007.07.025.
- Kosowska, M.; MAJCHER, A. M.; Fortuna, T. Volatile Compounds in Meat and Meat Products. Food Science and Technology (Campinas).2017, 37(1), 1–7. DOI: 10.1590/1678-457x.08416.
- Flemingjones, M. E.; Smith, R. E. Volatile Organic Compounds in Foods: A Five Year Study. Journal of Agricultural and Food Chemistry.2003, 51(27), 8120–8127. DOI: 10.1021/jf0303159.
- Bryant, R.; McClung, A. Volatile Profiles of Aromatic and Non-Aromatic Rice Cultivars Using SPME/GC–MS. Food Chemistry.2011, 124(2), 501–513. DOI: 10.1016/j.foodchem.2010.06.061.