ABSTRACT
The Xinjiang region is a major grape- and wine-production area in China, but the region’s notably high temperatures in the summer and year-round intense sun exposure play negative roles in the aroma, complexity, and elegance of Cabernet Sauvignon wine. In this study, Cabernet Sauvignon grapes harvested in this region were fermented on an industrial scale using four commercial yeast strains (L2323, D254, RVA, and CECA) and spontaneous yeast (NF). The results showed that a total of 123 volatile compounds were detected and 15 volatile compounds significantly contributed their flavor notes to the wine’s overall aroma. The use of RVA and CECA strains resulted in wine with higher concentrations of higher alcohols, terpenes and norisoprenoids. However, the D254-fermented wine showed high level of esters and carbonyl compounds. Wine fermented with the L2323 and D254 strain possessed a stronger fruity aroma, whereas the RVA strain enhanced the herbaceous, chemical, and fatty aromas in wine. Principal component analysis revealed that a significant aromatic feature difference was observed in these wines after alcoholic and malolactic fermentation. The use of different commercial yeast strains altered the aromatic profile of Cabernet Sauvignon wine.
Introduction
Wine is one of the most famous alcoholic beverages in the world and has attracted considerable attention in the field of food and nutritional sciences due to its nutritional value. Wine aromatic features play an essential role in the determination of wine quality.[Citation1,Citation2] Volatile compounds are the important compounds in wine and their composition determines the overall aroma of wine.[Citation3–Citation5] Regarding their origins, wine aroma can be classified into varietal, fermentative, and aging aromas. Varietal aromatic compounds in wine are largely derived from grapes, and these volatile compounds are extracted into wine during the maceration stage.[Citation3,Citation6] The fermentation process can also yield numerous volatile compounds in wine and these aromatic compounds significantly contribute to the fermentation scents in wine.[Citation7,Citation8] The wine-aging process can produce aging aromatic compounds via a series of redox reactions, which could further improve the complexity and maturation of wine.[Citation9]
It has been reported that more than 1000 volatile compounds are found in wine and approximately 400 volatile compounds result from wine fermentation.[Citation10] The major volatile compounds from wine fermentation include acetate esters, fatty esters, higher alcohols, medium- and long-chain volatile acids, and aldehydes.[Citation11–Citation13] Commercial yeast strains possess better sugar-to-alcohol conversion rates and SO2 tolerance during wine fermentation, and wine fermented with commercial yeast exhibit low levels of volatile acids. More importantly, commercial yeasts have been confirmed to exhibit larger effects on the volatiles composition and aromatic attributes of wine.[Citation14] For instance, it has been reported that different yeast strains showed different capacities for collapsing the cell wall in grapes during the maceration process. This phenomenon could directly alter the release rate of varietal volatile compounds from grapes to wine, which affects the varietal aroma of wine.[Citation15–Citation17] Additionally, different yeast strains possess different enzymes (proaroma compounds) to cleave the glycosidic bonds of aroma precursors to release volatile compounds in wine during the fermentation process.[Citation18] This effect could result in alteration of the volatiles composition and fermentation aroma in wine. Different aromatic compositions in wine after fermentation could further trigger different reactions during wine aging, which could lead to wine with different aging aromatic features.
Xinjiang province is a major wine-making region in China with a total annual grape yield of 1.1 million tons on a total 80,000-hm2 vineyard.[Citation19] Cabernet Sauvignon (Vitisvinifera L. cv) is a primary wine-making grape cultivar cultivated in this area. Compared to other wine-making regions in China, the Manasi county Xinjiang province belongs to a continental climate, with a 21.8°C annual average temperature; a 2000°C effectively accumulative temperature through July to September; a total 1780 h solar duration; and a total 200–300 mm annual rainfall.[Citation19] These climate factors could benefit the accumulation of sugar and lower the acid level in Cabernet Sauvignon grapes. However, unfortunately, the notably high-temperature condition in summer and year-round intense sun exposure play a negative role in the aroma, complexity, and elegance of Cabernet Sauvignon wine. Although several studies on the improvement of aroma in Cabernet Sauvignon wine in the Xinjiang region have been reported,[Citation20] the effects of various commercial yeast strains on the alteration of aroma feature of Cabernet Sauvignon wine in this area have not been thoroughly elucidated to date. Therefore, the main objective of this study is to apply four commercially available Saccharomyces cerevisiae yeast strains (D254, L2323, RVA, and CECA) to ferment wine on an industrial scale, and the volatiles composition in these wines were compared with the Cabernet Sauvignon wine under spontaneous fermentation (NF). The findings from this study could provide a better understanding of the effect of different yeasts on the volatile profile in wine, introducing a practical approach to improve the aroma of Cabernet Sauvignon wine produced in the Xinjiang region of China.
Materials and methods
Chemicals and standards
The volatile standards were purchased from Sigma-Aldrich (St. Louis, MO, USA), including 1-hexanol, (E)-3-hexen-1-ol, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, (Z)-2-hexen-1-ol, isobutanol, 1-butanol, isopentanol, 1-pentanol, 4-methyl-1-pentanol, 1-octanol, 2-nonanol, 1-decanol, 2-phenylethanol, 2-phenylethanol, 1-octen-3-ol, 2-ethyl-1-hexanol, nonanol, benzyl alcohol, ethyl acetate, isoamyl acetate, hexyl acetate, phenethyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl dodecanoate, ethyl lactate, methyl octanoate, isoamyl hexanoate, diethyl succinate, ethyl phenylacetate, methyl salicylate, ethyl nonanoate, propanoic acid, isobutyric acid, isovaleric acid, decanoic acid, acetoin, decanal, octanoic acid, hexanoic acid, nonanal, benzaldehyde, benzeneacetaldehyde, β-myrcene, cis-rose oxide, trans-rose oxide, citronellol, nerol, geranylacetone, D-limonene, α-terpineol, linalool, β-damascenone, β-lonone, phenol, 4-ethyl phenol, 2-isopropyl-3-methoxypyrazine, 2-methoxy-3-isobutylpyrazine, naphthalene, and 5-methyl-2-furfural. The internal standard 4-methyl-2-pentanol was also purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride (NaCl) was obtained from the Beijing Chemical Works (Beijing, China), and water was purified using a Milli-Q purification system (Millipore, Bedford, MA, USA).
Grapes and yeast strains
Ripe Cabernet Sauvignon (Vitisvinifera L. cv) grapes were harvested from the Guo-an Winery (N44°17′55′′, E86°12′2′′ with a 475-m altitude) in the Manasi County of the Xinjiang region in China; specifically, a 2012 vintage was employed. The Guo-an Winery has a “slit loam” soil type with a soil pH of 7.8–8.1. Vines in this winery were initially planted in 2000 and grown on their own roots under a north-south row orientation with a modified vertical shoot positioning trellis system. The grapes possessed a 249.6–256.4 g/L reducing sugar content and a 5.1–5.9 g/L total acidity (tartaric acid equivalents) with a pH of 3.76–3.87. Four commercially available yeast strains were used in this study to ferment the grape samples, including D254, L2323, RVA, and CECA. The D254 strain was a product of LAFFORT Fermented Beverages (LAFFORT, France), whereas the L2323 strain was from Lallemand Fermented Beverages (Blagnac, France). The RVA and CECA strains were purchased from Agrovin (Ciudad Real, Spain) and the Angel Yeast Co., Ltd. (Hubei, China), respectively.
Wine-making
The grape clusters, after removing poor grape clusters and impurities, were destemmed and crashed into grape must and later transferred into 200-HL capacity fermentation tanks with an 80% tank loading volume. Afterward, 60 mg/L sulfur dioxide was mixed with the must. The maceration process occurred at 8–10°C for 7 d, and during the maceration period, the must was automatically pumped over every 6 h. After the maceration process, the activated commercial yeasts (0.02 kg/HL) were mixed with the grape must to initiate wine alcoholic fermentation (AF) at 22–24°C. During alcoholic fermentation, the must was pumped over under the same time interval as the maceration process, and the wine density was measured. After 15 d of the AF of the wine (reducing sugar below 4 g/L), 0.001 kg/HL commercial LALVIN 31 lactic acid bacteria (Lallemand Fermented Beverages, Blagnac, France) were inoculated into the wine for malolactic fermentation (MLF) for 3 wk. Each fermentation was performed in triplicate. The wine samples in each yeast fermentation were collected in triplicate at the end of AF and MLF. The wine samples were immediately stored at −20°C until further analysis.
Physicochemical indexes
Physicochemical properties of the wine samples, including total sugar, alcohol, pH, and total acidity were measured according to the National Standard of the People’s Republic of China.[Citation21] The detailed parameters are listed in .
Table 1. Physicochemical parameters of different yeast strain fermented wines after alcoholic and malolactic fermentations.
Volatile compounds
Volatile compounds extraction in these wine samples followed previously published methods.[Citation22,Citation23] In brief, 5-mL wine samples were mixed with 1 g NaCl and 10 µL of 2.004 mg/mL 4-methyl-2-pentanol (internal standard) in a 15-mL vial. The vial was immediately capped with a polytetrafluoroethylene-silicon septum and equilibrated at 40°C for 30 min under agitation. Next, a DVB/Carboxen/PDMS fiber (50/30 µm, Supelco, Bellefonte, PA, USA) was inserted to the headspace of the vial for 30 min at the same temperature with the same agitation. After the absorption of the volatile compounds in the wine sample, the fiber was inserted into the GC injection port at 250°C for 25 min to release the volatile compounds to the GC. An Agilent 6890 GC equipped with an Agilent 5975 mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was used for the analysis of volatiles. A 60 m × 0.25 µm, 0.25 µm HP-INNOWAX column (J & W Scientific, Santa Clara, CA, USA) was used with a 1 mL/min helium flow rate (carrier gas). An oven temperature program was set as follows: 40°C for 5 min, 3°C per min up to 200°C, and then held for 2 min. A split-less mode was used with a 70-eV electron impact mode, and the interface of the mass spectrometer was set at 280°C in the selective ion mode with a mass scan range of m/z 20 to 450. Retention indices were calculated using a C7–C24 n-alkane series (Supelco, Bellefonte, PA, USA) under the same chromatographic conditions. Volatile compounds with the available reference standard were identified by comparing their retention indices and mass spectra to the standard. The volatiles without the available standard were identified by comparing the retention indices and mass spectra to the NIST Standard Reference Database and NIST11 library, respectively. A synthetic wine solution was prepared using 14% (v/v) ethanol and 3.0 g/L tartaric acid, and the pH was adjusted to 3.7 using 5 M NaOH. Standards were dissolved in the model solution with the same concentration of the internal standard, and the extraction and analysis of the standards in the model solution followed the same procedure as the samples. Quantitation of the volatile compounds with the reference standard was performed using the peak area ratio of the external standard to the internal standard versus the concentration of the external standard. Volatiles without the available standard, were quantified using the standard with the most similar structure or number of carbon atom.
Odor activity values and aroma series
The odor activity value (OAV) of a volatile compound is defined as the ratio of its concentration in wine over its perception threshold. The OAV is used to indicate the contribution of a volatile compound to the overall aroma in wine.[Citation7,Citation22,Citation24] A volatile with an OAV above 1 is suggested to exhibit significant contributions to the overall wine aroma.
The overall aroma in wine can be categorized into nine aroma series according to the wine aroma wheel, including (1) fruity, (2) floral, (3) herbaceous, (4) nutty, (5) caramel, (6) earthy, (7) chemical, (8) fatty, and (9) roasted aromas[Citation25] (Noble et al., 1987). Each aroma series was calculated by summing the OAVs of each significant volatile compound in each aromatic category.
Statistical analysis
Data are expressed as the mean ± standard deviation of triplicate tests. One-way analysis of variance was performed to judge the significance among the means under Duncan’s range test at a 0.05 significant level using SPSS 19.0 (Chicago, IL, USA). Principal component analysis was performed using all detected volatile compounds with OAV above 1 as variables to distinguish the volatile compositional similarity of these wine samples (http://www.metaboanalyst.ca, McGill University, Canada) after normalization of data.
Results and discussion
Physicochemical indexes
shows the physicochemical parameters of the wine fermented by different yeast strains at the end of the alcoholic and malolactic fermentations. These wine samples exhibited the difference in total sugar content at the end of the AF. For instance, the wine under spontaneous fermentation (NF wine) exhibited a total sugar level below 3 g/L, whereas the other commercial yeast strain-fermented wines had total sugar contents of 3–4 g/L. However, such differences in the total sugar content among these wines disappeared at the end of the MLF, and their sugar level ranged from 3.8 to 4.1 g/L. For total acidity, the D254 strain fermented wine at the end of the AF displayed the highest total acidity (6.9 g/L), whereas the other wine samples possessed a total acidity level of 6.1–6.3 g/L. After the MLF, these wine samples had a total acidity level of approximately 5.4 g/L, except for the CECA. These yeast strains did not significantly alter the pH of the wine at the end of the AF and MLF, and their pH was maintained at approximately 3.6–3.7. Similarly, the wine samples with these yeast strains after AF and MLF possessed a similar alcohol content (approximately 14%, v/v). The D254 and L2323 strains elevated the volatile acidity in the wine sample at the end of the AF, whereas the CECA-fermented wine showed the lowest volatile acidity. After the MLF, the NF wine appeared to have the highest volatile acidity compared to the other commercial yeast-fermented wines. Volatile acids had a negative effect on the sensory quality of wine.[Citation9] These commercial yeast strains might improve the sensory attributes of wine compared to the NF wine due to their low level of volatile acids.
Volatile compounds and odor activity value
A total of 123 volatile compounds were identified in these wine samples, including 25 alcohols, 49 esters, 8 acids, 8 carbonyl compounds, 21 terpenes and norisoprenoids, 3 volatile phenols, 2 pyrazines, 4 benzene derivatives, 2 furans, and 1 sulfur compound (). According to OAV, the volatile compounds that could significantly contribute their flavor notes to the overall aroma of the wines are listed in .
Table 2. Concentration and aromatic parameters of volatile compounds in different yeast strain fermented wines after alcoholic and malolactic fermentations (ug/L).
Table 3. Odor activity value (OAV) of main volatile compounds (OAV>0.1) in different yeast strain-fermented wines after alcoholic and malolactic fermentations.
Alcohols: C6 alcohols are reported as vegetal and herbaceous flavor notes, and their flavors and scents can negatively affect the overall aroma in wine.[Citation7] In the present study, five different C6 alcohols were detected in the wine samples. Only 1-hexanol exhibited an OAV value above 1, indicating that only this C6 alcohol could significantly contribute flavor notes to the overall aroma of the wine samples (). After the AF, the wine fermented with the L2323 and D254 strains contained less 1-hexanol compared to the other wine samples. However, the D254 strain fermented wine after the MLF exhibited the highest concentration of 1-hexanol, whereas the lowest level of 1-hexanol was found in the wine fermented by the L2323 and RVA strains ().
Higher alcohols, also known as fusel alcohols, are the main alcohols produced through yeast metabolism during wine fermentation. For example, glucose in the grape is reported to be converted into fusel alcohols by yeasts via the anabolic pathway.[Citation26,Citation27] Amino acids can also be metabolized through the catabolic pathway under the activity of enzymes released from yeasts to yield these compounds.[Citation28] In terms of flavor contributions, higher alcohols can contribute pungent scents to wine aromas, and their aromatic contribution is primarily dependent on their concentration in wine. It is reported that fusel alcohols could contribute a positive flavor contribution to wine when their concentration in wine is below 300 mg/L, whereas a negative aromatic contribution could be expected when wine contains fusel alcohols level above 400 mg/L.[Citation18,Citation29] In this study, the concentration of the total fusel alcohols in the different yeast strain-fermented wines appeared to be above 400 mg/L (), similar to the previous study.[Citation30] This result may be attributable to the cold maceration process, which could enhance the extraction of fusel alcohol precursors from grape skin, and these precursors could further be metabolized during wine fermentation to yield fusel alcohols.[Citation31] For the total fusel alcohol concentration, the wine with the RVA and CECA strains exhibited higher levels at the end of both AF and MLF, and levels accumulated faster in the CECA and RVA strain wines compared to the wine with the NF and L2323 strains from AF to MLF. It should be noted that the NF and L2323 strain-fermented wines showed a decrease in the total concentration of fusel alcohols from AF to the MLF. The NF wine exhibited the lowest total fusel alcohol concentration at the end of fermentation. Such an alteration in concentration in the NF wine was mainly attributed to the dramatic decrease in the concentration of isopentanol and benzyl alcohol. The CECA strain played a more important role in affecting the evolution of 1-octen-3-ol, 3-ethyl-4-methyl-1-pentanol, and 1-decanol in the wine. Regarding individual fusel alcohols, isopentanol appeared to be the dominant fusel alcohol in the wines (). This fusel alcohol could bring a sweet and bitter taste to wine and could also lead to feelings of dizziness[Citation18] (Swiegers, & Pretorius, 2005) The CECA wine contained higher levels of isopentanol than did the other wine samples.
Esters: Esters are the major volatiles that can be produced during wine fermentation process through two potential pathways.[Citation9] The chemical interactions between alcohols and acids could lead to the formation of esters. However, ester formation through this route is limited compared to the enzymatic reaction, due to the dynamic equilibrium of these volatile compounds.[Citation32] Yeast strains have been confirmed to release important enzymes that can trigger the formation of esters in wine during AF.[Citation12] Therefore, the ester composition in wine is primarily determined by the yeast strains used. A total of 49 esters were found in these wine samples (). Regarding their chemical nature, the esters were categorized as acetate esters, fatty acid ethyl esters, ethyl esters, and other esters. It has been reported that acetate esters could be produced through reactions between acetyl-CoA and higher alcohols during AF,[Citation9] and acetate esters have been indicated as the main flavor contributor to young wine.[Citation7,Citation33] In the present study, acetate esters were found to be the dominant esters, and their total concentration represented approximately 40–50% of the total ester content in these wine samples. Additionally, ethyl acetate and isoamyl acetate appeared to be the major individual acetate esters in these wine samples, and their level was observed to be higher in the NF, D254, and L2323 strain-fermented wine samples at the end of both AF and MLF (). Notably, the wine fermented with the RVA and CECA strains exhibited lower concentrations of acetate esters. It has been reported that the activity of alcohol acetyltransferases, rather than higher alcohols content (substrates), played a critical role in regulating the formation of acetate esters in wine.[Citation12] Therefore, we speculated that the RVA and CECA strains might possess less ability to biosynthesize alcohol acetyltransferases than do other strains to convert higher alcohols into acetate esters during wine AF, although these strain-fermented wines contained higher levels of higher alcohols.
These wine samples all contained 7 fatty acid ethyl esters (). Except for ethyl myristate and ethyl palmitate, these esters were found to contribute their flavor notes (fruity and floral scents) to the overall aroma in the wine samples due to their high OAV values (). In terms of the total concentration of these fatty acid ethyl esters, the wine fermented with the D254 strain appeared to contain the highest concentration at the end of the fermentation process. In addition, these fatty acid ethyl esters (except for ethyl butanoate and ethyl hexanoate) were also found to show the highest concentration in the D254 strain-fermented wine at the end of the AF and MLF compared to the other strain-fermented wines (). It should be noted that the RVA and CECA strain-fermented wine samples exhibited lower concentrations of these fatty acid ethyl esters, which indicated that these wines might display less fruity and floral flavor notes than the other wine samples after fermentation process. It has been reported that medium-chain fatty acid (C6-C12) are the substrates in wine that form fatty acid ethyl esters, and these esters could provide a pleasant flavor to the wine overall aroma.[Citation34] Therefore, we speculated that the D254 strain might improve the accumulation of medium-chain fatty acids in must during the maceration process, which could enhance the conversion of medium-chain fatty acids to fatty acid ethyl esters in wine during fermentation.
In addition to fatty acid ethyl esters, over the entire fermentation, these wine samples were also found to contain 18 ethyl esters (). Among these ethyl esters, ethyl lactate, ethyl 9-decenoate, and ethyl dihydrocinnamate were found to provide flavor notes to the overall aroma in these wines due to their high OAV (). Ethyl lactate was reported to exhibit fruity and buttery scents,[Citation35] and this volatile significantly accumulated at the end of the MLF in these wine samples. However, the concentration of ethyl 9-decenoate and ethyl dihydrocinnamate in these wine samples at the end of the MLF was lower than at the end of the AF. It should be noted that diethyl pentanedioate and ethyl 5-oxotetrahydro-2-furancarboxylate were only present in these wine samples at the end of the MLF.
In other ester categories, a total of 18 other esters were found in these wine samples, and their concentration in these wine samples was low at the end of both AF and MLF (). Notably, isopentyl lactate was not found in these wine samples at the end of the AF. However, this lactate’s concentration significantly accumulated in these wines at the end of the MLF, and its cream flavor note could be incorporated into the wine aroma due to its high OAV at the end of the fermentation.
Carbonyl compounds: A total of eightcarbonyl compounds, including six aldehydes and two ketones, were detected in these wine samples (). It has been reported that carbonyl compounds could indirectly contribute their flavor notes to the overall aroma in wine through a synergetic effect, even though their concentration in wine was relatively low.[Citation20] For instance, decanal and nonanal have been reported to contribute an unpleasant aroma to wine.[Citation36] In the present study, the RVA and CECA strain-fermented wines showed higher concentrations of nonanal at the end of the MLF than the other wines, indicating that these two wine samples might possess more unpleasant “green” flavors. In addition, benzeneacetaldehyde was found to be the only carbonyl compound that had a higher concentration than its perception threshold in these wines (). This finding indicated that the compound’s floral and honey aroma could be significantly incorporated into the wine overall aroma.[Citation24] Among these wine samples, the CECA strain-fermented wine possessed higher concentrations of benzenacetaldehyde than the other wines at the end of the MLF, which indicated that this wine might exhibit more floral and honey scents.
Terpenes and norisoprenoids: Terpenes and norisoprenoids are the major secondary metabolites synthesized during grape berry development.[Citation18] These volatile compounds play an essential role in determining the varietal flavor feature in wine, although they are not present at high levels in wine.[Citation18] It has been reported that yeasts can disrupt the cell wall of grape skin to release these volatile compounds.[Citation37] Meanwhile, yeast cells can initially adsorb these volatiles during wine AF and then release them via autolysis during MLF.[Citation38,Citation39] As shown in , 16 terpenes and 5 norisoprenoids were identified in these wines. It was observed that the NF, D254, and RVA wines contained higher concentrations of terpenes at the end of the AF. However, the wines fermented with the RVA and CECA strain exhibited a significantly higher level of terpenes at the end of the MLF. These indicated that the RVA and CECA strains could possess a greater capacity to disrupt grape skin cells to release the flavor volatiles of the varietal. These strains can adsorb these volatiles during AF and subsequently release these volatiles via cell autolysis during wine MLF. For example, the RVA and CECA strain-fermented wines contained higher concentrations of β-damascenone and β-lonone than the other wines after fermentation (). The β-damascenone has been reported to possess sweet and exotic flavor notes, whereas β-lonone was described as violet and rose aromas.[Citation7] Therefore, the flavor notes of these two volatiles could be significantly emphasized in RVA and CECA wines compared to other wine samples. In fact, the date of the appearance of ketones is a key factor for synthesizing β-damascenone, because when yeast is present, the addition of the ketones to the fermentation medium resulted in the formation of damascenone. No damascenone was detected in nonspiked reference fermentations.[Citation40]
Fatty acids: Fatty acid metabolism by yeast during wine fermentation is a main route to the production of fatty acids in wine, and medium-chain fatty acids could be further converted into long-chain fatty acids through the enzymatic activity of yeasts.[Citation18] Additionally, fatty acids can also be reacted with other compounds in wine to form esters, which are a main volatile class responsible for the fermentative flavor of wine. As a result, a total of eight acids were detected (). It has been reported that fatty acids can affect the complexity of wine aromas, and this impact depends on the concentration of acids in wine[Citation18] but is not associated with wine quality.[Citation41] For example, fatty acids at a concentration of 4–10 mg/L could enhance the complexity of wine, whereas the elegance of the wine aroma could be negatively affected when their concentration in wine is above 20 mg/L.[Citation42] Compared with this experiment, these fatty acids, except for acetic acid, exhibited concentrations below 4 mg/L, indicating that they may not significantly affect the wine aroma complexity.
Other volatiles: As shown in , a total of 12 other volatiles (3 volatile phenols, 4 benzene derivatives, 2 pyrazines, 2 furans, and 1 sulfur compound) were detected. Except for 2-isopropyl-3-methoxypyrazine (IPMP) and 2-methoxy-3-isobutylpyrazine (IBMP), the volatile compounds exhibited concentrations below their threshold in the wine samples at the end of the AF and MLF, indicating that these volatiles could not significantly contribute flavor notes to the overall aroma in these wines. Thus, these yeast strains did not result in large variations in the accumulation of these volatiles in wines. For IPMP, the musty, earthy, and leafy notes are incorporated into these wines due to their high OAV.[Citation43] However, these strains showed similar effect on the levels in wines during fermentation. Additionally, IBMP is one of the most abundant methoxypyrazines in wine, and is considered to be the main source of musty and green pepper aroma in wine.[Citation44] However, it can add complexity to the aroma of some high-quality wines in relatively high concentrations. In grape fruits, IBMP is mainly produced via the metabolism of amino acids in plant tissues. The L2323 strain increased levels in wine at the end of the AF, whereas the D254 strain-fermented wine exhibited higher levels of IBMP at the end of the MLF (). However, the content of IBMP increased significantly after 1 d of maceration and did not change after the end of AF and MLF.[Citation45]
Aroma series
To better understand the aromatic features of different yeast strain-fermented wine samples, volatile compounds with high OAV values were summarized to assess each aroma series in wine (). These wine samples after fermentation exhibited a similar aroma series order: fruity > fatty > herbaceous > floral > chemical > caramel. However, these wines showed differences in the contents of each aroma series, indicating that these yeast strains play an important role in altering the aromatic quality of the wines.
Figure 1. Total OAVs (∑OAV) of aroma series in different yeast strains treated wines after alcoholic fermentation (a) and malolactic fermentation (b). Different letters in the same aroma series represent the significant differences at a significant level of 0.05.
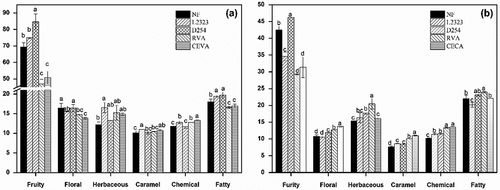
The fruity aroma was found to be the primary aromatic feature in these wine samples (), and its flavor was primarily contributed from the ethyl esters (including ethyl lactate, isoamyl acetate, ethyl butanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate), and β-damascenone ( and Supplementary Table 1). Among these wine samples at the end of the AF, the D254 strain-fermented wine appeared to possess the highest fruity aroma, followed by the L2323 and NF wines. The RVA- and CECA-fermented wines exhibited the least fruity aroma. In addition, the MLF resulted in a decrease in the fruity aroma in these wines. The D254 strain-fermented wine still displayed the highest fruity aroma compared to other wine samples at the end of the MLF, followed by the NF wine and then the L2323 wine. The least fruity aroma appeared to be found in the RVA and CECA fermented wines. These findings indicated that the D254 yeast could enhance fruity aromas in wine, consistent with other reports.[Citation46] The fatty aroma was a major featured aroma in these wine samples, and was mainly comprised of 2 fatty acids (hexanoic acid and octanoic acid), 1 higher alcohol (isopentanol), and 1 fatty acid ethyl ester (ethyl dodecanoate) ( and Supplementary Table 1). The D254 strain fermented wine at the end of the AF showed the highest fatty aroma, whereas the least fatty note was found in the RVA wine. However, the fatty aroma significantly accumulated in the RVA wine after the MLF. At the end of the MLF, the RVA and D254 strain-fermented wines possessed higher fatty aroma features than the other wine counterparts.
The floral series in the wine samples primarily resulted from 2-phenylethanol, ethyl octanoate, benzeneacetaldehyde, β-damascenone, and β-ionone ( and Supplementary Table 1). The D254 strain fermented wine showed the highest floral aroma compared to the other wine samples at the end of the AF. However, the wine fermented with the CECA strain significantly increased its floral scent during the MLF, and thus this wine had the highest floral aroma at the end of the MLF. Notably, a significant decrease was observed for 2-phenylethanol, ethyl octanoate, β-damascenone, and β-ionone in the NF wine sample at the end of the MLF (), which led to the NF wine with the least floral aroma. The floral feature in the CECA and RVA strain-fermented wines did not significantly change over the overall fermentation process.
The C6 compounds and pyrazines in these wine samples determined the herbaceous aroma of these wines, and an herbaceous aroma is considered a negative scent in red wine.[Citation7] Among these wine samples, the RVA strain-fermented wine had a high level of nonanal at the end of the MLF, which resulted in an increase in the herbaceous aroma in the RVA wine ( and ). Additionally, caramel flavor has been confirmed to enhance the comfortable and cheerful perception to wine. In the present study, isopentanol, benzeneacetaldehyde, and β-damascenone significantly contributed the caramel aroma to the wine samples. At the end of the AF, the L2323 strain-fermented wine showed the strongest caramel aroma, whereas the strongest caramel scent was found in the CECA strain fermented wine at the end of the MLF. The NF wine had the weakest least caramel flavor at the end of both AF and MLF compared to the other wine samples.
Fusel alcohols and volatile phenols in the present study contributed chemical aromas to these wines ( and Supplementary Table 1). These volatiles could improve wine complexity at a low concentration. However, high levels of these volatile compounds could negatively affect the fruity aroma of wine.[Citation47] The aroma series was found to be lower in the NF, L2323, and D254 wines at the end of the MLF than AF, whereas the RVA and CECA wine exhibited stronger chemical aromas at the end of the MLF.
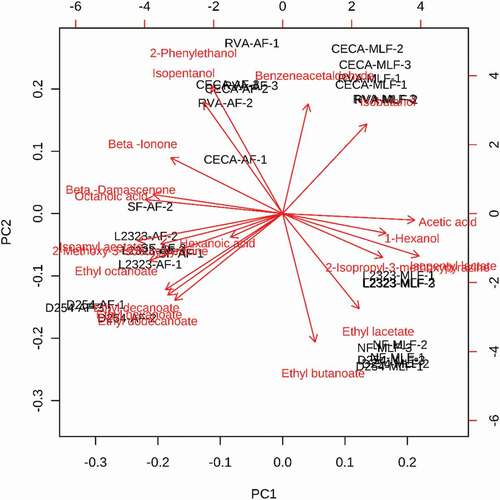
Principal component analysis
To investigate the diversity of the aromatic profile of these different yeast strain-fermented wines, principal component analysis was performed using volatile compounds with an OAV above 1 as the variable (), since these volatiles are the major volatiles that contributed to the overall aroma in these wine samples. Principal component 1 and 2 (PC1 and PC2) represented 72.7% of the total variance. It was observed that the wines at the end of the AF were placed on the left side of the PC1, whereas the wines at the end of the MLF were positioned on the positive scale of the PC1 (). This finding indicated that the aromatic feature of the wines after AF was different from that observed after MLF. In addition, the RVA and CECA strain-fermented wines were located at the positive position in the PC2, whereas the NF, L2323, and D254 wines were positioned at the negative side on the PC2. It should be noted that isopentanol, isobutanol, and benzeneacetaldehyde were positively correlated with PC2. Therefore, these volatile compounds play important roles in the aromatic feature of the RVA and CECA wines. In addition, ethyl decanoate, ethyl hexanoate, ethyl dodecanoate, and ethyl lactate showed a similar correlation as seen for the D254 wine to the PC1 and PC2, indicating that these fruity and floral aromatic compounds resulted in D254 wine with different aromas than the other wine samples. Isoamyl acetate, 2-methoxy-3-isobutylpyrazine, and ethyl octanoate appeared to be the major volatile compounds that distinguish the aroma feature of the L2323 wine from the other wines at the end of the AF. At the end of the MLF, the separation of the aroma feature of the L2323 wine resulted mainly from 2-isopropyl-3-methoxypyrazine and isopentyl lactate.
Conclusions
The effect of different commercial yeasts on volatiles composition and aromatic attributes were investigated in Cabernet Sauvignon wines made from the Xinjiang region of China. A total of 123 volatile compounds were detected. Among these volatile compounds, 15 volatiles were found to significantly contribute their flavor notes to the overall aroma of Cabernet Sauvignon wine in the Xinjiang region. The RVA and CECA yeast strain-fermented wines contained higher concentrations of higher alcohols, terpenes and norisoprenoids, whereas acetate esters, fatty acid ethyl esters, and ethyl esters carbonyl compounds were found to be higher in the D254 strain-fermented wine. The L2323 and NF wines exhibited higher concentrations of esters. Aroma series analysis revealed that the L2323 and D254 yeast strains could enhance the fruity aroma of wine, whereas wine fermented with the RVA strain possessed stronger herbaceous, chemical, and fatty aromas. The CECA strain resulted in wine with greater floral, caramel, and chemical features than the other wines. For this reason, we believe that this study is valuable for providing useful insights for future research and applications.
Supplemental Table
Download ()Acknowledgments
All of the authors are grateful to CITIC Guo-an Winery for their support in Cabernet Sauvignon wine-making.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, G. L.; Salinas, M. R. Complementary Effect of Cabernet Sauvignon on Monastrell Wines. Journal of Food Composition & Analysis 2008, 21(1), 54–61. DOI: 10.1016/j.jfca.2007.06.003.
- Tao, Y.-S.; Liu, Y.-Q.; Li, H. Sensory Characters of Cabernet Sauvignon Dry Red Wine from Changli County (China). Food Chemistry 2009, 114(2), 565–569. DOI: 10.1016/j.foodchem.2008.09.087.
- Rapp, A.;. Volatile Flavour of Wine: Correlation between Instrumental Analysis and Sensory Perception. Nahrung 1998, 42(6), 351–363. DOI: 10.1002/(SICI)1521-3803(199812)42:06<351::AID-FOOD351>3.3.CO;2-U.
- Rodríguez-Bencomo, J. J.; Conde, J. E.; Rodríguez-Delgado, M. A.; García-Montelongo, F.; Pérez-Trujillo, J. P. Determination of Esters in Dry and Sweet White Wines by Headspace Solid-Phase Microextraction and Gas Chromatography. Journal of Chromatography A 2002, 963(1–2), 213. DOI: 10.1016/S0021-9673(02)00551-4.
- Salinas, M. R.; Color, Polyphenol, and Aroma Compounds in Rose Wines after Prefermentative Maceration and Enzymatic Treatments. American Journal of Enology & Viticulture 2003, 54, 195–202.
- Girard, B.; Kopp, T. G.; Reynolds, A. G.; Cliff, M. Influence of Vinification Treatments on Aroma Constituents and Sensory Descriptors of Pinot Noir Wines. American Journal of Enology & Viticulture 1997, 48, 198–206.
- Ferreira, V.; Lopez, R.; Cacho, J. F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. Journal of the Science of Food & Agriculture 2000, 80(11), 1659–1667.
- Escudero, A.; Gogorza, B.; Melús, M. A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the Aroma of a Wine from Maccabeo. Key Role Played by Compounds with Low Odor Activity Values. Journal of Agricultural & Food Chemistry 2004, 52(11), 3516–3524. DOI: 10.1021/jf035341l.
- Li, H.; Wine Tasting; Science Press: Beijing China, 2006
- Nykanen, L.; Formation and Occurrence of Flavor Compounds in Wine and Distilled Alcoholic Beverages. American Journal of Enology & Viticulture 1986, 37, 84–96.
- Delfini, C.; Cocito, C.; Bonino, M.; Schellino, R.; Gaia, P.; Baiocchi, C. Definitive Evidence for the Actual Contribution of Yeast in the Transformation of Neutral Precursors of Grape Aromas. Journal of Agricultural & Food Chemistry 2001, 49(11), 5397–5408. DOI: 10.1021/jf010613a.
- Lilly, M.; Lambrechts, M. G.; Pretorius, I. S. Effect of Increased Yeast Alcohol Acetyltransferase Activity on Flavor Profiles of Wine and Distillates. Microbiology 2000, 66, 744–753.
- Estevez, P.; Gil, M. L.; Falque, E. Effects of Seven Yeast Strains on the Volatile Composition of Palomino Wines. International Journal of Food Science & Technology 2004, 39(1), 61–69. DOI: 10.1046/j.0950-5423.2003.00755.x.
- Fleet, G. H.;. Yeast Interactions and Wine Flavour. International Journal of Food Microbiology 2003, 86(1–2), 11–22. DOI: 10.1016/S0168-1605(03)00245-9.
- Gunata, Y. Z.; Bayonove, C. L.; Baumes, R. L.; Cordonnier, R. E. The Aroma of Grapes I. Extraction and Determination of Free and Glycosidically Bound Fractions of Some Grape Aroma Components. Journal of Chromatography A 1985, 331(1), 83–90. DOI: 10.1016/0021-9673(85)80009-1.
- Williams, P. J.; Sefton, M. A.; Wilson, B. Nonvolatile Conjugates of Secondary Metabolites as Precursors of Varietal Grape Flavor Components. Flavor Chemistry 1989, 388, 35–48.
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and Norisoprenoidic Glycoconjugates of Vitis Vinifera L. Cv. Melon B. As Precursors of Odorants in Muscadet Wines. Journal of Chromatography A 2001, 936(1–2), 145–157. DOI: 10.1016/S0021-9673(01)01150-5.
- Swiegers, J. H.; Pretorius, I. S. Yeast Modulation of Wine Flavor. Advances in Applied Microbiology 2005, 57, 131–175.
- Sun, D.-M.; Zhao, P.; Sun, Y.-Q. Analysis on Agro - Climatic Conditions for the Development of Grape Industry in Manasi County. Agriculture & Technology 2015, 17, 149–151.
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M. J.; Duan, C.-Q. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chemistry 2014, 154(2), 217–229. DOI: 10.1016/j.foodchem.2014.01.003.
- GB, T15038-2006. Analytical Methods of Wine and Fruit Wine; China Standard Press: Beijing, 2006.
- Wu, Y.-W.; Zhu, B.-Q.; Tu, C.; Duan, C.-Q.; Pan, Q.-H. Generation of Volatile Compounds in Litchi Wine during Winemaking and Short-Term Bottle Storage. Journal of Agricultural and Food Chemistry 2011, 59(9), 4923–4931. DOI: 10.1021/jf2001876.
- Zhang, M.-X.; Pan, Q.-H.; Yan, G.-L.; Duan, C.-Q. Using Headspace Solid Phase Micro-Extraction for Analysis of Aromatic Compounds during Alcoholic Fermentation of Red Wine. Food Chemistry 2011, 125(2), 743–749. DOI: 10.1016/j.foodchem.2010.09.008.
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Aroma Profile of Garnacha Tintorera-Based Sweet Wines by Chromatographic and Sensorial Analyses. Food Chemistry 2012, 134(4), 2313. DOI: 10.1016/j.foodchem.2012.03.105.
- Noble, A. C.; Arnold, R. A.; Buechsenstein, J.; Leach, E. J.; Schmidt, J. O.; Stern, P. M. Modification of a Standardized System of Wine Aroma Terminology. American Journal of Enology & Viticulture 1987, 38, 143–146.
- Li, H.; Tao, Y.-S.; Kang, W.-H.; Yin, C.-L. Wine Aroma Analytical Investigation Progress on GC. Journal of Food Science & Biotechnology 2006, 25, 99–104.
- Perestrelo, R.; Fernandes, A.; Albuquerque, F. F.; Marques, J. C.; Câmara, J. S. Analytical Characterization of the Aroma of Tinta Negra Mole Red Wine: Identification of the Main Odorants Compounds. Analytica Chimica Acta 2006, 563(1), 154–164. DOI: 10.1016/j.aca.2005.10.023.
- Ehrlich, F.; Ueber Das Natürliche Isomere Des Leucins. European Journal of Inorganic Chemistry. 1904, 37, 1809–1840.
- Rapp, A.; Versini, G. Influence of Nitrogen Compounds in Grapes on Aroma Compounds of Wines. Developments in Food Science 1997, 37(06), 1659–1694.
- Liu, J.-X.; Zhang, J.; Shi, T.-T.; Yu, Y.; Li, W.-J.; Sun, Y.-X. Effect of Different Commercial Yeast on Aroma Components of Wine. China Brewing 2015, 34, 42–46.
- Petropulos, V. I.; Bogeva, E.; Stafilov, T.; Stefova, M.; Siegmund, B.; Pabi, N.; Lankmayr, E. Study of the Influence of Maceration Time and Oenological Practices on the Aroma Profile of Vranec Wines. Food Chemistry 2014, 165(3), 506–514. DOI: 10.1016/j.foodchem.2014.05.144.
- Escudero, A.; Campo, E.; Fariña, L.; Juan Cacho, A.; Ferreira, V. Analytical Characterization of the Aroma of Five Premium Red Wines. Insights into the Role of Odor Families and the Concept of Fruitiness of Wines. Journal of Agricultural and Food Chemistry 2007, 55(11), 4501–4510. DOI: 10.1021/jf0636418.
- Pretorius, I. S.; Lambrechts, M. G. Yeast and Its Importance to Wine Aroma: A Review. South African Journal of Enology & Viticulture 2000, 21, 97–129.
- Saerens, S.; Delvaux, F.; Verstrepen, K.; Van Dijck, P.; Thevelein, J.; Delvaux, F. Parameters Affecting Ethyl Ester Production by Saccharomyces Cerevisiae during Fermentation. Applied and Environmental Microbiology 2008, 74(2), 454–461. DOI: 10.1128/AEM.01616-07.
- Peinado, R. A.; Moreno, J.; Bueno, J. E.; Moreno, J. A.; Mauricio, J. C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chemistry 2004, 84(4), 585–590. DOI: 10.1016/S0308-8146(03)00282-6.
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15(12), 9184–9196. DOI: 10.3390/molecules15129184.
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of Yeast Walls on the Behavior of Aroma Compounds in a Model Wine. American Journal of Enology & Viticulture 1994, 45, 29–33.
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast Derivatives (Extracts and Autolysates) in Winemaking: Release of Volatile Compounds and Effects on Wine Aroma Volatility. Food Chemistry 2006, 99(2), 217–230. DOI: 10.1016/j.foodchem.2005.06.049.
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine – A Review. Australian Journal of Grape & Wine Research 2006, 12(2), 119–127. DOI: 10.1111/j.1755-0238.2006.tb00051.x.
- Lloyd, N. D. R.; Capone, D. L.; Ugliano, M.; Taylor, D. K.; Skouroumounis, G. K.; Sefton, M. A.; Elsey, G. M. Formation of Damascenone under Both Commercial and Model Fermentation Conditions. Journal of Agricultural and Food Chemistry 2011, 59(4), 1338–1343. DOI: 10.1021/jf103741n.
- Varela, C.; Torrea, D.; Schmidt, S. A.; Ancin-Azpilicueta, C.; Henschke, P. A. Effect of Oxygen and Lipid Supplementation on the Volatile Composition of Chemically Defined Medium and Chardonnay Wine Fermented with Saccharomyces Cerevisiae. Food Chemistry 2012, 135(4), 2863–2871. DOI: 10.1016/j.foodchem.2012.06.127.
- Shinohara, T.; Gas Chromatographic Analysis of Volatile Fatty Acids in Wines. Journal of the Agricultural Chemical Society of Japan 2006, 49, 2211–2212.
- Dunlevy, J. D.; Kalua, C. M.; Keyzers, R. A.; Boss, P. K. The Production of Flavour & Aroma Compounds in Grape Berries. In In Grapevine Molecular Physiology & Biotechnology: Second Edition. Springer Netherlands; 2009; pp. 293–340.
- Sala, C.; Busto, O.; Guasch, J. Factors Affecting the Presence of 3-Alkyl-2-Methoxypyrazines in Grapes and Wines. A Review. Journal of Agricultural & Food Chemistry 2004, 55(2), 153–159.
- Sala, C.; Mestres, M.; Martı́, M. P.; Busto, O.; Guasch, J. Headspace Solid-Phase Microextraction Analysis of 3-Alkyl-2-Methoxypyrazines in Wines. Journal of Chromatography A 2002, 953(1–2), 1–6. DOI: 10.1016/S0021-9673(02)00123-1.
- Qin, W.-S.; Zhao, X.-J.; Zhang, N.; Zhai, H. Differential Synthesis of Aroma Compounds of Different Commercial Wine Yeasts Using SPME-GC-MS Analysis. Food & Fermentation Industries 2012, 38, 146–150.
- Styger, G.; Prior, B.; Bauer, F. F. Wine Flavor and Aroma. Journal of Industrial Microbiology & Biotechnology 2011, 38(9), 1145–1159. DOI: 10.1007/s10295-011-1018-4.