ABSTRACT
A comparative analysis of egg yolk proteome of three major poultry species, namely, chicken, duck, and quail, was carried out with two-dimensional (2D) gel electrophoresis patterns and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry. Proteins identified from these three poultry species shared high degrees of sequence and structure homology, which might be related to the bird’s adaptability under different environmental stresses. Few specific proteins were found in this study. Comparative 2D gel electrophoresis patterns of chicken, duck, and quail revealed that some protein-specific regions on gels could be used for authentic identification of poultry eggs. These findings could provide a fundamental understanding of different poultry egg protein profiles and showed us some new sights in egg yolk nutrients related to certain properties and functions.
Introduction
Egg yolk, well known for its high value [Citation1–Citation3], can not only provide almost all essential nutrients for embryo development, but may also elicit specific biological functions. It consists of 17% protein, 33% lipid, and 48% water. Various avian species may show differential egg protein patterns due to the adaptation under varied environments and evolution. The amino acid diversity of homologous proteins also reflects their environmental adaptability and their relationship with evolution to some extent. For example, the homology comparison of the amino acid sequences of ovalbumin-specific peptides of different avian eggs showed diversities related to their evolution. [Citation4] By constructing a phylogenetic tree of albumin in different animals, chicken has closer genetic relationship with quail rather than duck.[Citation5]
Comparative proteomics offers the possibility to analyze the differential expression patterns of all proteins in the same tissue at specific times and under specific environmental conditions. Several studies of chicken egg yolk proteome[Citation6,Citation7] and comparative proteomics analyses[Citation2,Citation8,Citation9] have profiled its protein patterns in different states. The egg yolk protein profile compared to other poultry species remains unknown. As three most consumed avian eggs all over the world, chicken, quail, and duck eggs might have different egg protein patterns due to the adaptation under varied environments and evolution. According to the phylogenetic analysis with whole mitochondrial genome[Citation10] and egg white proteome[Citation11], Japanese quail (Coturnix japonica) and chicken had a much closer relationship than duck. These data implied that the protein patterns of these three avian egg yolks were different, which might be of significant importance in biology. Therefore, comparative proteomics of different avian eggs is essential to determine the unique protein pattern of each species. Analyzing the diversity of amino acid sequences and expression patterns of the same protein family among different species not only helps us to further understand the evolutionary relationship between populations, but also implicates the adaptability to the environment.
Besides, recent various egg products, such as liquid egg, egg powder, frozen egg, and other egg-based products, are gaining increasing popularity in China with their convenience and safety. However, unlike those of shell eggs, adulteration cannot be identified directly with the naked eyes. Adulteration occurs frequently in liquid egg products, because the source, type, purity, freshness, and other quality information of these products are not easily distinguishable. In fact, adulteration might lead to a number of potential health risks, such as egg allergy, one of the most common food hypersensitivity especially in infants and young children. Most of the egg allergens are mainly found in egg whites, but serum albumin and chicken egg yolk antibody (IgY) in egg yolks could also cause allergic reactions.[Citation12] In addition, allergic reactions to different avian eggs in the reference human groups are not the same, which indicates the interspecific-different allergies. Different avian egg yolk IgYs and their fractions, for instance, seemed to show different stabilities and antigenic activities.[Citation13,Citation14] Anibarro reported that some people with no history of allergy to chicken eggs became allergic when eating duck and goose eggs due to the different ovalbumin-specific epitopes between chicken and lying geese mesh (including duck and geese).[Citation15] Caro Contreras reported a case of quail egg allergy without chicken egg allergy, for the interaction of transferrin and IgE in quail eggs was not found in other eggs.[Citation16] Therefore, comparative proteomics of different avian eggs is essential to define the unique protein pattern of each species to ensure food quality and safety.
This study was aimed to identify and compare the protein patterns of egg yolks of three poultry species (duck, quail, and chicken) using two-dimensional gel electrophoresis (2D PAGE) and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry (MALDI-TOF-MS/MS) analysis. The unique protein expression pattern of each species was obtained by analyzing the amino acid sequences of the proteins identified in this study. Moreover, protein-specific regions found in this study could be used as biomarkers to provide some reference for the authentic identification.
Materials and methods
Egg yolk sampling
Fresh eggs of chicken, quail, and duck were collected randomly from the Poultry Research Centre farm of Huazhong Agricultural University within 24 h after laid and utilized in this study. Each sample consisted of five eggs. Egg yolk plasma protein extraction was performed basically according to the previously described method reported by Mann and Mann.[Citation7]
Two-dimensional gel electrophoresis analysis and protein identification
2D gel electrophoresis analysis for three egg yolk proteins was performed with the Ettan IPGphor 3 System (GE Healthcare, USA) for the first dimension isoelectric focusing and the Ettan DALT Six System (GE Healthcare, USA) for SDS-PAGE in the second dimension, respectively. The process of 2D PAGEs followed the method of Yin[Citation17] and Naveena[Citation18], and the range of pH was 4–7. The protein spots were visualized via silver staining. Each gel was replicated three times. The differences of 2D data were evaluated by ANOVA and Tukey’s significance test (p < 0.01) using SPSS 13.0 (SPSS, Chicago, IL). Mass spectrometry analysis protein spots of good reproducibility have been selected, and the isolated protein spots from 2D gels of chicken, quail, and duck egg yolk were excised manually, then destained, washed, and digested with sequencing-grade trypsin (Promega, Madison, WI, USA) and alkylation. The samples mixed with an equivalent matrix solution (HCCA) were applied for further MALDI-TOF-MS/MS analysis using a fuzzy logic feedback control system (Ultraflex MALDI-TOF-TOF mass spectrometer (Bruker, Karlsruhe, Germany)). Proteins were identified by searching the MASCOT program (http://www.matrixscience.com) in the nonredundant sequence database (NCBInr), and those with low success rate identification (score < 30) were abandoned. Additional sequence homology analysis was with the Basic Local Alignment Search Tool (BLAST).
Results and discussion
Egg yolk proteins from different poultry species (duck, quail, and chicken) were analyzed by 2D PAGE, as shown in . Protein spots of quail and duck were more similar in terms of distance distribution than those of chicken protein spots (). Five distinct regions, the first three regions in particular, were found in all the three poultries. All egg yolk protein spots on the 2D gels of these three poultry species were subsequently identified by MALDI-TOF MS/MS. The detected peptides of each protein spot were listed in and Table S1. A total of 32, 35, and 32 protein spots representing 12 different proteins were identified successfully in the egg yolk of duck, quail, and chicken, respectively. Plenty of protein spots were identified as the same protein cluster though with different MWs (molecular weights) or pIs (isoelectric points), which may be caused by altered post-translational modification (phosphorylation, glycosylation, alternative splicing, etc.). Several common proteins have been identified in all these three gels, such as vitellogenin (VTG)-related proteins and serum albumin, although these homologous proteins have different positional distributions on 2D gels. On the other hand, specific proteins were found in only one or two of the poultry egg yolks, such as hemopexin and riboflavin-binding protein which were only identified in duck egg yolk and β-2-glycoprotein 1 and VTG-3 which were only observed in that of chicken. Apoptosis antagonizing transcription factor (AATF) and retinol-binding protein 4 were absent from that of chicken.
Table 1. Proteins identification by mass spectrometry (MALDI-TOF-MS/MS) after two-dimensional polyacrylamide gel electrophoresis (2DE) analysis.
Figure 1. 2D electrophoresis of three poultry egg yolk proteins. Spots that identified successfully were indicated by numbers and arrows via MALDI-TOF-MS/MS. A, duck egg yolk; B, quail egg yolk; C, chicken egg yolk.
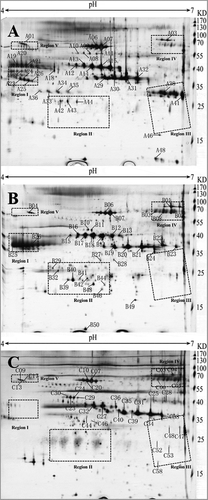
Figure 2. Phylogenetic tree constructed based on amino acid sequences of different avian egg albumin and VTG-related proteins. (a) Serum albumin; (b) VTG-related protein; GI: accession numbers of matched proteins according to the National Center for Biotechnology Information nonredundant sequence (NCBInr) database.
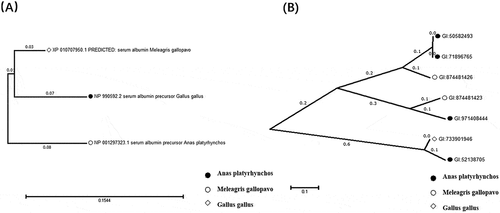
Some proteins were identified in these three poultry egg yolks with different location distributions (), such as VTG-related proteins, serum albumin, and ovalbumin. Meanwhile, the amino acids of these proteins showed interspecies differences. VTG, as a phospholipoglycoprotein widely existing in egg-laying non-mammal, could not only promote the growth and differentiation of animal oocytes, but also provide developing embryos nutrients, such as amino acids, fats, carbohydrates, phosphorus, sulfur, calcium, zinc, iron, etc.[Citation19,Citation20] Three VTG isoforms (VTG-1, VTG-2, and VTG-3) have been identified from these three avian species egg yolk, and these differences were induced by hormones in primary sequence and structure. Spots A38 and A41 were identified as VTG-2 isoform X1 (Anas platyrhynchos) on region III of duck egg yolk protein gel, while on the corresponding region of chicken, spots C47 and C48 were identified as VTG-3 (Gallus gallus), as well as C58 representing VTG-1 precursor (Gallus gallus) (). VTG consist of three parts: lipovitellin I (N-terminal), phosvitin (middle part), and some C-terminal proteins (lipovitellin II, yolk glycoprotein, and ovovitellin).[Citation21,Citation22] Among these proteins, VTG-2 is most similar to VTG-1 (37% sequence identity), and VTG-3 showed 35% sequence identity to VTG-1. A phylogenetic tree among proteins was constructed based on amino acid sequences of the VTG-related proteins identified in this study, as shown in . The phylogenetic tree indicated that chicken and quail shared closer distance through the comparison of these egg proteins. This may be owing to the fact that both chicken and quail are Galliformes, while duck is Anseriformes. The sequence of VTG-1 from chicken contains more large open β-sheets forming DUF1943 domain which could promote the phagocytosis of E. coli and S. aureus by carp macrophages recombinant proteins.[Citation23,Citation24] This different structure may be formed on account of various living conditions during the evolution.
Serum albumins were identified in the egg yolks of these kinds of poultry eggs and located in the middle and upper parts of the 2D gels (spots B06, B07, B10, B11, C07, C10, C20, C23, C24, C29, C30, and C44 in ). The serum albumin spot distributions on chicken and quail egg yolk gels are more similar than that of duck. The phylogenetic relationships of serum albumin based on amino acid sequences were shown in . It was found that chicken and quail have closer evolutionary relationship through the analysis of the results.
Protein spots on region V were identified as homologous proteins. Spot B04 on quail egg yolk gel and spots C09, C13, and C17 on that of chicken were designated as PIT 54 protein precursor, while its homologous protein DMBT1 was also identified on the coordinate region of the duck gel (spot A01). The sequence of DMBT 1 was 87% identical to PIT 54 protein precursor. Based on this, PIT 54 and DMBT1 can be used to distinguish egg products between chicken/quail and duck by antigen–antibody experiments. In human, DMBT1 may play an important role in the processes of infection and inflammation as a scavenger receptor cysteine-rich (SRCR) superfamily.[Citation25] PIT 54 has antibacterial activity[Citation26] and antioxidant activity[Citation27], which might lead to the different shelf life of different poultry eggs.
Besides common proteins observed in the present study, some specific proteins were only identified in one or two poultry egg yolk without designated regions on 2D gels. The identification of these proteins by comparison with their homologous sequences in databases gave a clue of differential environmental adaptability of different poultry species.
The living environments of these three poultry species are quite different. In order to adapt to the environment, homologous proteins in three poultry eggs have evolved corresponding subtypes. Three isoforms of HX were identified in duck egg yolk (A08, A09, and A11, GI:971378941), and their spots distribution on 2D gel was similar to that on chicken egg yolk.[Citation28,Citation29] The experimental MW (57.5 kDa) was slightly higher than its theoretical value (49.7 kDa), which is most likely combined with heme.[Citation28] Compared to chicken HX, hydrophilic analysis revealed that less hydrophobic amino acid residues of HX from duck were exposed on the protein surface, which means that the duck HX is more hydrophilic and stable. It can be seen that the isoforms of AATF from quail are more acidic than that from duck ( and ), indicating more phosphorylated subtypes of AATF from quail. It is reported that the phosphorylated form of AATF helps to counteract ionizing radiation.[Citation30] Compared with the aquatic environment, ionizing radiation from terrestrial and airborne environments may be stronger, which in turn causes to require more phosphorylated subtypes of AATF in quail to counteract ionizing radiation. Furthermore, the MWs of all these spots on the 2D gels are lower than their theoretical value, which may be caused by degradation. These fragments are all N-terminal sequences of AATF according to the sequence analysis (). The C-terminal region of AATF is reported to be involved in the transcription process as an adaptor during the transportation of proteins and vesicles. The lack of C-terminal may lead to less directed transporting of intercellular materials during the embryonic development and it is a topic worth of further study.
Based on the protein-specific regions (regions I–IV), 2D gel patterns could be used for authentic identification. Two regions, namely regions I and II, were absent on the chicken egg yolk gel. For region I, four densely distributed protein spots (A23–A26) were found on duck egg yolk gel, comparing to the corresponding area (including spots B25 and B26) of quail egg yolk gel. No spot was identified successfully in the counterpart (or equivalent region) on chicken egg yolk gel. Spots B25 and B26 were designated as VTG-1-like. Prominently shown with high intensities in region I, four protein spots (A23–A26) from duck egg yolk were identified as riboflavin protein (RfBP), while were not found in the corresponding areas of chicken and quail (). The shifting of chicken RfBP out of the gel range in this study might be due to its lower pI caused by phosphorylation.[Citation31,Citation32] RfBP acts as a bitter inhibitor/protein sweetener. Its presence or absence may, to some extent, influence the flavors of different kinds of eggs.[Citation33] The main biological function of RfBP is to store vitamin B2 and deliver it to chicken embryos for the development of the embryo. Their different sources (e.g. chicken egg white, quail egg white, or yolk) showed various glycosylation patterns as well as diverse tertiary structures[Citation34,Citation35], indicating their distinct biological functions during embryonic development.
In the case of region II, three spots (A42–A44) with high intensity were detected as Apo A-I (Anas platyrhynchos) on the egg yolk gel of duck, while the closely distributed spots (B29, B32, B39–B44, and B46) were also identified as Apo A-I (Coturnix japonica) on the quail coordinates. But no obvious protein spot was identified successfully there on chicken egg yolk gel. Apo A-I plays a vital role in lipid metabolism and is proved to prevent atherosclerosis and coronary heart disease.[Citation36] Regions I and II could be used as biomarker locations to verify duck egg and quail egg proteins.
For region III, it was absent on the quail egg yolk gel. The abundance of duck spots (A38 and A41) was higher than those from chicken (C47, C48, C52, C53, and C58). Therefore, region III could be used to distinguish whether quail egg yolks are mixed by chicken or duck egg yolk products. Region III contains two kinds of egg yolk proteins: Ig γ chain and VTG-related protein. Spots C52 and C53 were designated as Ig γ chain (clone 36) (Gallus gallus), which is a fraction of IgY heavy chain.
As to region IV, some protein spots showed high intensity on chicken and quail gels, while the corresponding spots showed lower intensity in duck. Among these spots, B01, B02, B03, C03, and C04 were identified as ovotransferrin (Gallus gallus), and A03 was assigned to transferrin (Meleagris gallopavo), which shares 90% sequence identity with Gallus ovotransferrin. These protein sequences identified from chicken and duck all lack C-terminal sequences. B08 was designated to ovoinhibitor precursor (Gallus gallus), while duck ovoinhibitor was not identified in duck. As a trypsin and chymotrypsin inhibitor,[Citation37] ovoinhibitor has been found in the egg white of chicken, quail, and ostrich. [Citation11] Its deficiency in duck egg white may suggest weaker inhibition of endogenous protease activity and shorter storage period than those of chicken and quail eggs.
Conclusion
In conclusion, major proteins of three different poultry species (duck, quail, and chicken) egg yolks were compared based on 2D PAGE and MALDI-TOF/TOF. Twelve proteins were identified respectively in every single kind of egg yolks. The comparison of 2D gels suggested that some regions could be used as biomarkers for authentication. The detected proteins in these regions (regions I–V) showed interspecies differences. These variations of different poultry species egg proteins may be related to the diverse physiological needs, such as cell defense or regulation of embryonic development, for adapting to distinct living environments. These findings provide a deeper understanding of the unique protein expression patterns of related poultry species, and make better sources of different egg proteins in human health and food industry.
Additional information
Funding
References
- Kovacs-Nolan, J.; Phillips, M.; Mine, Y. Advances in the Value of Eggs and Egg Components for Human Health. Journal of Agricultural and Food Chemistry 2005, 53, 8421–8431. DOI: 10.1021/jf050964f.
- Rehault-Godbert, S.; Mann, K.; Bourin, M.; Brionne, A.; Nys, Y. Effect of Embryonic Development on the Chicken Egg Yolk Plasma Proteome after 12 Days of Incubation. Journal Agricultural Food Chemical 2014, 62, 2531–2540. DOI:10.1021/jf404512x.
- Gouda, M.; Zu, L.; Ma, S.; Sheng, L.; Ma, M. Influence of Bio-Active Terpenes on the Characteristics and Functional Properties of Egg Yolk. Food Hydrocolloids 2018, 80, 222–230. DOI:10.1016/j.foodhyd.2018.02.009.
- Ren, J.; Hu, J.; Chen, L.; Liu, Y.; Xu, X.; He, J.; Shen, J.; Lu, L. Identification of Egg White Proteins and Divergence in the Regulatory Region of the Ovalbumin Gene in Avians. Protein and Peptide Letters 2017, 24, 12–25. DOI: 10.2174/0929866523666161025120953.
- Li, S.; Cao, Y.; Geng, F. Genome-Wide Identification and Comparative Analysis of Albumin Family in Vertebrates. Evolutionary Bioinformatics 2017, 13. http://journals.sagepub.com/doi/abs/10.1177/1176934317716089?url_ver=Z1176934317716039.1176934317716088-1176934317712003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%1176934317716083dpubmed (accessed June 19, 2017).
- Farinazzo, A.; Restuccia, U.; Bachi, A.; Guerrier, L.; Fortis, F.; Boschetti, E.; Fasoli, E.; Citterio, A.; Righetti, P. G. Chicken Egg Yolk Cytoplasmic Proteome, Mined via Combinatorial Peptide Ligand Libraries. Journal of Chromatography A 2009, 1216, 1241–1252. DOI:10.1016/j.chroma.2008.11.051.
- Mann, K.; Mann, M. The Chicken Egg Yolk Plasma and Granule Proteomes. Proteomics 2008, 8, 178–191. DOI:10.1002/pmic.200700790.
- Padliya, N. D.; Qian, M.; Mimi Roy, S.; Chu, P.; Zheng, H.; Tess, A.; Dariani, M.; Hariri, R. J. The Impact of Fertilization on the Chicken Egg Yolk Plasma and Granule Proteome 24 Hours Post-Lay at Room Temperature: Capitalizing on high-pH/low-pH Reverse Phase Chromatography in Conjunction with Tandem Mass Tag (TMT) Technology. Food & Function 2015, 6, 2303–2314. DOI: 10.1039/c5fo00304k.
- Gao, D.; Qiu, N.; Liu, Y.; Ma, M. Comparative Proteome Analysis of Egg Yolk Plasma Proteins during Storage. Journal of the Science of Food and Agriculture 2017, 97, 2392–2400. DOI: 10.1002/jsfa.8052.
- Nishibori, M.; Tsudzuki, M.; Hayashi, T.; Yamamoto, Y.; Yasue, H. Complete Nucleotide Sequence of the Coturnix Chinensis (Blue-Breasted Quail) Mitochondorial Genome and a Phylogenetic Analysis with Related Species. The Journal of Heredity 2002, 93, 439–444. DOI: 10.1093/jhered/93.6.439.
- Hu, S.; Qiu, N.; Liu, Y.; Zhao, H.; Gao, D.; Song, R.; Ma, M. Identification and Comparative Proteomic Study of Quail and Duck Egg White Protein Using 2-Dimensional Gel Electrophoresis and Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Tandem Mass Spectrometry Analysis. Poultry Science 2016, 95, 1137–1144. DOI: 10.3382/ps/pew033.
- Szepfalusi, Z.; Ebner, C.; Pandjaitan, R.; Orlicek, F.; Scheiner, O.; Boltz-Nitulescu, G.; Kraft, D.; Ebner, H. Egg Yolk Alpha-Livetin (Chicken Serum Albumin) Is a Cross-Reactive Allergen in the Bird-Egg Syndrome. The Journal of Allergy and Clinical Immunology 1994, 93, 932–942. DOI: 10.1016/0091-6749(94)90388-3.
- Dias Da Silva, W.; Tambourgi, D. V. IgY: A Promising Antibody for Use in Immunodiagnostic and in Immunotherapy. Veterinary Immunology and Immunopathology 2010, 135, 173–180. DOI: 10.1016/j.vetimm.2009.12.011.
- Quirce, S.; Diez-Gomez, M. L.; Eiras, P.; Cuevas, M.; Baz, G.; Losada, E. Inhalant Allergy to Egg Yolk and Egg White Proteins. Clinical Experiments Allergy 1998, 28, 478–485. DOI: 10.1046/j.1365-2222.1998.00253.x.
- Anibarro, B.; Seoane, F. J.; Vila, C.; Lombardero, M. Allergy to Eggs from Duck and Goose without Sensitization to Hen Egg Proteins. Journal Allergy Clinical Immunity 2000, 105, 834–836. DOI: 10.1067/mai.2000.104547.
- Caro Contreras, F. J.; Giner Munoz, M. T.; Martin Mateos, M. A.; Plaza Martin, A. M.; Sierra Martinez, J. I.; Lombardero, M. Allergy to Quail’s Egg without Allergy to Chicken’s Egg. Case Report. Allergology Immunopathol (Madrid) 2008, 36, 234–237. DOI: 10.1016/s0301-0546(08)72555-2.
- Yin, Q.; Zhang, Y.; Dong, D.; Lei, M.; Zhang, S.; Liao, -C.-C.; Pan, Y.-H. Maintenance of Neural Activities in Torpid Rhinolophus Ferrumequinum Bats Revealed by 2D Gel-Based Proteome Analysis. . Biochimica Et Biophysica Acta (BBA) - Proteins and Proteomics 2017, 1865, 1004–1019. DOI: 10.1016/j.bbapap.2017.04.006.
- Naveena, B. M.; Jagadeesh, D. S.; Kamuni, V.; Muthukumar, M.; Kulkarni, V. V.; Kiran, M.; Rapole, S. In‐Gel and OFFGEL‐based Proteomic Approach for Authentication of Meat Species from Minced Meat and Meat Products. Journal Sciences Food Agricultural 2017, 98. DOI: 10.1002/jsfa.8572
- Sato, M.; Kawashima, T.; Aosasa, M.; Horiuchi, H.; Furusawa, S.; Matsuda, H., Excision of Foreign Gene Product with Cathepsin D in Chicken Hepatoma Cell Line. Biochemical and Biophysical Research Communications 2005, 330, 533–539. DOI: 10.1016/j.bbrc.2005.03.008.
- Choi, B. K.; Chitwood, D. J.; Paik, Y. K. Proteomic Changes during Disturbance of Cholesterol Metabolism by Azacoprostane Treatment in Caenorhabditis Elegans. Molecular Cellular Proteomics 2003, 2, 1086–1095. DOI: 10.1074/mcp.M300036-MCP200.
- Banaszak, L.; Sharrock, W.; Timmins, P. Structure and Function of a Lipoprotein: Lipovitellin. Annual Reviews Biophysics Biophysics Chemical 1991, 20, 221–246. DOI: 10.1146/annurev.biophys.20.1.221.
- Wallace, R. A.; Hoch, K. L.; Carnevali, O. Placement of Small Lipovitellin Subunits within the Vitellogenin Precursor in Xenopus Laevis. Journal of Molecular Biology 1990, 213, 407–409. DOI: 10.1016/s0022-2836(05)80203-7.
- Sun, C.; Hu, L.; Liu, S.; Gao, Z.; Zhang, S. Functional Analysis of Domain of Unknown Function (DUF) 1943, DUF1944 and Von Willebrand Factor Type D Domain (VWD) in Vitellogenin2 in Zebrafish. Developmental and Comparative Immunology 2013, 41, 469–476. DOI: 10.1016/j.dci.2013.07.005.
- Li, Z. J.; Zhang, S. C.; Liu, Q. H. Vitellogenin Functions as a Multivalent Pattern Recognition Receptor with an Opsonic Activity. PLoS One 2008, 3. DOI: 10.1371/journal.pone.0001940
- Deng, H.; Gao, Y. B.; Wang, H. F.; Jin, X. L.; Xiao, J. C. Expression of Deleted in Malignant Brain Tumours 1 (DMBT1) Relates to the Proliferation and Malignant Transformation of Hepatic Progenitor Cells in Hepatitis B Virus-Related Liver Diseases. Histopathology 2012, 60, 249–260. DOI: 10.1111/j.1365-2559.2011.04082.x.
- Georgieva, T. M.; Koinarski, V. N.; Urumova, V. S.; Marutsov, P. D.; Christov, T. T.; Nikolov, J.; Chaprazov, T.; Walshe, K.; Karov, R. S.; Georgiev, I. P.; et al. Effects of Escherichia Coli Infection and Eimeria Tenella Invasion on Blood Concentrations of Some Positive Acute Phase Proteins (Haptoglobin (PIT 54), Fibrinogen and Ceruloplasmin) in Chickens. Reviews Medica Vet-Toulouse 2010, 161, 84–89.
- Iwasaki, K.; Morimatsu, M.; Inanami, O.; Uchida, E.; Syuto, B.; Kuwabara, M.; Niiyama, M. Isolation, Characterization, and cDNA Cloning of Chicken Turpentine-Induced Protein, a New Member of the Scavenger Receptor Cysteine-Rich (SRCR) Family of Proteins. The Journal of Biological Chemistry 2001, 276, 9400–9405. DOI: 10.1074/jbc.M011713200.
- Wellner, D.; Cheng, K.-C.; Muller-Eberhard, U. N-Terminal Amino Acid Sequences of the Hemopexins from Chicken, Rat and Rabbit. Biochemical and Biophysical Research Communications 1988, 155, 622–625. DOI: 10.1016/s0006-291x(88)80540-0.
- Wang, J.; Liang, Y.; Omana, D. A.; Kav, N. N.; Wu, J. Proteomics Analysis of Egg White Proteins from Different Egg Varieties. Journal Agricultural Food Chemical 2012, 60, 272–282. DOI: 10.1021/jf2033973.
- Aristide Floridi, M. F.;. Che-1: A New Effector of Checkpoints Signaling. Cell Cycle (Georgetown, Tex.) 2007, 6, 804–806. DOI: 10.4161/cc.6.7.4043.
- Fenselau, C.; Heller, D. N.; Miller, M. S.; White, H. B., 3rd. Phosphorylation Sites in Riboflavin-Binding Protein Characterized by Fast Atom Bombardment Mass Spectrometry. Analytical Biochemistry 1985, 150, 309–314. DOI: 10.1016/0003-2697(85)90515-9.
- Mega, T.; Hamazume, Y.; Nong, Y.-M.; Ikenaka, T. Studies on the Methods for the Determination of Phosphorylation Sites in Highly Phosphorylated Peptides or Proteins: Phosphorylation Sites of Hen Egg White Riboflavin Binding Protein. The Journal of Biochemistry 1986, 100, 1109–1116.
- Maehashi, K.; Matano, M.; Nonaka, M.; Udaka, S.; Yamamoto, Y. Riboflavin-Binding Protein Is a Novel Bitter Inhibitor. Chemical Senses 2008, 33, 57–63. DOI: 10.1093/chemse/bjm062.
- Amoresano, A.; Brancaccio, A.; Andolfo, A.; Perduca, M.; Monaco, H. L.; Marino, G. The Carbohydrates of the Isoforms of Three Avian Riboflavin-Binding Proteins. European Journal of Biochemistry/FEBS 1999, 263, 849–858. DOI: 10.1046/j.1432-1327.1999.00570.x.
- Walker, M.; Stevens, L.; Duncan, D.; Price, N. C.; Kelly, S. M. A Comparative Study of the Structure of Egg-White Riboflavin Binding Protein from the Domestic Fowl and Japanese Quail. Comparative Biochemistry and Physiology B, Comparative Biochemistry 1991, 100, 77–81. DOI: 10.1016/0305-0491(91)90088-u.
- Miguel, M.; Manso, M. A.; López-Fandiño, R.; Ramos, M. Comparative Study of Egg White Proteins from Different Species by Chromatographic and Electrophoretic Methods. European Food Researcher Technological 2005, 221, 542–546. DOI: 10.1007/s00217-005-1182-8.
- Tomimatsu, Y.; Clary, J. J.; Bartulovich, J. J. Physical Characterization of Ovoinhibitor, a Trypsin and Chymotrypsin Inhibitor from Chicken Egg White. Archives of Biochemistry and Biophysics 1966, 115, 536–544. DOI: 10.1016/0003-9861(66)90073-7.
