ABSTRACT
Cereals like sorghum and millet are splendid sources of phenolics, dependent on their genetic makeup, and possess varied levels of flavonoids, flavanols, flavanones, anthocyanins, and condensed tannins. In this study, chemical composition and anthocyanin and tannin content of seven sorghum and two millet varieties were evaluated. American Association of Cereal Chemists (AACC) respective methods were used for chemical composition. Anthocyanins were determined through HPLC/UV-Vis, while condensed tannins were determined spectrophotometrically. Four anthocyanins determined included apigeninidin, kuromanin, pelargonidin, and cyanidin. Among sorghum varieties, JS-263 had the highest apigeninidin (16 µg/g). Other anthocyanins were not found in appreciable quantities. None of the anthocyanins were detected in millet varieties. Condensed tannins were highest in sorghum variety PC-1 (179.76 mg/100g) and millet variety S. Bajra-2011 (172.75 mg/100g). Two varieties, one each from sorghum and millet, were selected for extraction of bran as a separate fraction. Brans were modified by size reduction and enzymatic treatment (xylanase and cellulase). Enzymatic treatment favorably affected the extraction of phenolic compounds like anthocyanins. Sorghum and millet flour- and bran-supplemented breads were also developed and evaluated for sensory acceptability.
Introduction
Sorghum and millet might be considered as food security crops as they outperform other cereals under harsh environmental conditions and are economical to produce. These cereal crops have their significance as a staple food in semi-arid tropical regions of Asia and Africa.[Citation1,Citation2] The production of sorghum and millet in Pakistan was recorded as 161,000 and 299,000 tons, respectively.[Citation3] These grains are important sources of dietary calories, protein, minerals, and dietary fiber.[Citation4]
The critical botanical constituents of cereal grains include endosperm, germ, and bran. Bran is a rich source of important nutrients like minerals, vitamins, dietary fiber, non-starch polysaccharides, and phenolic compounds including phenolic acids, flavonoids, anthocyanins, and tannins.[Citation5] The coarse grains, i.e., sorghum and millet, are preferably consumed as whole grains, but bran can be isolated for the formulation of functional foods. The use of whole grains and/or bran in products, particularly the baked goods, is limited because of the technical problems associated with them at levels which could wield health benefits.[Citation6] The bran could be pretreated prior to its use to make it pliable either by physical treatments like size reduction, heat treatments, soaking, etc., or bioprocessing like enzymatic treatments or fermentation.[Citation7] Xylanase; hydrolytic enzymes could cleave arabinoxylans backbone, while cellulases might bring out catalysis of cellulose to glucose. The tough gritty structure of bran which creates problem during product development and in finished products could be modified by treating bran with these enzymes.[Citation8,Citation9]
Like other cereals, starch is the predominant portion of sorghum and millet grains. The protein quotient of millet is comparable to sorghum, wheat, and corn along with good amounts of fat and fiber.[Citation10] Sorghum and millet contain substantially higher levels of phenols and antioxidants. The health-promoting properties, antioxidant activity, and uses as nutraceuticals in foods have been well documented. The sorghum phenolic compounds profile could be affected by genetic factors regulating the color and thickness of its pericarp, secondary plant colors, and presence of pigmented testa. The phenolic profile of sorghum is also altered under the action of biotic or abiotic stresses. The phenolic compounds of importance in sorghum include proanthocyanidins, flavones, and 3-deoxyanthocyanidins. These phenolic compounds are helpful in modulation of gut microbiota and regulation of non-communicable diseases such as cancer, dyslipidemia, cardiovascular diseases, diabetes, and obesity.[Citation1]
An array of free phenolic compounds as well as their conjugates with glycosides is associated with cell wall polysaccharides in cereal grains. Phenolic compounds in millet mainly include free or conjugated hydroxybenzoic or hydroxycinnamic acid derivatives. Additionally, flavonoids including anthocyanidins, flavanols, flavanones, flavones, and aminophenolic compounds are also found in millets.[Citation11]
Anthocyanins contribute to bright red, purple, and blue colors in different plants, plant parts, and fruit. These are water-soluble compounds, and commonly their six classes are found in different foods including cyanidin, delphinidin, malvinidin, pelargonidin, petunidin, and peonidin.[Citation12] Anthocyanins are advantageous because of antioxidant, anti-inflammatory, anti-cancer, anti-allergic, and gastroprotective properties.[Citation13–Citation16] Sorghum possesses unique anthocyanins, i.e., they lack OH in the third position of C6-C3-C6 ring, making them more stable under different processing conditions and changes in pH. Millets contain proanthocyanidins, whereas no anthocyanins have ever been reported in millet varieties.[Citation17]
Condensed tannins, also known as proanthocyanidins, are comprised of polymerized flavanol units. They contribute to astringency in foods and generally considered as anti-nutritional because of their properties of binding or chelating minerals and proteins, making them less bioavailable for absorption. Condensed tannins are beneficial for health because of having antioxidant, anticarcinogenic, gastroprotective, anti-ulcerogenic, and cholesterol-lowering properties and improved urinary tract functioning.[Citation4,Citation18]
Composite flour with good end-use quality could be developed with sorghum and millet flours. Sorghum and millet flours have functional advantages associated with them. Sorghum being bland in flavor and light in color has outstanding processing properties. Millet flour has a strong flavor and darker color. Both these cereals are gluten free and are an excellent choice for diabetics, celiacs, and ethnic groups owing to their slow hydrolysis.[Citation19] In short, beneficial functional properties are associated with sorghum and millet perhaps because of the phenolic compounds, e.g., anthocyanins and tannins that have potential health benefits. Therefore, the study was aimed at exploring the chemical profile, anthocyanins, condensed tannins, and end-use quality of sorghum and millet. Furthermore, the effect of modifications like size reduction and enzymatic treatment was also evaluated on total anthocyanin content (TAC), condensed tannins, and bread-making properties of sorghum and millet bran.
Materials and methods
Procurement of materials
Commercial cultivars of sorghum and millet were procured from Ayub Agricultural Research Institute, Faisalabad, Pakistan. Chemicals and reagents were purchased from Sigma–Aldrich and local market. The grains were milled in Quadrumat senior mill, and four fractions obtained were blended together to make whole grain flour. Bran from sorghum variety JS-263 and millet variety MB-87 was also collected as a separate fraction for modifications and evaluation of chemical composition, TAC, condensed tannins, and development of bread with bran-supplemented flours. The selection of these two varieties for isolation of bran was based on chemical composition, phenolic compounds, and end-use quality.
Modification of bran
The sorghum and millet brans were modified by size reduction and enzymatic treatments. For size reduction, the native bran (as is obtained from the mill) was further ground to allow it to sieve through mesh #10 and 18 sieves with the final sizes 1 mm termed as fine and 2 mm named as coarse bran. For enzymatic modification, xylanase and cellulase enzymes were used at the rate of 1 U/ml in 10% bran suspension in water. The mixture was shaken for 14 h at 30°C at 150 rpm followed by centrifugation at 4000g for 10 min. The pellets were collected, dried in dehydrator, and stored in refrigerator for analyses. Thus, five types of brans from both sorghum and millet were analyzed, i.e., native (as is), fine (1 mm), coarse (2 mm), xylanase treated (E1), and cellulase treated (E2).[Citation20]
Chemical analysis
Sorghum and millet flours were analyzed for chemical composition, i.e., moisture, ash, crude fat, crude fiber, and crude protein content.[Citation21] The protein conversion factor was 6.25.
Isolation of anthocyanins
Anthocyanins in sorghum and millet flour samples were extracted using acidified methanol as solvent. Briefly, 0.5 g sample in 10ml solvent was taken in 50 ml centrifuge tubes and shaken the samples for 2 h at low speed. The samples were stored overnight at −20°C. Samples were brought to room temperature and centrifuged at 7000g for 10 min and decanted. Samples were extracted thrice with the solvent. The three aliquots were mixed and kept in the dark at less than −20°C until analyzed.[Citation22]
Preparation of standards
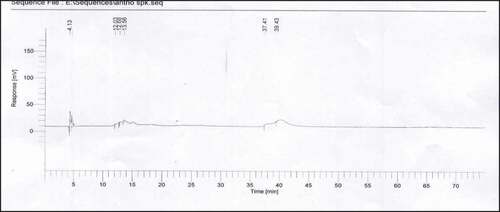
Four standards were used for anthocyanins, namely: apigeninidin chloride, pelargonidin chloride, cyanidin chloride, and kuromanin chloride. Stock solutions (1000 ppm) were prepared by dissolving in mobile phase B. Working standard solutions of 100 ppm were made for each standard.
Characterization and quantification of anthocyanins
Anthocyanins were characterized using HPLC (Perkin Elmer, USA) outfitted with UV-Vis detector, column C18 (5 µm particle size, Restek Pinnacle ODS, 250 × 4.6 mm) thermostatted at 40°C. Injection volume was 20 µl, flow rate was 0.5 ml/min, column temperature was 35°C, and detection was done at 210–600 nm. The mobile phase A was 10% formic acid in water, and mobile phase B was acetonitrile:water:formic acid (5:4:1). The HPLC gradient was: 0–3 min, 88% A; 3–10 min, 88–70% A; 10–15 min, 70% A; 15–20 min, 70–60% A; 20–30 min, 60% A; 30–40 min, 60–0% A; 60–63 min, 0–88% A; and 63–75 min, 88% A.[Citation22]
Extraction of condensed tannins
Condensed tannins in sorghum and millet flour and bran samples were extracted with vanillin-HCl. Samples (0.5 g each) were weighed in 250 ml conical flask, 75 ml distilled water was added, and boiled for 30 min subsequently centrifuged at 318 g for 20 min. Supernatant was collected in 100 ml flask and volume made with vanillin-HCl.[Citation23]
Condensed tannins
The standard used for condensed tannin determination was catechin. The stock solution was made with 40 ppm catechin in 1% acidified (HCl) methanol. Working standard solutions of 0.2, 0.4, 0.6, 0.8, and 1 ml were prepared. Vanillin-HCl reagent up to 5 ml was added into each test tube. Standards and sample solutions were measured spectrophotometrically at 500 nm after 20 min.
Total anthocyanin content
TAC in bran samples was determined following the method of Abdel-Aal and Hucl.[Citation24] Samples were extracted with 24 ml acidified (1 N HCl) methanol (85: 15; v/v) for 30 min. The pH was adjusted to 1 at the start of the reaction and rechecked after 15 and 30 min of reaction. After extraction, centrifugation of samples was done at 21,000g for 20 min (4°C). The samples after centrifugation were refrigerated for 48 h. Extracts after 48 h were again centrifuged at 21,000g for 20 min (4°C) and were concentrated to 2 ml under continuous nitrogen. The supernatants were decanted in 50 ml volumetric flask, and volume was made with acidified methanol. The absorbance was read at 535 nm on spectrophotometer. TAC in bran samples was calculated as µg/g according to the original method.[Citation24]
Preparation of bread
Sorghum and millet flour were added () during dough making, and bread was prepared with straight dough method given in AACC (2000) method No. 10–10. Bran-supplemented breads were prepared by addition of 20% of each type of bran with straight dough method.[Citation21]
Table 1. Treatment plan for product (bread) development.
Sensory evaluation
Sensory evaluation of breads was carried out for external and internal characteristics on a 9-point hedonic scale by a panel of eight judges according to the method described by Meilgaard et al.[Citation25]
Statistical analysis
The statistical model used for interpretation of data was complete randomized design, and mean comparison was obtained with Duncan’s multiple range test and Tukey test.[Citation26]
Results and discussion
Chemical composition
The proximate composition of seven sorghum and two pearl millet genotypes has been presented in . The values for moisture content extended from 5.5% (JS-2002) to 10.0% (F-114) for sorghum cultivars and from 8.1% (Sargodha Bajra-2011) to 9.3% (MB-87) for millet varieties. The ash content varied from 1.61% (JS-263) to 3.3% (Hegari) for sorghum and from 1.5% (MB-87) to 2.0% (S. Bajra-2011) for millet cultivars. Crude fiber ranged between the minimum value of 2.6% for MR-Sorghum genotype and the maximum value of 3.9% for JS-263 genotype and in millet from 2.3% (MB-87) to 3.0% (S. Bajra-2011). The fat content ranged from 2.7% (F-114) to 5.6% (Hegari) and from 3.7% (MB-87) to 3.8% (S. Bajra-2011). Crude protein of these genotypes investigated ranged from 12.6% (F-114) to 17.0% (Sandal Bar) and from 15.7% (MB-87) to 16.1% (S. Bajra-2011). Present studies were found to be in line with previous research outcomes.[Citation27–Citation30]
Table 2. Proximate composition of sorghum and millet varieties.
The moisture content possesses economic significance as well as determinant of the shelf life of milled products, i.e., sorghum and millet flour in this case. The varieties having lower moisture content were brittle, and varieties with higher moisture content were tough. Ash content represents minerals/inorganics present in a food. Higher ash content implies high mineral content. Sorghum varieties showed higher ash content in comparison with millet varieties, indicating higher total mineral content. Fat plays an important role as a source of energy and in controlling shelf life and an important determinant of baking quality of flour. Sorghum varieties demonstrated higher fat content compared to millet varieties. The crude fiber content was also found slightly higher in sorghum varieties compared to millet varieties. Protein content is also a very important determining factor for baking quality. The protein in sorghum and millet flours was substantial, but unlike wheat, these two cereal grains could be considered as gluten-free grains. The variations in chemical composition might be owing to genetic factors, climatic conditions, and soil fertility conditions.[Citation31]
Anthocyanins by HPLC
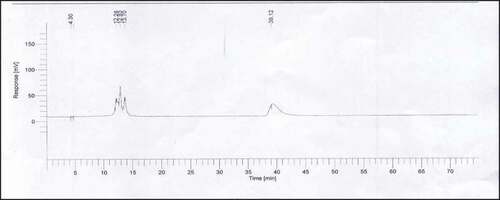
The values for anthocyanin apigeninidin, cyanidin, kuromanin, and pelargonidin of different sorghum and millet varieties have been presented in . The chromatograms of individual standard anthocyanins and mixed standard have been depicted in –, respectively. The chromatograms for representative samples have been given in and . Apigeninidin is most commonly found in sorghum as it is 3-deoxyanthocyanin which are commonly found in higher quantities in sorghum as compared to other common anthocyanins like cyanidin, pelargonidin, etc. Apigeninidin was detected in the highest amount in sorghum variety JS-263 (16.56 µg/g) and in the lowest amount in sorghum variety Hegari (0.031 µg/g). The amounts of apigeninidin in other sorghum varieties were JS-2002 (0.082 µg/g), PC-1 (0.084 µg/g), Sandal Bar (0.46 µg/g), F-114 (0.037 µg/g), and MR- Sorghum (0.04 µg/g).
Table 3. Anthocyanin content in different sorghum and millet varieties.
Cyanidin was detected only in PC-1 (0.015 µg/g). Kuromanin was detected in four varieties of sorghum among which the highest amount was found in Sandal Bar (0.56 µg/g) and the lowest amount was detected in JS-2002 (0.0081 µg/g). Other two varieties which were found to contain kuromanin included PC-1 (0.21 µg/g) and MR-Sorghum (0.015 µg/g). Pelargonidin was found to be present in minute quantities in two sorghum varieties PC-1 (0.28 µg/g) and MR-sorghum (0.0058 µg/g).
The results were identical to findings reported by Dykes and Rooney[Citation17] who proposed that most predominant anthocyanins in sorghum are 3-deoxyanthocyanins. The levels of anthocyanins in Pakistani sorghum varieties were found to be much less than black and red sorghum brans up to10,100 µg/g and 3600 µg/g, respectively, as reported by Awika and Rooney.[Citation13] A very important factor determining the levels and amounts of anthocyanins in sorghum is the production of 3-deoxyanthocyanin as phytoalexin. Sorghum plant produces and accumulates 3-deoxyanthocyanins like apigeninidin in cellular inclusions in response to fungal attack.[Citation32] Variations in levels of anthocyanins in sorghum varieties could also be attributed to varietal differences, color of pericarp, and soil and climatic conditions.[Citation33] No anthocyanins were detected in millet varieties which might be due to the genetic makeup of millet as it has been reported by Zhu [Citation34] that apigeninidin- and luteolinidin-type compounds were detected in finger millet with ethyl acetate extract only. The anthocyanin profile of millets is unclear and needs further studies.
Total anthocyanin content
TAC gives a measure of total anthocyanins present in a food sample. Native and modified sorghum and millet bran were evaluated for TAC to assess the effect of modifications on TAC of the brans. The values for TAC for different sorghum and millet bran have been given in . Among different sorghum brans, the lowest value was indicated by fine sorghum bran (21.42 mg/kg) and the highest by xylanase-treated sorghum bran (133.11 mg/kg). The TAC in sorghum bran was found to be lower than in [Citation35] who reported 0.58 mg/g TAC in sorghum bran. Regarding TAC values in different millet brans, the lowest value for TAC was demonstrated by fine millet bran (21.47 mg/kg) and the highest by cellulase-treated millet bran (63.43 mg/kg). Enzymatic treatment favored the extraction and detection of anthocyanins as the highest values for TAC were observed in enzymatically treated brans. Other determining factors for anthocyanin content might include the choice of extraction solvent, maturity and ripening stage of grain, insect infestation, or mold attack because anthocyanins being phytoalexins are produced in response to mold invasion.[Citation32]
Table 4. Total anthocyanin content (TAC) of sorghum and millet bran.
Condensed tannins
The condensed tannins are predominantly present in sorghum varieties containing B1 and B2 genes, which are the major phenolic compounds in these varieties. Tannins are generally considered as anti-nutritional because they have the ability to bind proteins or chelate minerals, decreasing the bioavailability of these nutrients. The values for condensed tannins of sorghum and millet flour have been presented in . Condensed tannin values in sorghum ranged from 172.10 mg/100g to 179.76 mg/100g. The highest tannin content was observed in PC-1 (179.76 mg/100g) and the lowest was exhibited by F-114 (172.10 mg/100g). Condensed tannin content ranged in millet from 172.75 mg/100g to 174.18 mg/100g. The results for condensed tannins in sorghum and millet flour were identical to findings reported by Dykes and Rooney [Citation17] and Chandrasekara et al.[Citation36], respectively. The variations in tannin content could be accredited to varietal differences, ripening stage, climatic factors, or the interaction between these factors. Pearl millet normally does not contain condensed tannins but gives positive results for vanillin-HCl assay. The data suggest that the condensed tannin content in sorghum and millet is affected by cultivar differences.[Citation37]
Table 5. Condensed tannins (mg/100g) in different sorghum and millet cultivars.
The condensed tannin content in different types of bran samples has been presented in . The condensed tannin content in different types of sorghum brans was recorded lowest for fine sorghum bran (172.38 mg/100g) and highest for coarse sorghum bran (179.77 mg/100 g). The values for condensed tannins in sorghum bran were lower compared to results reported by Ayala-Soto et al.[Citation35] who reported 126–3500 mg/100 g condensed tannins in sorghum bran. The condensed tannin content in millet brans was lower for fine millet bran (171.80 mg/100 g) while higher for coarse millet bran (175.81 mg/100 g). The results indicated that bran particle size reduction aided in the reduction of condensed tannins. Enzymatic treatment of bran was also effective in bringing down tannin content of bran.
Table 6. Condensed tannins in sorghum and millet bran.
Sensory evaluation of bread
Breads were developed with the incorporation of sorghum and millet flour in wheat flour. The varieties Hegari, F-114, and JS-263 were added in wheat flour at the rate of 20–50%, while Sandal Bar, JS-2002, MR-Sorghum, and PC-1 were added at 20–30%. Both millet varieties were supplemented at 10–40%. The breads were evaluated for external characters (crust) and internal characters (crumb). The sensory score for overall acceptability ranged from 6.2 to 8.8 in wheat: sorghum bread and 5.5 to 8.2 in wheat: millet bread out of total 10. Breads made with sorghum variety F-114 and millet variety MB-87 scored better for different sensory parameters. The overall acceptability of bread was influenced by genetic variations, varietal differences, and level of millet and sorghum in wheat flour. The score for texture (total score 15) of bread made with sorghum ranged from 11.0 to 12.8. The score for the texture of bread was significantly higher in F-114 (T1), while the score for the texture of bread made with millet was significantly higher in MB-87 (T1). The lowest score was found in S. Bajra-2011. Sorghum flour being bland could be successfully added even at 50% replacement of wheat flour in bread formulations but producing 100% sorghum flour could be a challenge as observed by Abdelghafor et al. [Citation38] who reported that these breads were unappealing for consumers. The sorghum or pearl millet flour at 70% could be incorporated with the addition of cassava starch and some other improvers like fugal amylase and fat, etc., as reported by Olatunji et al.[Citation39] Millet flour addition although produced acceptable breads at the rate of 40%, but acceptability for millet breads was higher at 10% as because at higher rates millet flour produces grit and slightly bitter taste in the product.
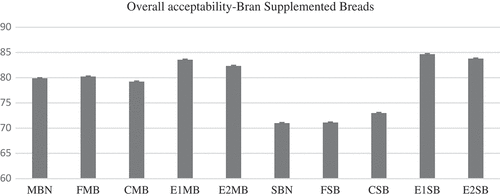
Bran-supplemented breads scored for overall acceptability () ranging from 71 (native sorghum bran) to 84.67 (xylanase-treated sorghum bran) and 79.2 (coarse millet bran) to 83.56 (xylanase-treated millet bran). It was observed that enzymatically treated bran-supplemented breads had higher scores for overall acceptability compared to native bran-supplemented breads, implying that bran modification positively alters the bran morphology, mellowing it and making more pliable and acceptable for consumers.[Citation40]
Figure 8. Overall acceptability of sorghum and millet bran supplemented breads. MBN: native millet bran; FMB: fine millet bran; CMB: coarse millet bran; E1MB: xylanase-treated millet bran; E2MB: cellulase-treated millet bran; SBN: native sorghum bran; FSB: fine sorghum bran; CSB: coarse sorghum bran; E1SB: xylanase-treated sorghum bran; E2SB: cellulase-treated sorghum bran.
Conclusion
The research outcomes of the present study concluded that sorghum flour of JS-263 cultivar was a good source of anthocyanins (16 µg/g) compared to other sorghum cultivars. All other sorghum cultivars contained lower amounts of apigeninidin (3-deoxyanthocyanin). None of the anthocyanins were found in millet cultivars. Condensed tannins were in higher amount in sorghum cultivar PC-1 (179.76 mg/100g). Among millet varieties, condensed tannins were found to be in higher quantity in MB-87 (174.76 mg/100g). The sorghum and millet are superior to other cereals as sources of antioxidants. They could be beneficial for health if blended with wheat flour substituting a portion of wheat preferably 30%. Cereal brans can be isolated as a functional ingredient, and the properties could be modified either physically or through bioprocessing. It was gathered from the results that bran could be altered structurally without any negative implications on its nutrition. Hence, this functional ingredient might be made suitable for value addition and end-use.
Acknowledgments
The research work is a part of the project funded by the Higher Education Commission (HEC) of Pakistan under NRPU. The authors are grateful to HEC for providing sponsorship.
References
- Cardoso, L. M.; Pinheiro, S. S.; De Carvalho, C. W. P.; Queiroz, V. A. V.; De Menezes, C. B.; Moreira, A. B.; De Barros, F. A. R.; Awika, J. M.; Martino, H. S. D.; Pinheiro-Sant’Ana, H. M. Phenolic Compounds Profile in Sorghum Processed by Extrusion Cooking and Dry Heat in a Conventional Oven. Journal of Cereal Science 2015, 65, 220–226. DOI: 10.1016/j.jcs.2015.06.015.
- FAOSTAT. Food and Agricultural Organization of the United Nations. [cited 2017 Nov 03]. Available from: http://faostat.fao.org/ .
- GOP (Government of Pakistan). Economic Survey of Pakistan; Economic Affairs Division, Ministry of Finance: Islamabad, Pakistan, 2016–2017.
- Taylor, J. R. N.; Grain Production and Consumption: Africa. In Encyclopedia of Grain Science; Wrigley, C., Corke, H., Walker, C. E., Eds.; Elsevier: London, 2004; pp 70–78.
- Patel, S.;. Cereal Bran Fortified-Functional Foods for Obesity and Diabetes Management: Triumphs, Hurdles and Possibilities. Journal of Functional Foods 2015, 14, 255–269. DOI: 10.1016/j.jff.2015.02.010.
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the Addition of Bran Fractions on Bread Properties. Journal of Cereal Science 2013, 57, 325–332.
- Brewer, L. R.; Kubola, J.; Siriamornpun, S.; Herald, T. J.; Shi, Y. Wheat Bran Particle Size Influence on Phytochemical Extractability and Antioxidant Properties. Food Chemistry 2014, 152, 483–490. DOI: 10.1016/j.foodchem.2013.11.128.
- Santala, O.; Lehtinen, P.; Nordlund, E.; Suortti, T.; Poutanen, K. Impact of Water Content on the Solubilisation of Arabinoxylan during Xylanase Treatment of Wheat Bran. Journal of Cereal Science 2011, 54, 187–194. DOI: 10.1016/j.jcs.2011.02.013.
- Santala, O. K.; Nordlund, E. A.; Poutanen, K. S. Treatments with Xylanase at High (90%) and Low (40%) Water Content Have Different Impacts on Physicochemical Properties of Wheat Bran. Food Bioprocess Technology 2013, 6, 3102–3112. DOI: 10.1007/s11947-012-0967-6.
- Kaur, D. K.; Jha, A.; Latha, S.; Singh, A. K. Significance of Coarse Cereals in Health and Nutrition: A Review. Journal of Food Science and Technology 2012, 11, 612–619.
- Shahidi, F.; Chandrasekar, A. Millet Grain Phenolics and Their Role in Disease Risk Reduction and Health Promotion: A Review. Journal of Functional Foods 2013, 5, 570–581.
- Dykes, L.; Seitz, L. M.; Rooney, W. L.; Rooney, L. W. Flavonoid Composition of Lemon-Yellow Sorghum Genotypes. Food Chemistry 2011, 128, 173–179. DOI: 10.1016/j.foodchem.2011.03.020.
- Awika, J. M.; Rooney, L. W. Sorghum Phytochemicals and Their Potential Impact on Human Health. Phytochemistry 2004, 65, 1199–1221. DOI: 10.1016/j.phytochem.2004.04.001.
- Gu, L.; Kelm, M. A.; Hammerstone, J. F.; Beecher, G.; Chunnigham, D.; Vannozi, S.; Prior, L. Fractionation of Polymeric Procyanins from Lowbush Blueberry and Quantification of Procyanins in Selected Foods with an Optimized Normal-Phase HPLC-MS Fluorescent Detection Method. Journal of Agriculture and Food Chemistry 2002, 50, 4852. DOI: 10.1021/jf020214v.
- Harborne, J. B.; William, C. A. Advances in Flavonoids Research since 1992. Phytochemistry 2000, 55, 481. DOI: 10.1016/S0031-9422(00)00235-1.
- Yao, L. H.; Jian, Y. M.; Shi, J.; Thomas-Barberan, F. A.; Datta, N.; Singanusong, R.; Chen, S. S. Flavonoids in Food and Their Health Benefits. Plant Foods for Human Nutrition 2004, 59, 113.
- Dykes, L.; Rooney, L. W. Sorghum and Millet Phenols and Antioxidants. Journal of Cereal Science 2006, 44, 236–251. DOI: 10.1016/j.jcs.2006.06.007.
- Waniska, R. D.; Rooney, L. W. Structure and Chemistry of Sorghum Caryopsis. In Sorghum; Origin, History, Technology and Production; Wayne-Smith, C., Fredricksen, R. A., Eds.; John Wiley and Sons: New York, 2000. pp 648–688.
- Prior, R. L.; Gu, L. Occurrence and Biological Significance of Proanthocyanin in American Diet. Phytochemistry 2005, 66, 2264. DOI: 10.1016/j.phytochem.2005.03.025.
- Douge, M.; Nonus, M.; Thomasset, T.; Teissier, P.; Barbeau, J. Y. ESEM Study of the Effects of Hydrolytic Enzymes on Wheat Bran Structure. Microscopy and Analysis 2004, 18, 21–22.
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, 2000.
- Awika, J. M.; Rooney, L. W.; Waniska, R. D. Anthoycanins from Black Sorghum and Their Antioxidant Properties. Food Chemistry 2004a, 90, 293–301. DOI: 10.1016/j.foodchem.2004.03.058.
- Broadhurst, R. B.; Jones, W. T. Analysis of Condensed Tannins Using Acidified Vanillin. Journal of Science of Food and Agriculture 1978, 29, 788–794. DOI: 10.1002/jsfa.2740290908.
- Abdel-Aal, E. S. M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chemistry 1999, 76, 350–354. DOI: 10.1094/CCHEM.1999.76.3.350.
- Meilgaard, M.; Civille, G. V.; Carr, B. T. Overall Difference Tests: Does a Sensory Difference Exist between Samples. Sensory Evaluation Techniques 2007, 4, 63–104.
- Steel, R. G. D.; Torrie, J. H.; Dickey, D. A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed ed.; McGraw Hill Book Co. Inc.: New York, 1997.
- Abdalla, A. A.; El-Tinay, A. H.; Mohamed, B. E.; Abdalla, A. H. Proximate Composition, Starch, Phytate and Mineral Content of 10 Pearl Millet Genotypes. Food Chemistry 1997, 63, 243–246. DOI: 10.1016/S0308-8146(97)00228-8.
- Irén, L.;. Sorghum and Millets, in Cultivated Plants, Primarily as Food Sources. In Encyclopedia of Life Support Systems (EOLSS); György, F., Ed.; Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, U.K., 2004.
- Saha, S.; Gupta, A.; Singh, S. R. K.; Bharti, N.; Singh, K. P.; Mahajan, V.; Gupta, H. S. Compositional and Varietal Influence of Finger Millet Flour on Rheological Properties of Dough and Quality of Biscuit. LWT - Food Science and Technology 2011, 44, 616–621. DOI: 10.1016/j.lwt.2010.08.009.
- Liu, L.; Herald, T. J.; Wang, D.; Wilson, J. D.; Bean, S. R.; Aramouni, F. M. Characterization of Sorghum Grain and Evaluation of Sorghum Flour in a Chinese Egg Noodle System. Journal of Cereal Science 2012, 55, 31–36. DOI: 10.1016/j.jcs.2011.09.007.
- Kent, N. L.; Evers, A. D. Technology of Cereals, 4th ed.; Programon Press: Oxford, 1994.
- Escribano-Bailon, M. T.; Santos-Buelga, C.; Rivas-Gonzalo, J. C. Anthocyanins in Cereals. Journal of Chromatography A 2004, 1054, 129–141.
- Louise, S.; Sekwati-Monang, B.; Lutz, D. L.; Schieber, A.; Micheal, G. Phenolic Acids and Flavonoids in Non-Fermented and Fermented Red Sorghum. Journal of Agricultural and Food Chemistry 2010, 58, 9214–9220. DOI: 10.1021/jf101504v.
- Zhu, F.;. Anthocyanins in Cereals: Composition and Health Effects. Food Research International 2018, 109. DOI: 10.1016/j.foodres.2018.04.015
- Ayala-Soto, F. E.; Serna-Saldívar, S. O.; Welti-Chanes, J.; Gutierrez-Uribe, J. A. Phenolic Compounds, Antioxidant Capacity and Gelling Properties of Glucoarabinoxylans from Three Types of Sorghum Brans. Journal of Cereal Science 2015, 65, 277–284.
- Chandrasekara, A.; Bioactivities, S. F. Antiradical Properties of Millet Grains and Hulls. Journal of Agricultural and Food Chemistry 2011, 59, 9563–9571. DOI: 10.1021/jf201849d.
- Taylor, J. R. N.; Schober, T. J.; Bean, S. R. Novel Food and Non-Food Uses for Sorghum and Millet. Journal of Cereal Science. 2006, 252–271. doi:10.1016/j.jcs.2006.06.009.
- Abdelghafor, R. F.; Mustafa, A. I.; Ibrahim, A. M. H.; Krishnan, P. G. Quality of Bread from Composite Flour of Sorghum and Hard White Winter Wheat. Advance Journal of Food Science and Technology 2011, 3, 9–15.
- Olatunji, O.; Koleoso, O. A.; Oniwinde, A. B. Recent Experience on the Milling of Sorghum, Millet, and Maize for Making Nonwheat Bread, Cake, and Sausage in Nigeria. In Utilization of Sorghum and Millets; Gomez, M. I., House, L. R., Rooney, L. W., Dendy, D. A. V., Eds..; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 1992a. pp 83–88.
- Coda, R.; Katina, K.; Rizzello, C. G. Bran Bioprocessing for Enhanced Functional Properties. Current Opinion in Food Science 2015, 1, 50–55. DOI: 10.1016/j.cofs.2014.11.007.