ABSTRACT
This study aimed to investigate the effect of limited hydrolysis on conformational and antioxidant properties of soy protein isolate-maltodextrin (SPI-Md) conjugates. Extrinsic fluorescence analysis showed unfolding of the protein molecule and exposure of hydrophobic groups in SPI-Md conjugate hydrolysates. Free amino acid analysis showed that, the contents of hydrophobic amino acids in SPI-Md conjugates increased after hydrolysis. The contents of leucine, isoleucine, phenylalanine increased from 0.32, 0.30 and 0.54 to 1.36, 1.86 and 2.60, respectively, when the hydrolysis degree (DH) gradually increased from 0% to 5.7%. The FT-IR spectrum showed that C = O absorption of the amide group formed by glycosylation continued unabated after limited hydrolysis (DH 2.9%). The glycated SPI products showed good reducing power and superior resistance to lipid oxidation (34%, 12 mg mL−1), whereas the limit hydrolysates (DH 2.9%) of SPI-Md conjugates showed more efficient radical-scavenging capacity (89.5%, at 12 mg mL−1) and iron-chelation activity (91.3%, at 12 mg mL−1). Results of this study indicated that, slight enzymatic hydrolysis (DH 0–2.9%) could help partially unfolding the globular structure of SPI-Md conjugates without deteriorating amide bonds and had a positive effect on their antioxidant properties.
Introduction
Vegetable proteins, especially soy protein, are relatively cheap, rich in nutrients, and have good health functions, making them realistic alternatives to animal proteins[Citation1] (casein and whey protein) and synthetic polymers in some applications (as wall material for active component microencapsulation).[Citation2] However, the compact globular structure of native soy protein vastly restricts its molecular flexibility and emulsifying capability compared with other animal proteins, such as casein and whey protein.[Citation3] Highly sensitivity to food processing conditions, such as heating, pH, and ionic strength, also limits the application of native soy protein.[Citation4] This put forward imperative requirements for improvement of soy protein functional properties to expand its application in food processing or developing novel foods with desirable functionalities.
Much efforts have been made for developing new food ingredients with improved functional properties, utilising various physical, chemical, and enzymatic methods.[Citation5,Citation6] Protein–saccharide graft reactions are based on Maillard reactions between protein amino groups and saccharide reducing-end carbonyl groups of saccharides.[Citation7] It was proposed as an effective way for improving the functional properties of proteins without using chemicals. The Maillard reaction products (MRPs) were reported to have good antioxidant activities [Citation8] and emulsifying properties due to their amphiphilic character.[Citation9] Recently, the application of modified proteins in formulated food products increased because of their improved functional activities.[Citation10] Many studies aimed to investigate the constituent of MRPs and how they work, however, the antioxidant mechanism and active ingredients remain poorly understood.[Citation7]
Controlled enzymatic hydrolysis was well accepted as a convenient and efficient method for improving the functional properties of proteins. Protein hydrolysates derived from different sources, such as milk, soy and rice exhibited good antioxidant activities and was considered to be a good source of antioxidant peptides for food and nutraceutical applications.[Citation11,Citation12] It was inferred that the antioxidant activity of protein hydrolysates might be associated with their reduced molecular weight, amino acid composition, and hydrophobicity.[Citation13] Some aromatic residues exposed after hydrolysis could act as antioxidants by donating proton to electron deficient radicals, such as tryptophan, tyrosine, and phenylalanine.[Citation14]
Therefore, controlled enzymatic hydrolysis was used to modify the Maillard Reaction products of soybean protein (SPI-Md conjugates), and expected to further improve their functionality.[Citation6] Based on our previous research results, the oxidative stabilities (peroxide value and headspace propanal) of microcapsule particles prepared by combined modified soy protein isolate (SPI) products were apparently improved [Citation15], which obtained through combined modification of limited hydrolysis and Maillard Reaction. It was proved to be an effective method for preparing emulsifiers with favourable antioxidant activities through the combined modification of controlled enzymatic hydrolysis and protein-polysaccharide conjugation.[Citation6] However, no information is available regarding the mechanism by which these combined modified SPI products execute their antioxidative effects. Besides, we are not very clear about the effect of enzymatic hydrolysis on the complex structure of SPI-Md conjugates formed by Maillard Reaction. Therefore, a further study is needed to help in understanding the mechanism of the antioxidant capacity and the structural changes of the combined modified SPI products in order to design better functional ingredients in the future.
Herein, the effects of limited enzymatic hydrolysis with varying DH on the antioxidative capacities and structural features of SPI-Md conjugates were assessed. The structure of hydrolyzed SPI-Md conjugates was characterized by measuring extrinsic emission fluorescence spectra, infrared spectra as well as free amino acid analysis. Based on these findings, the relationship between structure modification and antioxidative capacity was discussed, and the validity of approach used to enhance the functional properties of SPI-Md conjugates was confirmed.
Materials and methods
Materials
The defatted soybean meal was purchased from Anyang Mantianxue Food Manufacturing Company (Henan, China). The maltodextrin (Md) (DE 8–10) was obtained from Baolingbao Biology Company (Shandong, China). Lecithin was obtained from East China Normal University Chemical Reagent Co., Ltd (Shanghai, China). The enzyme Neutrase (0.8 AU/g), an endo protease was produced by Bacillus subtilis, from Novozymes (Jiangsu, China). Other chemicals and solvents used were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of soy protein isolate and SPI-Md conjugate and hydrolysates
SPI was prepared using alkali-solution and acid-isolation extraction method described by Zhang et al.[Citation6]
The preparation of SPI-Md conjugate and its hydrolysates was according to the method of Zhang et al.[Citation16] SPI was dissolved into distilled water (4%, w/v, protein) and adequately mixed with maltodextrin at the ratio of 2/1 (SPI/maltodextrin, w/w). After that, the mixed solution was kept at 80°C (pH 7.0) for 80 min, and the degree of glycosylation (DG) was 33%, determined by the modified o-phthaldialdehyde (OPA) method.[Citation17]
The SPI-Md conjugate hydrolysates were prepared in according to the pH-stat method.[Citation18] The glycosylated solution was adjusted to 54°C, pH 7.0, and hydrolyzed by Neutrase (E/S = 0.5%, 2%, 4%, 5%, 6%) for 25 min. The degree of hydrolysis (DH) of the hydrolysates ultimately was 1.8%, 2.9%, 4.6%, 5.7%, 6.2%, respectively. After this reaction, the enzyme was inactivated by heating at 80°C for 10 min. As control, native SPI was also incubated at the same heating conditions (90 min at 80°C, pH 7.0).
Extrinsic emission fluorescence spectra
Extrinsic emission fluorescence spectra of the protein samples were determined according to the method of Zhuo et al. [Citation19] with small modifications. And the measurements were performed using a Hitachi F-7000 fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan). In this method, ANS was used as a fluorescence probe. The ANS dispersions (8 mmol L−1) and protein solutions (1 mg mL−1) were prepared in 50 mmol L−1 phosphate buffer (pH 7.0), and then 20 μL of ANS dispersions and 4 mL of protein solutions was mixed together by shaking on a vortex mixer for about 5 s. Fluorescence intensity (FI) was measured at wavelengths of 390 nm (excitation) at 20 ± 0.5 ◦C and the ANS fluorescence emission was registered between 300 and 500 nm, with a constant slit width of 5 nm. All fluorescence experiments were performed in triplicate.
Measurement of infrared spectra
The protein samples were prepared using potassium bromide (KBr) pellet method.[Citation20] Infrared spectra were measured with a Fourier transform infrared spectrophotometer (Nicolet iS10) (Thermo, USA) at 20°C. Full-wave scanning was collected with 32 scans in the 4000–400 cm−1 region. The measurement of each sample was carried out in triplicate.
Free amino acid analysis
Free amino acid content of SPI and its modified samples was determined according to the method reported by Eric et al.[Citation21] An equivalent volume of trichloroacetic acid (TCA) was added to the sample to precipitate peptides and/or proteins. The supernatant was submitted to high-performance liquid chromatography (HPLC) analysis in Agilent 1100 (Agilent Technology, Palo Alto, CA, USA) assembly system using an ODS Hypersil C18 column (4.6 × 250 mm), running at 1.0 mL min−1. The results acquired were analyzed with the aid of Chem Station for LC 3D software (Agilent Technology, Palo Alto, CA, USA). Results were expressed as percentage (%) of free amino acid of the measurement.
Determination of antioxidant capacity
We applied four models (DPPH radical scavenging activity, Fe2+ Chelating activity, Inhibiting activity of lipid oxidation and Reducing power) for evaluation of antioxidant capacity.
DPPH radical scavenging activity was determined according to the method of Gu et al.[Citation22]
Fe2+ Chelating activity of SPI-Md conjugates was determined by the method of Gu et al.[Citation23]
The reducing power of SPI and its modified samples were determined according to the method of Gu et al.[Citation23] Inhibition of lipid peroxidation was determined according to the method described by Huang et al.[Citation24]
Statistical analysis
The experiments were carried out in triplicate, and values were reported as mean ± standard error (SD). All data were analyzed using analysis of variance (ANOVA), and the differences were evaluated by Duncan’s multiple range test. Statistical analysis was performed assuming a significance level of p < 0.05. The correlation between the variables was performed by Pearson Correlation Coefficient.
Results and discussion
Extrinsic fluorescence emission spectroscopy
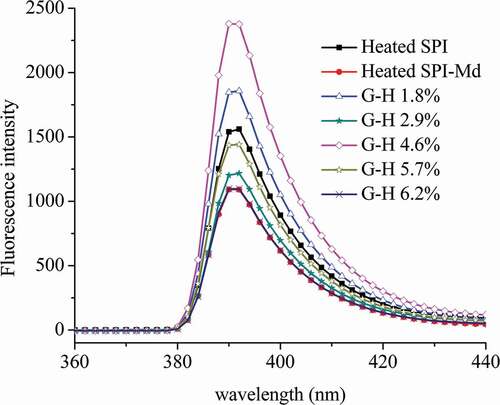
Fluorescence spectroscopy serves as a powerful sensing tool for the investigation of local environment of the fluorophore yielding structural information.[Citation25] Extrinsic fluorescence probe such as excited state charge, proton and electron transfer molecules can be used to study conformational changes of protein structure with the variation of its solvent characteristics.[Citation26] The external fluorescence spectra of SPI, SPI-Md conjugate, and SPI-Md conjugate hydrolysates are shown in . The spectra showed an obvious decrease in fluorescence intensity after glycosylation of native SPI and Md, which demonstrated the change of the microenvironment around the fluorophore in protein solution. This phenomenon was probably due to the shielding effect of the polysaccharide chain, resulting in lower extent of interaction with quenching agents either in a solvent or in the protein itself.[Citation27] However, it was noticed that fluorescence intensity of all hydrolyzed samples of SPI-Md conjugates increased again after enzymatic hydrolysis at varying DH, except the hydrolyzed sample of DH 6.2%. Besides, there was a blue shift (392–390 nm) after the enzymatic hydrolysis. This might be due to the exposure of hydrophobic groups in the inner part of SPI after limited proteolysis, leading to the enhanced hydrophobicity of the microenvironment of ANS probe.[Citation28]
FT-IR spectroscopy
FT-IR spectroscopy is a particularly useful technique for the study on protein–carbohydrate structure, because there are several readily identifiable regions of the mid-infrared spectrum where the chemical fingerprints of carbohydrates and proteins do not overlap significantly.[Citation29,Citation30] As for the formation of the covalent bonds between food protein and reducing saccharide, the most distinctive spectral feature is the increase of the number of hydroxy. As shown in , peaks located at 3700~3200 cm−1 became much wider and stronger, and the IR absorption peaks shifted to lower wave numbers due to the H-bonds which indicated the increase of the hydroxyl number. In the region of 1260~1000 cm−1, a new peak appeared, representing the C-C, C-O, and C-H stretching vibrations of carbohydrates.[Citation27] Furthermore, the absorption peak located at 1076 cm−1 was enhanced, which might be attributed to the introduction of butylated hydroxy groups and the bending vibration of -OH.
Figure 2. FT-IR spectrum of SPI, Md, SPI/Md mixture, SPI-Md conjugates, and hydrolysates of SPI-Md conjugates (DH 2.9%).
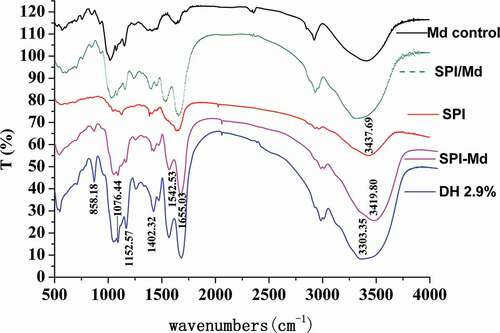
After enzymatic hydrolysis, the absorption peaks located at 3303 cm−1 became wider and shifted to a lower wave number, while the –OH stretching vibration showed no decrease in intensity. The absorption peaks found at 1655 cm−1 was related to the C = O stretching vibration in amide groups, indicating the presence of peptide bonds. The absorption located at 1152~1076 cm−1 was assigned to be the C-O-C glucosidic bond. As shown in , both the O-H and C = O absorptions were enhanced after the glycosylation, and they were not reduced by further enzymatic hydrolysis. Considering these phenomena, it could be inferred that amide bonds, which were generated by glycosylation of the amino acid residues in native SPI and carbonyl groups in Md, had not been destroyed by enzymatic hydrolysis.
Amino acid analysis
During combined modification, soy protein may undergo two chemical changes: first, proteins cross-link with the maltodextrin to form high molecular weight SPI-Md conjugates; then, the conjugates were hydrolyzed into smaller fractions and amino acids. As shown in , the amounts of the free amino acids showed a significant (p < 0.05) difference between the hydrolysates, SPI-Md conjugates, and SPI samples, indicating that the combined modification had some influence on free amino acids. The content of many amino acids represented by lysine and histidine decreased after the glycosylation between native SPI and maltodextrin, which might result from their participation in conjugate formation with the polysaccharides.[Citation23]
Table 1. Changes in free amino acid content (mg/100 ml) of SPI, SPI-Md, and hydrolysates of SPI-Md conjugates.
Afterwards, there was a further change in free amino acids. The content of the hydrophobic amino acids such as leucine, isoleucine, phenylalanine increased from 0.32, 0.30 and 0.54 to 1.36, 1.86 and 2.60, respectively, as the hydrolysis progresses, which might be because the peptide chain located within the globular molecule was destroyed and consequently released some of the hydrophobic amino acids.[Citation13] Besides, this change was of great importance in improving the antioxidant capacities of the compound modified products. Research has shown that proteins and polypeptides containing hydrophobic amino acids or aromatic amino acids (Leu, Ile, Val, Phe) had stronger scavenging and chelating abilities.[Citation31] Most hydrophobic amino acids were found in the middle of the peptide chain and were deeply embedded in the molecular structure of protein. From the above, it was suggested that the enzyme sites of Neutrase were located in the protein molecular structure [Citation32], which was conducive to unfolding of the protein tertiary structure during enzymatic hydrolysis.
DPPH radical-scavenging activity
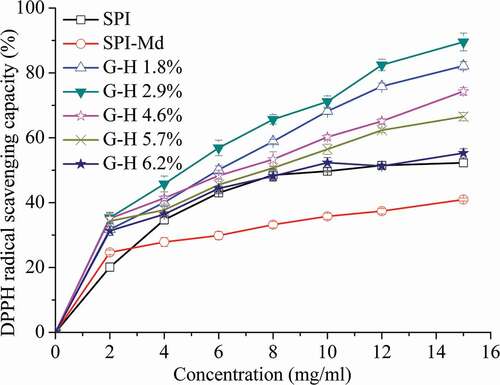
DPPH free radicals show maximal absorbance at 517 nm in ethanol. When DPPH encounters with a proton-donating substance (H+), the colour changes from purple to yellow, and the absorbance decreases, indicating the scavenging of DPPH radical.[Citation33] As shown in , all samples showed increased antioxidant activity with the increase of concentration. The DPPH radical scavenging activity of SPI-Md conjugates enhanced after hydrolysis (P < 0.05) . According to the correlation analysis, there existed a weak yet positive relation between the DPPH radical scavenging activity and DH (). This phenomenon was due to an increase in hydrophobic amino acids identified by free amino acid analysis, as discussed above. The most powerful DPPH radical scavenging activity was observed (89.5%, at the concentration of 12 mg mL−1), when the hydrolysis degree reached 2.9%. It was reported that polypeptides containing hydrophobic amino acids or aromatic amino acids (Leu, Ile, Val, Phe) has stronger scavenging and chelating abilities.[Citation31] Increased hydrophobicity of proteins could help to chelate or store metal ions which are important prooxidants in food lipids. The DPPH scavenging process occurred via the donation of electron or hydrogen from the protein hydrolysates, which made the DPPH radical form a stable DPPH-H molecule, and terminated the oxidation.[Citation33]
Table 2. Correlation coefficient of DH and antioxidant activity.
Fe2+ chelating ability
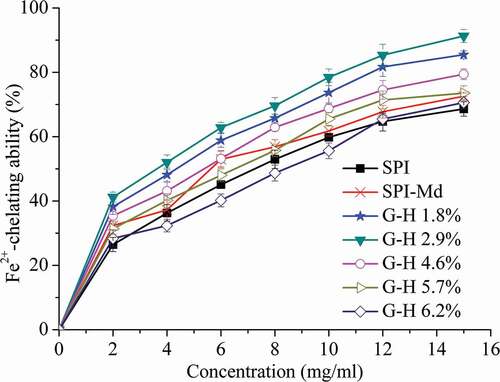
Transition metals are found in all foods since they are common constituents of raw food materials, water, ingredients, and packaging materials.[Citation34] Transition metals such as Fe2+ and Cu2+ are well-known stimuli of lipid peroxidation and their chelation helps to retard the peroxidation and thus prevent food rancidity.[Citation35] We therefore assessed the chelating activity of native SPI and modified SPI samples against Fe2+, as shown in . For all samples, the chelating activity was enhanced as the concentration increased. After the glycosylation of SPI and Md, an increasing trend was observed under the same concentrations. It was widely acknowledged that MRPs, especially melanoidins, could inhibit the occurrence of lipid peroxidation through chelating iron ion in the system.[Citation36] A significant enhancement (p < 0.05) of the chelating activity was observed after the initial enzymatic hydrolysis of SPI-Md conjugates. However, the chelating activity decreased significantly (P < 0.05), when the DH exceeded 2.9%, which might be related to the change in molecular weight. And moderate negative correlation was significantly shown between the Fe2+ chelating ability and DH (). Research has shown that hydrolysates of high molecular weight possess much stronger Fe2+-chelating abilities.[Citation35] Limited hydrolysis (DH, 2.1–5.4%) was proved to be sufficient to improve the amphiphilic properties of peanut protein isolate, via the cleavage of peptide bonds and unfolding of the compacted globular structure.[Citation37] Therefore, in addition to the enhancement of interfacial properties, this combined modification method was also effective for improving the antioxidant activities of the SPI-Md conjugates.
Reducing power
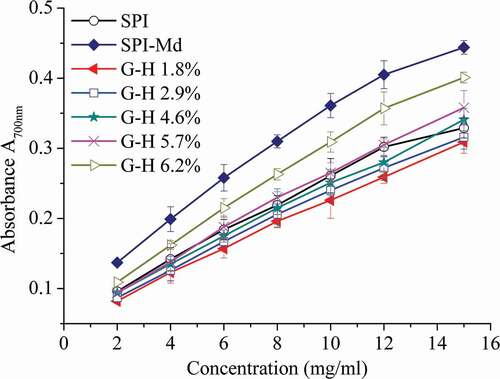
The reducing power assay, the presence of reductants in the tested samples could inhibit or reduce the conversion of Fe3+/ferricyanide to the ferrous form (Fe2+). The Fe2+ can thus be monitored by measuring the formation of Prussian blue at 700 nm.[Citation23] The reducing power of SPI-Md conjugate hydrolysates exhibited a mild increase with the increase of DH value (P < 0.05), compared to SPI, though lower than that of no-hydrolyzed SPI-Md conjugates (). From the result of correlation analysis, the reducing power had low negative correlation with DH (). It was confirmed that the increased availability of hydrogen ions (protons and electrons) derived from the cleavage of protein chains could cause enhancement of the reducing power.[Citation38]
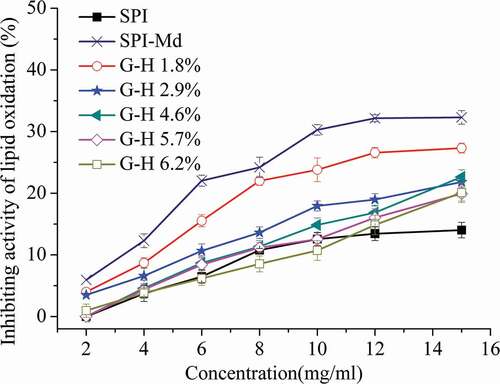
Inhibiting activity of lipid oxidation
For the SPI-Md conjugate, a remarkable increase of the inhibiting activity was observed after conjugation with maltodextrin (). Transition metals such as Fe2+ and Cu2+ are well-known stimuli of lipid peroxidation, and their chelation helps to retard the peroxidation. Potentially, the protein conjugates could prevent oxidation in the lecithin system via greater iron-chelating potential.[Citation22,Citation33] However, the inhibiting activity of SPI-Md conjugate hydrolysates decreased significantly (P < 0.05) as the DH increased (). Correlation analysis showed that the relationships were highly significant (P < 0.05) negative between DH and the inhibiting activity (). Previous studies had demonstrated that higher molecular weight (MW) hydrolysates had higher chelating activities against Fe2+ than those lower-MW hydrolysates.[Citation35]
Figure 6. Lipid oxidation resistance of SPI, SPI-Md, and hydrolysates of SPI-Md conjugates at pH = 7.0.
In assessing the link between two different antioxidant indexes, we found there was indeed a connection under our experimental conditions. As shown in , we found the reducing power had a significant (P < 0.05) negative correlation with the DPPH scavenging activities, a moderate negative correlation with Fe2+ chelating ability. These results were also in agreement with the previous reports of Gu et al. (2009) [Citation23], who described that a report of higher reducing power did not necessarily suggest a stronger DPPH scavenging ability. It was considered that the reducing power of protein hydrolysates might be associated with the reduced molecular, the number of hydroxyl groups and the amino acid composition.[Citation13] Besides, the inhibiting activity of lipid oxidation had a moderate positive correlation with reducing power, DPPH radical-scavenging activity, and Fe2+ chelating ability, respectively. That could be because the inhibiting activity of lipid oxidation was the result of multiple antioxidation mechanism. It was reported that the antioxidant ability of protein hydrolysates or peptides was due to the hydrophobic amino acids of the residues, which might increase the presence of the peptides at the water–lipid interface and thereby facilitate access to scavenge hydrophobic radical species or free radicals generated in the lipid phase.[Citation13] However, it is difficult to make a comparison between different studies due to the lack of antioxidant standard and considerable influence by radical concentration and experimental conditions. More investigation needs to be conducted to discuss the antioxidant effect in the complex system and their synergistic effects.
Conclusion
In this study, neutrase-assisted controlled hydrolysis of SPI-Md conjugates led to significant beneficial changes to their characteristics and antioxidant activities. Fluorescence emission spectroscopy analysis indicated that limited proteolysis partially released hydrophobic groups from the inner part of the SPI and help the hydrophobic amino acids such as leucine, isoleucine, phenylalanine expose, as confirmed by the analysis of amino acids composition. FTIR spectroscopy demonstrated that the amide bonds generated by the glycosylation between amino acid residues in the native SPI and carbonyl group in Md were not destroyed after enzymatic hydrolysis. Antioxidant activity assay showed that limit hydrolysates of SPI-Md conjugates exhibited excellent iron-chelation and radical-scavenging activities, whereas glycosylated SPI showed better reducing power and resistance to lipid oxidation. Although more investigation needs to be conducted, this study may provide a reference for further modification of proteins to improve functional properties.
Acknowledgments
This research was financially supported by the Program of “Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province”, Tianjin municipal education commission scientific research project (Project No. 2017KJ169) and the National 863 Program of China (2013AA102204).
Additional information
Funding
References
- Shao, Y.Y.; Lin, K.H.; Kao, Y.J. Modification of Foaming Properties of Commercial Soy Protein Isolates and Concentrates by Heat Treatments[J]. JOURNAL of FOOD QUALITY 2016, 39, 1745–4557. DOI: 10.1111/jfq.12241.
- Nesterenko, A.; Alric, I.; Silvestre, F.; et al. Influence of Soy Protein’s Structural Modifications on Their Microencapsulation Properties: α-Tocopherol Microparticle preparation[J]. Food Research International 2012, 48(2), 387–396.
- Li, W.; Zhao, H.; He, Z.; et al. Modification of Soy Protein Hydrolysates by Maillard Reaction: Effects of Carbohydrate Chain Length on Structural and Interfacial properties[J]. Colloids and Surfaces B: Biointerfaces 2016, 138, 70–77. DOI: 10.1016/j.colsurfb.2015.11.038.
- Jiang, J.; Xiong, Y.L.; Chen, J. pH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different pH, Salt Concentration, and Temperature Conditions[J]. Journal of Agricultural and Food Chemistry 2010, 58(13), 8035–8042. DOI: 10.1021/jf101045b.
- Sponton, O.E.; Perez, A.A.; Carrara, C.; et al. Effect of Limited Enzymatic Hydrolysis on Linoleic Acid Binding Properties of β-lactoglobulin[J]. Food Chemistry 2014, 146, 577–582. DOI: 10.1016/j.foodchem.2013.09.089.
- Zhang, Y.; Tan, C.; Zhang, X.; et al. Effects of Maltodextrin Glycosylation following Limited Enzymatic Hydrolysis on the Functional and Conformational Properties of Soybean Protein isolate[J]. European Food Research and Technology 2014, 238(6), 957–968.
- Wang, W.Q.; Bao, Y.H.; Chen, Y. Characteristics and Antioxidant Activity of Water-Soluble Mail Lard Reaction Products from Interactions in a Whey Protein Isolate and Sugars system[J]. Food Chemistry 2013, 139(1–4), 355–361. DOI: 10.1016/j.foodchem.2013.01.072.
- Jiang, Z.M.; Brodkorb, A. Structure and Antioxidant Activity of Mail Lard Reaction Products from Alpha-Lactalbumin and Beta-Lactoglobulin with Ribose in an Aqueous Model system[J]. Food Chemistry 2012, 133(3), 960–968. DOI: 10.1016/j.foodchem.2012.02.016.
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying Properties of Soy Whey Protein Isolate-Fenugreek Gum Conjugates in Oil-In-Water Emulsion Model system[J]. Food Hydrocolloids 2013, 30(2), 691–697. DOI: 10.1016/j.foodhyd.2012.09.002.
- Zilic, S.; Akillioglu, G.; Serpen, A.; et al. Effects of Isolation, Enzymatic Hydrolysis, Heating, Hydratation and Maillard Reaction on the Antioxidant Capacity of Cereal and Legume proteins[J]. Food Research International 2012, 49(1), 1–6.
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; et al. Isolation and Identification of Antioxidant Peptides from Enzymatically Hydrolyzed Rice Bran protein[J]. Food Chemistry 2016, 192, 156–162. DOI: 10.1016/j.foodchem.2015.06.057.
- Uraipong, C.; Zhao, J. Identification and Functional Characterisation of Bioactive Peptides in Rice Bran Albumin hydrolysates[J]. International Journal of Food Science & Technology 2016, 51(10), 2201–2208. DOI: 10.1111/ijfs.13204.
- Zhang, H.J.; Wang, J.; Zhang, B.H.; et al. Antioxidant Activities of the Fractionated Protein Hydrolysates from Heat Stable Defatted Rice bran[J]. INTERNATIONAL JOURNAL of FOOD SCIENCE and TECHNOLOGY 2014, 49(5), 1330–1336.
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; et al. Effects of Isolation, Enzymatic Hydrolysis, Heating, Hydratation and Maillard Reaction on the Antioxidant Capacity of Cereal and Legume proteins[J]. Food Research International 2012, 49(1), 1–6.
- Zhang, Y.; Tan, C.; Abbas, S.; et al. Modified SPI Improves the Emulsion Properties and Oxidative Stability of Fish Oil microcapsules[J]. Food Hydrocolloids 2015, 51, 108–117. DOI: 10.1016/j.foodhyd.2015.05.001.
- Zhang, Y.; Tan, C.; Abbas, S.; et al. The Effect of Soy Protein Structural Modification on Emulsion Properties and Oxidative Stability of Fish Oil microcapsules[J]. Colloids and Surfaces B: Biointerfaces 2014, 120, 63–70. DOI: 10.1016/j.colsurfb.2014.05.006.
- Zhang, Y.; Tan, C.; Eric, K.; et al. Effect of Limited Enzymatic Hydrolysis on Physico-Chemical Properties of Soybean Protein Isolate-Maltodextrin conjugates[J]. International Journal of Food Science & Technology 2015, 50(1), 226–232.
- Adler-Nissen, J.;. Enzymic Hydrolysis of Food proteins[M]. Elsevier Applied Science Publishers.1986, 172(8), 1783-1785.
- Zhuo, X.-Y.; Qi, J.-R.; S-W, Y.; et al. Formation of Soy Protein Isolate-Dextran Conjugates by Moderate Maillard Reaction in Macromolecular Crowding conditions[J]. JOURNAL of the SCIENCE of FOOD and AGRICULTURE 2013, 93(2), 316–323.
- French, D.L.; Arakawa, T.; Li, T.S. Fourier Transformed Infrared Spectroscopic Investigation of Protein Conformation in Spray-Dried Protein/Trehalose powders[J]. Biopolymers 2004, 73(4), 524–531. DOI: 10.1002/bip.10558.
- Eric, K.; Raymond, L.V.; Huang, M.; et al. Sensory Attributes and Antioxidant Capacity of Maillard Reaction Products Derived from Xylose, Cysteine and Sunflower Protein Hydrolysate Model system[J]. Food Research International 2013, 54(2), 1437–1447.
- Gu, F.-L.; Kim, J.M.; Abbas, S.; et al. Structure and Antioxidant Activity of High Molecular Weight Maillard Reaction Products from casein–Glucose[J]. Food Chemistry 2010, 120(2), 505–511.
- Gu, F.; Kim, J.M.; Hayat, K.; et al. Characteristics and Antioxidant Activity of Ultrafiltrated Maillard Reaction Products from a Casein–Glucose Model system[J]. Food Chemistry 2009, 117(1), 48–54.
- Huang, M.G.; Liu, P.; Song, S.Q.; et al. Contribution of Sulfur-Containing Compounds to the Colour-Inhibiting Effect and Improved Antioxidant Activity of Maillard Reaction Products of Soybean Protein hydrolysates[J]. JOURNAL of the SCIENCE of FOOD and AGRICULTURE 2011, 91(4), 710–720.
- Samanta, A.; Paul, B.K.; Guchhait, N. Spectroscopic Probe Analysis for Exploring Probe-Protein Interaction: A Mapping of Native, Unfolding and Refolding of Protein Bovine Serum Albumin by Extrinsic Fluorescence Probe[J]. Biophysical Chemistry 2011, 156(2–3), 128–139. DOI: 10.1016/j.bpc.2011.03.008.
- Paul, B.K.; Samanta, A.; Guchhait, N. Exploring Hydrophobic Subdomain IIA of the Protein Bovine Serum Albumin in the Native, Intermediate, Unfolded, and Refolded States by a Small Fluorescence Molecular Reporter[J]. Journal of Physical Chemistry B 2010, 114(18), 6183–6196. DOI: 10.1021/jp100004t.
- Liu JH, Ru QM, Ding YT. Glycation a Promising Method for Food Protein Modification: Physicochemical Properties and Structure, a review[J]. Food Research International. 2012, 49(1), 170–183. doi:10.1016/j.foodres.2012.07.034.
- Chen, L.; Chen, J.S.; Ren, J.Y.; et al. Modifications of Soy Protein Isolates Using Combined Extrusion Pre-Treatment and Controlled Enzymatic Hydrolysis for Improved Emulsifying properties[J]. Food Hydrocolloids 2011, 25(5), 887–897.
- Farhat, I.A.; Orset, S.; Moreau, P.; et al. FTIR Study of Hydration Phenomena in Protein-Sugar systems[J]. JOURNAL of COLLOID and INTERFACE SCIENCE 1998, 207(2), 200–208.
- Turner, J.A.; Sivasundaram, L.R.; Ottenhof, M.A.; et al. Monitoring Chemical and Physical Changes during Thermal Flavor generation[J]. Journal of Agricultural and Food Chemistry 2002, 50(19), 5406–5411.
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum Sativum L.) Enzymatic Protein Hydrolysate Fractions[J]. Journal of Agricultural and Food Chemistry 2010, 58(8), 4712–4718. DOI: 10.1021/jf904456r.
- Thamnarathip, P.; Jangchud, K.; Jangchud, A.; et al. Extraction and Characterisation of Riceberry Bran Protein Hydrolysate Using Enzymatic hydrolysis[J]. International Journal of Food Science & Technology 2016, 51(1), 194–202.
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; et al. Antioxidative Activity of Caramelisation Products and Their Preventive Effect on Lipid Oxidation in Fish mince[J]. Food Chemistry 2005, 90(1–2), 231–239.
- Hu, M.; McClements, D.J.; Decker, E.A. Impact of Chelators on the Oxidative Stability of Whey Protein Isolate-Stabilized Oil-In-Water Emulsions Containing Omega-3 Fatty acids[J]. Food Chemistry 2004, 88(1), 57–62. DOI: 10.1016/j.foodchem.2004.01.022.
- Zhang, L.; Li, J.R.; Zhou, K.Q. Chelating and Radical Scavenging Activities of Soy Protein Hydrolysates Prepared from Microbial Proteases and Their Effect on Meat Lipid peroxidation[J]. BIORESOURCE TECHNOLOGY 2010, 101(7), 2084–2089. DOI: 10.1016/j.biortech.2009.11.078.
- Liu, P.; Huang, M.G.; Song, S.Q.; et al. Sensory Characteristics and Antioxidant Activities of Maillard Reaction Products from Soy Protein Hydrolysates with Different Molecular Weight Distribution[J]. Food and Bioprocess Technology 2012, 5(5), 1775–1789.
- Martinez, K.D.; Sanchez, C.C.; Ruiz-Henestrosa, V.P.; et al. Effect of Limited Hydrolysis of Soy Protein on the Interactions with Polysaccharides at the Air-Water interface[J]. Food Hydrocolloids 2007, 21(5–6), 813–822.
- Liu, Q.; Kong, B.; Xiong, Y.L.; et al. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate as Influenced by the Degree of hydrolysis[J]. Food Chemistry 2010, 118(2), 403–410.