 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to evaluate the effect of setting temperatures (30°C, 35°C, 40°C, 45°C, and 50°C) on gel properties and protein profiles of paste gels derived from silver carp (Hypophthalmichthys molitrix) and chicken meat. The mixture composed of 50% (w/w) chicken meat and 50% (w/w) silver carp meat, and the three paste gels, were assessed based on color, gel strength, TPA, water distribution, chemical interactions, and SDS-PAGE. Chicken gels had better gel properties and a higher content of immobilized water than the mixture or fish gels, regardless of setting conditions. On the other hand, an appropriate setting temperature for the three paste gels promoted aggregation of the myosin heavy chain (MHC) and the formation of hydrophobic interactions and disulfide bonds, which resulted in superior gel properties. Pre-incubation at 40°C enhanced gel properties of fish meat, but pre-incubation at 45°C and 50°C were appropriate for achieving better gels for the mixture and chicken, respectively. These results indicated that there is the potential to obtain mixed products and new meat products by utilizing chicken and fish meat that have improved gel properties.
Introduction
Silver carp (Hypophthalmichthys molitrix) is one of the widely cultivated freshwater fish in the aquaculture industry in China, with a production of 4.51 million tons in 2017,[Citation1] which makes silver carp one of the most important freshwater food fish in the world. Unfortunately, the commercial benefit of silver carp has been limited despite its high production, which is mainly caused by its limited regional distribution and short shelf-life after harvest.[Citation2] Silver carp is a potential alternative to marine fish for surimi processing due to its high nutritional value, especially, its high content of unsaturated fatty acids.[Citation3] However, silver carp possess poor gel characteristics, which may be related to the modori.[Citation4] To overcome this weakness, a number of studies have been conducted to combine freshwater fish pastes with meat and meat by-products, such as pork mince, porcine hearts, and bovine hearts.[Citation5–Citation7] The addition of fish meat may improve the textural property and flavor of meat.[Citation8] However, as a healthy dietary component and a widely accepted material for surimi processing,[Citation9,Citation10] information on the gel-forming characteristics of chicken/fish mixtures is limited.
In general, thermal fish gels are typically formed in a two-step, heating process.[Citation11] The first step involves an appropriate setting that enhances gel strength of surimi-based products by forming a continuous matrix from original paste.[Citation12] Then, this matrix changes into a rigid, opaque gel by raising the heating temperature to approximately 90°C. In previous studies of several surimi gels from silver carp and pork, pre-incubation at 40°C significantly (P < 0.05) increased the breaking force of the cooked fish meat samples.[Citation13] Additionally, surimi from tropical species showed different gel properties when set at medium or high temperatures prior to heating.[Citation14] Gel properties are important for meat and meat products.[Citation15] Hence, it is important to investigate the effects of setting temperatures on paste gel properties of chicken and fish meat.
To provide a theoretical foundation to improve the gel properties of silver carp meat with chicken meat and to provide a comprehensive understanding of paste gel processing, this study investigated the characteristic of gels from silver carp meat, chicken breast meat, and a mixture composed of 50% (w/w) chicken meat and 50% (w/w) fish meat at various temperatures. The hypothesis was that the gelation properties of meat paste may be improved by restructuring the paste with chicken and silver carp meat. The indicators of gel properties (breaking force, deformation, gel strength, whiteness, and water distribution), we studied protein patterns using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE analysis) at different temperatures.
Materials and methods
Materials
Silver carp with an average weight of 1.5–2.0 kg were obtained from an aquatic products market, Beijing, China, and transported to the laboratory alive in water. Fish were stunned, beheaded, gutted, filleted, and washed with cold distilled water within 2 h. White dorsal muscle was collected manually and minced. Chicken breast meat stored at 4°C was purchased from a Wumart supermarket, Beijing, China, and the fat and connective tissue was removed. All chemicals used were of analytical grade.
Sample preparation
Chicken and fish meat were chopped separately with a food processer (Joyoung JYL-D055, Beijing, China). The minced meat was washed twice in cold distilled water (4°C, 2 volumes, w/v) and once in 0.5% NaCl (4°C, 2 volumes, w/v), and stirred for 1 h at a low speed with a JJ-1 electrical blender (Jintan Ronghua Instrument Manufacture Co, Ltd, Jintan, China). The pastes (chicken and fish meat) were centrifuged at 5,000 g for 8 min, and the supernatant was discarded. Finally, the pastes with 2.5% (w/w) NaCl were adjusted to a final moisture content of 82% with distilled water.
The salted pastes of fish and chicken meat were prepared at ratios (w/w) of 2:0, 1:1, and 0:2. The mixed pastes were stuffed in 50 mL centrifuge tubes (2 cm diameter, 20 cm length) and centrifuged at 3,000 g for 10 min to remove any air bubbles in the slurry. Afterwards, the salted fish meat paste samples were cultured in a water bath at different setting temperatures (30°C, 35°C, 40°C, 45°C, and 50°C) for 1 h followed at 90°C for 0.5 h. All gels were chilled quickly with ice/water and stored at 4°C overnight before analysis.
Determination of color and whiteness
The color of paste gels was determined using a JP7100F colorimeter (Juki Corp, Tokyo, Japan), which included L* (lightness), a* (redness/greenness), b* (yellowness/blueness), and whiteness values. Whiteness was calculated using the following equation:[Citation16]
Measurement of gel strength
The paste gels were cut into 2.0-cm-high cylindrical specimens. Measurements of gel strength were obtained using a CT3 texture analyzer (Brookfield, WI). Breaking force (g) and deformation (mm) were measured by employing a 5.0-mm-diameter spherical head with 50% compression at a test speed of 1 mm/s. Gel strength was calculated using the following equation:
Texture profile analysis (TPA)
The textural properties of the paste gels were analyzed by using a CT3 texture analyzer (Brookfield, WI), following the method of Li et al.[Citation8] A square probe (TA3/100) was pressed against the 2.0-cm-high samples at a speed of 1 mm/s for two consecutive cycles using 30% compression. The textural characteristics were expressed as hardness, springiness, cohesiveness, gumminess, and chewiness. Hardness is the resistance at maximum compression during the first compression; springiness refers to the ability of the sample to recover its original form after the deforming force is removed; cohesiveness is the extent to which the sample could be deformed before rupture; gumminess is the amount of energy needed to separate a semi-solid food to a pre-swallowing state; chewiness indicates the work needed to chew a solid sample to a steady state for swallowing.[Citation17] All samples were analyzed at room temperature.
Measurements of NMR water distribution
Low-field NMR relaxation measurements were taken using a Niumag Benchtop Pulsed NMR Analyser PQ001 (Niumag Electric Corporation, Shanghai, China), which operated at a resonance frequency of 23.0 Hz, following the methods of Han et al.[Citation18] The samples were cut into cubes (10 * 10 * 10 mm) and packed in plastic films, and then they were put into the NMR probe. Afterwards, water distribution was determined using the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence at 32°C, with a τ-value (time between 90 pulse and 180 pulses) of 120 μs.[Citation19] After four scan repetitions, we acquired about 3,200 echoes as constant factors.
To better analyze the data, a multi-exponential fitting analysis was performed using a MultiExp Inv Analysis software from Niumag Electric Corporation. This analysis generated a plot of relaxation amplitude for individual relaxation processes compared to relaxation time. The proportion of water molecules and water distribution was calculated and exhibited by relaxation time and peak position.[Citation20]
Determination of protein solubility
Gels of protein solubility were determined using the method developed by Liu, Gao, Ren, & Zhao[Citation21], with minor modifications. The agents that cleave or destroy different types of inter-molecular bonds were applied to identified types of molecular bonding: 0.05 M NaCl (SA), 0.6 M NaCl (SB), 0.6 M NaCl + 1.5 M urea (SC), and 0.6 M NaCl + 8 M urea (SD).[Citation22] About 2 g of gel samples was homogenized for 30 s with 10 mL of each solution,and the blended solutions were centrifuged at 10, 000 g for 15 min. The protein concentration in supernatants was determined by the Biuret method.[Citation22]
Measurements of sulfhydryl (SH) groups
Sulfhydryl (SH) groups were determined using the method of Ellman[Citation23] and Liu et al.[Citation13] with some modifications. Four grams of chopped gel samples were added to 11 mL of 20 mM Tris- HCl solutions, which contained 0.6 M KCl, pH 7.0. The mixtures were then homogenized for 30 s and preserved at 4°C for 1 h. Afterwards, the homogenate was centrifuged at 10,000 g for 10 min. The protein concentration in the supernatant was adjusted to 2 mg/mL by the Biuret method[Citation22] and used to determine the concentration of SH groups using the Ellman method.[Citation23] The amount of total SH was measured at 412 nm with a molar extinction coefficient of 13,600 L/(mol•cm) using a spectrophotometer (Model DU640, Beckman Instruments).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
To prepare the protein sample, 2 g of chopped gels from each group was added to 18 mL 5% SDS, homogenized for 1 min, and heated at 85°C in a water bath for 1 h. The mixtures were centrifuged at 10,000 g for 10 min. The protein concentration in supernatants was adjusted to 2 mg/mL using the Biuret method.[Citation22] Afterwards, protein samples were mixed with a sample buffer (with and without dithiothreitol (DTT)) at the ratio of 1:1 (v/v), boiled for 5 min, and stored at −20°C until use. SDS-PAGE gel was conducted using a 10% running gel and a 4% acrylamide stacking gel. Twenty micrograms protein was loaded onto the sample well in the stacking gel and subjected by electrophoresis at an initial voltage of 80 V, until the sample was completely pressed into a straight line. Immediately after entering the separation gel, the voltage was increased to 120 V. Electrophoresis time was about 1.5 h, and then the sample was fixed, dyed, and decolored until the protein bands were clear and distinct.
Data analysis
All analysis was conducted in triplicate, and the data were presented as the mean ± standard deviation. Statistical analysis was performed using SPSS19.0 software, and significant (P < 0.05) differences were determined between means using Duncan’s multiple range test.
Results and discussion
Color and whiteness
The color of fish gel is an important factor for consumers, and whiteness of gel is regarded as a useful index.[Citation24] Whiteness of the three paste gels was different across setting temperatures (30°C, 35°C, 40°C, 45°C, and 50°C) (). The L*, a*, and whiteness of fish gels increased slightly with setting temperatures from 30°C to 40°C, and then they decreased significantly (P < 0.05) to the lowest level at 45°C. For chicken and mixture gels, the values of L*, a*, and whiteness increased slightly in the setting range of 30°C to 40°C, but decreased markedly from 40°C to 50°C. The b* values increased sharply for chicken gels with setting temperatures from 30°C to 50°C. Values for mixture gels for L*, a*, b*, and whiteness were between the values for fish and chicken meat. Weerasinghe et al.[Citation25] suggested that the decrease in whiteness of fish gels at 45°C-50°C was due to the modori phenomenon. Similarly, the L* and whiteness values of fish gels decreased significantly (P < 0.05) from the maximum of 67.02 (40°C) to 64.03 (45°C) in our study, which indicated the presence of modori for endogenous proteinases in muscle protein of silver carp.
Figure 1. Effects of setting temperatures on whiteness and color of the mixture gels composed of fish and chicken meat.
Note: a. Results are presented as mean ± standard deviations.b. Different lowercase letters (a-c) in the same species indicate significant differences across heating treatments (P < 0.05). Different capital letters (A-C) indicate significant differences among fish, chicken, and mixture samples at the same treatment (P < 0.05).
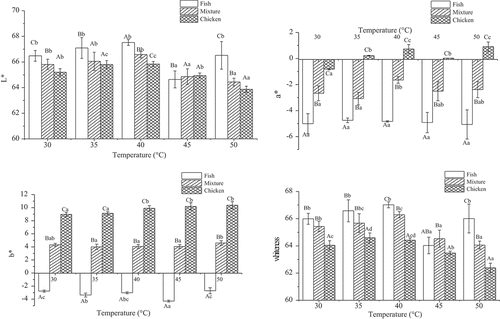
Regardless of setting conditions, the values of whiteness of fish gels were significantly (P < 0.05) higher than that in chicken gels (), which may be due to species differences.[Citation26] The whiteness of fish surimi or surimi-like material is related to the efficiency of removal of the sarcoplasmic protein from muscle[Citation27] and the elimination of heme pigments[Citation26], which depend exclusively on the characteristics of the raw material. Additionally, the whiteness of mixture gels was higher than the other two gels when pre-incubated at 45°C, which was possibly caused by protein-protein cross-linking from fish and chicken gels. Similarly, Liu, Zhao, Xiong, Xie, & Liu, (2007) studied on gel properties of silver carp and white croaker blended surimi, found that the whiteness of the blended surimi was higher than that of white croaker alone.[Citation2] Buamard, N., & Benjakul, S.[Citation28] also concluded that addition of oxidised phenolic compounds resulted in the difference in whiteness of sardine surimi gel, and the denatured or unfolded muscle proteins were more polymerised by protein cross-linkers in coconut husk extract during heating.
Gel properties
For fish samples, breaking force and gel strength were improved slightly with setting temperatures from 30°C to 40°C, with peak values (138.5 g and 688.7 g•mm, respectively) at 40°C (). Breaking force and gel strength of the mixture gels was almost constant in the range of 30°C-45°C, which reached a plateau (158.75 g and 785.2 g•mm, respectively) at 45°C, but dropped significantly (P < 0.05) at 50°C. Meanwhile, breaking force and gel strength of chicken meat samples were similar to the mixture from 30°C to 45°C, but increased significantly (P < 0.05) and reached the highest values (184.0 g and 912.3 g•mm, respectively) at 50°C.
Figure 2. Effects of setting temperatures on gel strength of the mixture gels composed of fish and chicken meat.
Note: a. Results are presented as mean ± standard deviations.b. Different lowercase letters (a-b) in the same species indicate significant differences across heating treatments (P < 0.05). Different capital letters (A-C) indicate significant differences among fish, chicken, and mixture samples at the same treatment (P < 0.05).
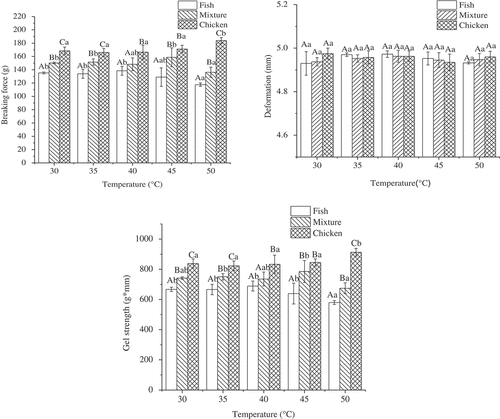
Luo et al.[Citation29] reported that better breaking force and gel strength for silver carp was at 40°C, which was caused by the denaturation of myosin protein. The first endothermic transitions of myofibrillar proteins from chicken meat were approximately 50°C, and the first transition peak had a direct connection with myosin.[Citation30] In sum, the reason for the improved mixture gels at a setting temperature at 45°C was likely due to a myosin protein–protein interaction between fish meat protein and chicken meat protein. When the setting temperatures were at 45°C and 50°C, the properties of fish and mixture gels were poorer than chicken gels, which might be attributed to modori due to endogenous acid proteinases.[Citation4]
In contrast, no marked difference was observed in deformation among fish, mixtures, or chicken meat samples at all setting temperatures (). Thus, the effects of different setting temperatures for gels were exhibited on the breaking force rather than on deformation. Furthermore, breaking force and gel strength of chicken gels were significantly (P < 0.05) higher than those of fish gels, and chicken gels had higher values compared to the mixture gels, although these were not significant. Lan et al.[Citation4] also reported that the gel properties of myofibrillar proteins from chicken gels were stronger than fish gels.
Texture profile analysis (TPA)
For fish meat samples, hardness (the most important feature of texture), cohesiveness, springiness, gumminess (the extent of flesh height), and chewiness increased with setting temperatures from 30°C to 40°C, then dropped from 40°C to 50°C (). Hardness, cohesiveness, gumminess, and chewiness of mixture gels were enhanced in the range of 30°C-45°C, but decreased at 50°C. Hardness and cohesiveness of chicken gels improved from 30°C to 50°C. For mixture and chicken gels, springiness and cohesiveness fluctuated across setting temperatures, but the highest springiness occurred at 2.74 and 2.76 mm, respectively, and the highest cohesiveness occurred at 0.88 and 0.89, respectively. Additionally, the textural properties of mixture gels were dramatically higher than those of fish gels at the same setting temperature. Moreover, fish, mixture, and chicken gels had better textural characteristics at 40°C, 45°C, and 50°C, respectively, which was in agreement with the changes in the gel properties in our study.
Table 1. Effects of setting temperatures on texture properties of the mixture gels composed of fish and chicken meat.
Measurements of NMR water distribution
The water states of gels are composed primarily of bound moisture, immobilized water, and free water, with the vast majority of water being immobilized water (more than 88%). The bound moisture of fish and mixture gels fluctuated across setting temperatures (). However, setting temperatures had little effect on the bound moisture of chicken gels. Meanwhile, the proportion of immobilized water of fish gels reached a maximum (approximately 91%) at setting temperatures of 35°C and 40°C. On the contrary, the proportion of free water was lower (7.6%) than other treatments when pre-incubated at 40°C for fish gels. For mixture gels, there were no significant (P > 0.05) changes for immobilized water and free water when setting temperatures ranged from 30°C to 45°C. Nevertheless, the proportion of immobilized water and free water fluctuated at different setting treatments for chicken meat samples. However, the proportion of immobilized water was the highest (91.87%) at 45°C, and free water was lower than 7.5% for chicken gels. Similar variations in the proportion of water in chicken gels were also investigated by Qin, Xu, Zhou, & Wang[Citation31], who reported that mobility and content of water increased, which led to better WHC of chicken myosin gels. Shao et al.[Citation32] revealed that the T21 component corresponded to immobilized water, which was the predominant water component that was trapped within the gel matrix. Additionally, changes in water distribution were correlated with protein conformation and gel structure, because Sánchez-González et al.[Citation33] demonstrated that an increase in the I3220/3400 ratio occurred in the transition from small water interstices to greater domains of water. Based on the above changes and the research of Stevenson, Liu, & Lanier[Citation34], we concluded that water may have shifted from free water to immobilized water to form better gels.
Table 2. Effect of setting temperatures on the water distribution of the mixture gels composed of fish and chicken meat.
Protein solubility
Proteins can be partially solubilized in relevant chemical agents, which might be used to distinguish the existence of ionic bonds (difference between SB and SA), hydrogen bonds (difference between SC and SB), and hydrophobic interactions (difference between SD and SC). The primary associative forces involved in gels were ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds.[Citation35,Citation36] For fish and mixture gels, the proportion of ionic bonds declined to the lowest level when the setting temperatures were 35°C (9.22%) and 40°C (7.91%), respectively (). Then, the proportion of ionic bonds rebounded at a higher setting temperature. In contrast, the proportion of ionic bonds of chicken gels increased continuously across setting temperatures. Regardless of setting temperatures, the proportion of ionic bonds of chicken gels was significantly (P < 0.05) lower than that in fish gels. In addition, a lower proportion of ionic bonds (not significant) was observed in chicken gels than in mixture gels. Similarly, Zhang et al.[Citation37] reported that high temperatures strongly affected the changes in concentration of ionic bonds. Similar variations in ionic bonds were also investigated by Liu et al.[Citation21] This might be the reason that more electrostatic interactions in proteins of chicken gels changed the conformational structure than other paste gels.
Table 3. Effects of setting temperatures on soluble protein of the mixture gels composed of fish and chicken meat.
Hydrogen bonds are primary intermolecular forces that affect the secondary structure of proteins.[Citation38] With increasing temperatures, the proportion of hydrogen bonds decreased, followed by an increase for fish and mixture gels (). The minimum proportion was obtained for fish (4.57%) gels and mixture (6.12%) gels at 40°C. On the contrary, the proportion of hydrogen bonds of chicken gels declined from 30°C to 50°C. Changes in hydrogen bonds may be correlated with protein conformation and gel structure, because transformation from α-helix to β-sheet structure occurs through a rearrangement of hydrogen bonds during gelation.[Citation39]
Conversely, the proportion of hydrophobic interactions of fish and mixture gels increased from 30°C to 40°C, followed by a decrease from 40°C to 50°C (). The minimum proportions of hydrophobic interactions were obtained at 40°C for fish gels (87.59%) and mixture gels (81.89%). However, the proportion of hydrophobic bonds increased from 30°C to 50°C for chicken gels. Hydrophobic interactions from the exposure of hydrophobic amino acid groups were further promoted by setting.[Citation40] Moreover, the proportion of hydrophobic interactions of chicken gels were significantly (P < 0.05) higher than that in fish gels.
The thermal process in gels favors the formation of hydrophobic interactions.[Citation39] Therefore, abundant hydrophobic interactions were observed in our three paste gels, which accounted for at least 73% of all non-covalent bonds in all two-step heating samples. Conversely, ionic and hydrogen bonds accounted for smaller proportions than hydrophobic interactions for all chemical bonds in the three paste gels, which suggested that ionic and hydrogen bonds may not be major forces in gel formation. To some extent, this was consistent with breaking force and gel strength.
In addition to the non-covalent bonds as mentioned above, the sum of protein solubility into different solvents may also reflect the changes in chemical linkages during the gelation process.[Citation38] The sum of protein solubility for fish and mixture gels declined, then they were enhanced with increasing temperatures, with the lowest solubility at 35°C (9.96 mg/mL) and 45°C (12.01 mg/mL), respectively. Protein solubility for chicken gels decreased continuously from 30°C (13.06 mg/mL) to 50°C (12.59 mg/mL). Decreased protein solubility indicated the formation of non-disulfide covalent bonds induced by endogenous TGase.[Citation21] Therefore, it is possible that the low solubility of the three paste gels that were heated at appropriate temperatures was due to significant non-disulfide, covalent cross-linking, which was possibly due to either high TGase activity or the reactivity of myofibrillar proteins in the TGase-induced reaction. In general, we observed good agreement with the tendency of ionic bonds, which were correlated strongly with better gel properties in the three paste gels.
Measurement of sulfhydryl groups
Traditionally, heating leads to cleavage of existing disulfide bonds or activation of buried sulfhydryl (SH) groups through protein unfolding, which indicates that SH groups may form new intermolecular disulfide bonds that are essential for aggregation formation.[Citation41] Therefore, the SH group was lower for fish and mixture gels at settings of 40°C and 45°C, respectively (). For chicken gel samples, disulfide bonds from SH groups increased when the setting temperatures increased from 30°C to 50°C. Based on these results, breaking force and gel strength of the mixture gels obviously increased at the corresponding appropriate temperatures than at other setting conditions, which highlighted the important role of disulfide bonds in gelling. A similar phenomenon has been reported for exposed sulphydryl groups that were linked with each other and formed disulfide bonds in surimi from silver carp when the incubation temperature was 40°C.[Citation21] These results were consistent with our data on gel properties.
Figure 3. Effects of setting temperatures on SH group of the mixture gels composed of fish and chicken meat.
Note: a. Results are presented as mean ± standard deviations.b. Different lowercase letters (a-b) in the same species indicate significant differences across heating treatments (P < 0.05). Different capital letters (A-C) indicate significant differences among fish, chicken, and mixture samples at the same treatment (P < 0.05).
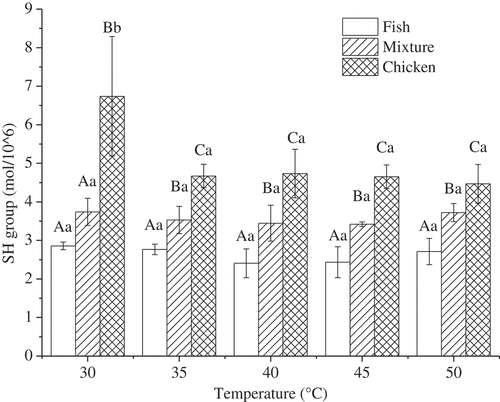
Figure 4. Effects of setting temperatures on SDS-PAGE pattern of the mixture gels composed of fish and chicken meat.
MHC - myosin heavy chain, A1- actin.a,b and c, electrophoresis diagram of gels from sliver carp, mixture and chicken gel with addition of DTT, respectively; d,e and f, electrophoresis diagram of gels from sliver carp meat, blended meat and chicken meat without addition of DTT, respectively.
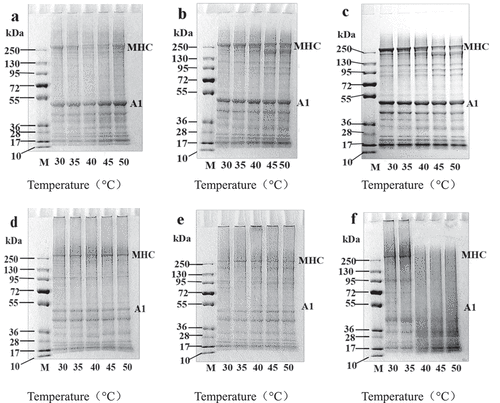
Sds-page
Regardless of whether we added dithiothreitol (DTT) or not, the band intensity of the myosin heavy chain (MHC) in the electrophoretic pattern decreased for fish and chicken mixture gels at different setting conditions (). In general, a lightened band intensity of MHC across setting temperatures indicated that the polyacrylamide gels blocked the entrance of oversized, high-molecular-weight fractions that were generated from MHC cross-linking.[Citation39] For fish gels, a lighter MHC band at 40°C was observed with or without the addition of DTT. It was evident that the band intensity of MHC for mixture and chicken gels were lighter than other treatments, regardless of the addition of DTT with setting temperatures at 45°C and 50°C, respectively. The reduction in MHC band intensity was concomitant with increased breaking force and gel strength (). However, no marked difference in actin bands (43 kDa) was observed during the setting and heat treatments. Actin might interact with myosin through non-covalent bonds, such as hydrophobic interactions, but actin was less responsible for the formation of the gel network.[Citation42]
The intensity of MHC bands with addition of DTT for three paste gels was sharply lower compared to the gels without DTT. The reason may be that DTT destroys and cuts high-molecular-weight fractions (HMWF) that were too large to enter the polyacrylamide gel, especially by breaking the three-dimensional net structure that consisted of disulfide bonds.[Citation43] As a consequence, the intensity of MHC and actin increased, which suggested that disulfide bonds were the major force in the three paste gels. These changes explained the stronger gel properties for fish, mixture, and chicken meat pastes at settings of 40°C, 45°C, and 50°C, respectively.
Conclusion
The present study indicated that different setting temperatures affected the chemical interactions and gel properties for the three paste gels dramatically. Pre-incubation of fish meat pastes at 40°C enhanced breaking force, gel strength, and the content of immobilized water, and they exhibited better whiteness than at other setting conditions. Moreover, higher gel properties and better whiteness were obtained from mixture and chicken pastes at 45°C and 50°C, respectively. Additionally, the gel properties of mixture samples were significantly higher than fish gels at the same setting temperature. Appropriate setting temperatures for the three paste gels promoted the aggregation of MHC and the formation of hydrophobic interactions and disulfide bonds, which resulted in superior gel properties. Therefore, the gel properties of fish meat samples were significantly (P < 0.05) improved by adding chicken. The results of our study may be useful for the food industry for the production of mixture gels that consist of chicken and fish meat. However, further studies are needed to confirm the mechanism, detailed procedures, and appropriate proportions of chicken and fish meat that should be used.
Additional information
Funding
References
- The Ministry of Agriculture Fishery and Fishery Administration. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, 2017.
- Liu, L.; Luo, Y.; Song, Y.; Shen, H.; Hong, H. Study on Gel Properties of Silver Carp (Hypophthalmichthys Molitrix) and White Croaker (Argyrosomus Argentatus) Blended Surimi at Different Setting Conditions. Journal of Aquatic Food Product Technology 2013, 22(1), 36–46. DOI: 10.1080/10498850.2011.623263.
- Buchtová, H.;. Fatty Acids Profile in Mechanically Recovered Meat from Common Carp (Cyprinus Carpio) and Silver Carp (Hypophthalmichthys Molitrix). Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis 2011, 59(1), 15–22. DOI: 10.11118/actaun201159010015.
- Lan, Y.H.; Novakofski, J.; Mccusker, R.H.; Brewer, M.S.; Carr, T.R.; Mckeith, F.K. Thermal Gelation of Pork, Beef, Fish, Chicken and Turkey Muscles as Affected by Heating Rate and pH. Journal of Food Science 2010, 60(5), 936–940. DOI: 10.1111/j.1365-2621.1995.tb06265.x.
- Kang, G.H.; Yang, H.S.; Jeong, J.Y.; Moon, S.H.; Hur, S.J.; Park, G.B.; Joo, S.T. Gel Color and Texture of Surimi-Like Pork from Muscles at Different Rigor States Post-Mortem. Asian Australasian Journal of Animal Sciences 2007, 20(7), 1127–1134. DOI: 10.5713/ajas.2007.1127.
- Kang, G.H.; Yang, H.S.; Jeong, J.Y.; Moon, S.H.; Joo, S.T.; Park, G.B. Gel Properties of Surimi-Like Materials from Cardiac and Skeletal Muscle of Pigs. Asian Australasian Journal of Animal Sciences 2007, 20(8), 1292–1296. DOI: 10.5713/ajas.2007.1292.
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Effect of Bovine and Fish Gelatin in Combination with Microbial Transglutaminase on Gel Properties of Threadfin Bream Surimi. International Aquatic Research 2012, 4(1), 1–13. DOI: 10.1186/2008-6970-4-12.
- Li, Q.; Gui, P.; Huang, Z.; Feng, L.; Luo, Y. Effect of Transglutaminase on Quality and Gel Properties of Pork and Fish Mince Mixtures. Journal of Texture Studies 2017, 49(1), 56–64. DOI: 10.1111/jtxs.12281.
- Chen, X.; Li, Y.; Zhou, R.; Liu, Z.; Lu, F.; Lin, H.; Xu, X.; Zhou, G. L‐Histidine Improves Water Retention of Heat‐Induced Gel of Chicken Breast Myofibrillar Proteins in Low Ionic Strength Solution. International Journal of Food Science & Technology 2016, 51(5), 1195–1203. DOI: 10.1111/ijfs.13086.
- Moreno, H.; Herranz, B.; Pérezmateos, M.; Sánchezalonso, I.; Borderías, A.J. New Alternatives in Seafood Restructured Products. Critical Reviews in Food Science and Nutrition 2016, 56(2), 237–248. DOI: 10.1080/10408398.2012.719942.
- Luo, J.B.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. The Impaired Intestinal Mucosal Immune System by Valine Deficiency for Young Grass Carp (Ctenopharyngodon Idella) Is Associated with Decreasing Immune Status and Regulating Tight Junction Proteins Transcript Abundance in the Intestine. Fish & Shellfish Immunology 2014, 40(1), 197–207. DOI: 10.1016/j.fsi.2014.07.003.
- Fukushima, H.; Okazaki, E.; Fukuda, Y.; Watabe, S. Rheological Properties of Selected Fish Paste at Selected Temperature Pertaining to Shaping of Surimi-Based Products. Journal of Food Engineering 2007, 81(2), 492–499. DOI: 10.1016/j.jfoodeng.2006.11.029.
- Liu, R.; Zhao, S.M.; Xie, B.J.; Xiong, S.B. Contribution of Protein Conformation and Intermolecular Bonds to Fish and Pork Gelation Properties. Food Hydrocolloids 2011, 25(5), 898–906. DOI: 10.1016/j.foodhyd.2010.08.016.
- Benjakul, S.; Visessanguan, W.; Chantarasuwan, C. Effect of High-Temperature Setting on Gelling Characteristic of Surimi from Some Tropical Fish. International Journal of Food Science & Technology 2004, 39(6), 671–680. DOI: 10.1016/s0308-8146(03)00012-8.
- Li, K.; Chen, L.; Zhao, Y.Y.; Li, Y.P.; Wu, N.; Sun, H.; Xu, X.L.; Zhou, G.H. A Comparative Study of Chemical Composition, Color, and Thermal Gelling Properties of Normal and PSE-Like Chicken Breast Meat. CyTA - Journal of Food 2015, 13(2), 213–219. DOI: 10.1080/19476337.2014.941411.
- Truong, B.Q.; Buckow, R.; Nguyen, M.H.; Furst, J. Gelation of Barramundi (Lates Calcarifer) Minced Muscle as Affected by Pressure and Thermal Treatments at Low Salt Concentration. Journal of the Science of Food & Agriculture 2017, 97(11), 3781–3789. DOI: 10.1002/jsfa.8242.
- Ramirezsuarez, J.C.; Morrissey, M.T. Effect of High Pressure Processing (HPP) on Shelf Life of Albacore Tuna (Thunnus Alalunga) Minced Muscle. Innovative Food Science & Emerging Technologies 2006, 7(1–2), 19–27. DOI: 10.1016/j.ifset.2005.08.004.
- Han, M.; Wang, P.; Xu, X.; Zhou, G., Low-Field NMR Study of Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Microstructural Characteristics. Food Research International 2014, 62, 1175–1182. DOI: 10.1016/j.foodres.2014.05.062.
- Meiboom, S.; Gill, D. Modified Spin‐Echo Method for Measuring Nuclear Relaxation Times. Review of Scientific Instruments 1958, 29(8), 688–691. DOI: 10.1063/1.1716296.
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C., Effects of Konjac Glucomannan on Heat-Induced Changes of Physicochemical and Structural Properties of Surimi Gels. Food Research International 2016, 83, 152–161. DOI: 10.1016/j.foodres.2016.03.007.
- Liu, H.; Gao, L.; Ren, Y.; Zhao, Q. Chemical Interactions and Protein Conformation Changes during Silver Carp (Hypophthalmichthys Molitrix) Surimi Gel Formation. International Journal of Food Properties 2014, 17(8), 1702–1713. DOI: 10.1080/10942912.2012.700538.
- Torten, J.; Whitaker, J.R. Evaluation of the Biuret and Dye-Binding Methods for Protein Determination in Meats. Journal of Food Science 2010, 29(2), 168–174. DOI: 10.1111/j.1365-2621.1964.tb01713.x.
- Ellman, G.L.;. Tissue Sulfhydryl Groups. Archives of Biochemistry & Biophysics 1959, 82(1), 70–77. DOI: 10.1016/0003-9861(59)90090-6.
- Cortés-Ruiz, J.A.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Carvallo-Ruiz, M.G.; García-Sánchez, G. Production and Functional Evaluation of a Protein Concentrate from Giant Squid (Dosidicus Gigas) by Acid Dissolution and Isoelectric Precipitation. Food Chemistry 2008, 110(2), 486–492. DOI: 10.1016/j.foodchem.2008.02.030.
- An, H.; Weerasinghe, V.; Seymour, T.A.; Morrissey, M.T. Cathepsin Degradation of Pacific Whiting Surimi Proteins. Journal of Food Science 2010, 59(5), 1013–1017. DOI: 10.1111/j.1365-2621.1994.tb08179.x.
- Cortezvega, W.R.; Fonseca, G.G.; Prentice, C. Comparisons of the Properties of Whitemouth Croaker (Micropogonias Furnieri) Surimi and Mechanically Deboned Chicken Meat Surimi-Like Material. Food & Nutrition Sciences 2012, 3(11), 1480–1483. DOI: 10.4236/fns.2012.311192.
- Kang, G.H.; Kim, S.H.; Kim, J.H.; Kang, H.K.; Kim, D.W.; Na, J.C.; Yu, D.J.; Suh, O.S.; Choi, Y.H. Effects of Washing Methods on Gel Properties of Chicken Surimi Prepared from Spent Hen Breast Muscle. Poultry Science 2009, 88(7), 1438–1443. DOI: 10.3382/ps.2008-00212.
- Buamard, N.; Benjakul, S., Improvement of Gel Properties of Sardine (Sardinella Albella) Surimi Using Coconut Husk Extracts. Food Hydrocolloids 2015, 51, 146–155. DOI: 10.1016/j.foodhyd.2015.05.011.
- Luo, Y.; Shen, H.; Pan, D. Gel-Forming Ability of Surimi from Grass Carp (Ctenopharyngodon Idellus): Influence of Heat Treatment and Soy Protein Isolate. Journal of the Science of Food & Agriculture 2006, 86(5), 687–693. DOI: 10.1002/jsfa.2395.
- Zhao, X.; Xing, T.; Chen, X.; Han, M.Y.; Xu, X.L.; Zhou, G.H., Yield, Thermal Denaturation, and Microstructure of Proteins Isolated from Pale, Soft, Exudative Chicken Breast Meat by Using Isoelectric Solubilization/Precipitation. Process Biochemistry 2017, 58, 167–173. DOI: 10.1016/j.procbio.2017.04.035.
- Qin, H.; Xu, P.; Zhou, C.; Wang, Y. Effects of L-Arginine on Water Holding Capacity and Texture of Heat-Induced Gel of Salt-Soluble Proteins from Breast Muscle. LWT - Food Science and Technology 2015, 63(2), 912–918. DOI: 10.1016/j.lwt.2015.04.048.
- Shao, J.J.; Zou, Y.F.; Xu, X.L.; Zhou, G.H.; Sun, J.X. Effects of NaCl on Water Characteristics of Heat-Induced Gels Made from Chicken Breast Proteins Treated by Isoelectric Solubilization/Precipitation. CyTA - Journal of Food 2016, 14(1), 145–153. DOI: 10.1080/19476337.2015.1071432.
- Sanchezgonzalez, I.; Carmona, P.; Moreno, P.; Borderias, J.; Sanchezalonso, I.; Rodriguezcasado, A.; Careche, M. Protein and Water Structural Changes in Fish Surimi during Gelation as Revealed by Isotopic H/D Exchange and Raman Spectroscopy. Food Chemistry 2008, 106(1), 56–64. DOI: 10.1016/j.foodchem.2007.05.067.
- Stevenson, C.D.; Liu, W.; Lanier, T.C. Rapid Heating of Alaska Pollock and Chicken Breast Myofibrillar Protein Gels as Affecting Water-Holding Properties. Journal of Agricultural & Food Chemistry 2012, 60(40), 10111–10117. DOI: 10.1021/jf3032292.
- Pérezmateos, M.; Lourenço, H.; Montero, P.; Borderías, A.J. Rheological and Biochemical Characteristics of High-Pressure- and Heat-Induced Gels from Blue Whiting (Micromesistius Poutassou) Muscle Proteins. Journal of Agricultural & Food Chemistry 1997, 45(1), 44–49. DOI: 10.1021/jf960185m.
- Gomez-Guillen, M.C.; Borderias, A.J.; Montero, P. Chemical Interactions of Nonmuscle Proteins in the Network of Sardine (Sardina Pilchardus) Muscle Gels ☆. LWT - Food Science and Technology 1997, 30(6), 602–608. DOI: 10.1006/fstl.1997.0239.
- Zhang, L.; Yong, X.; Jie, X.; Li, Z.; Xue, C. Effects of High-Temperature Treatment (⩾100°C) on Alaska Pollock (Theragra Chalcogramma) Gels. Journal of Food Engineering 2013, 115(1), 115–120. DOI: 10.1016/j.jfoodeng.2012.10.006.
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y., Changes in Chemical Interactions and Gel Properties of Heat-Induced Surimi Gels from Silver Carp (Hypophthalmichthys Molitrix) Fillets during Setting and Heating: Effects of Different Washing Solutions. Food Hydrocolloids 2017, 75, 116–124. DOI: 10.1016/j.foodhyd.2017.09.007.
- Nakai, S.; Li-Chan, E. Hydrophobic Interactions in Food Systems; CRC Press: Boca Raton, 1988. DOi: 10.1201/9781351073318.
- Weng, W.; Zheng, W. Effect of Setting Temperature on Glucono‐δ‐Lactone‐Induced Gelation of Silver Carp Surimi. Journal of the Science of Food & Agriculture 2015, 95(7), 1528–1534. DOI: 10.1002/jsfa.6857.
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Comprehensive Reviews in Food Science & Food Safety 2011, 10(1), 33–51. DOI: 10.1111/j.1541-4337.2010.00137.x.
- Jimenezcolmenero, F.; Cofrades, S.; Carballo, J.; Fernandez, P.; Fernandezmartin, F. Heating of Chicken and Pork Meat Batters under Pressure Conditions: Protein Interactions. Journal of Agricultural & Food Chemistry 1998, 46(11), 4706–4711. DOI: 10.1021/jf980354y.
- Tammatinna, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Gelling Properties of White Shrimp (Penaeus Vannamei) Meat as Influenced by Setting Condition and Microbial Transglutaminase. LWT - Food Science and Technology 2007, 40(9), 1489–1497. DOI: 10.1016/j.lwt.2006.11.017.
