ABSTRACT
Crocodiles have been hunted and consumed for centuries for skins, nutrients, and medicines. These indomitable trends have overpowered restrictions from wildlife and conservation agencies, continuing the illegal trades of crocodiles across the world. This paper described the development of a very stable, fast, and secured polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay for the confirmed detection of Crocodylus porosus under any matrices and decomposing treatments. Two very short-sites (77 and 127-bp) of atp6 and cytb genes of C. porosus were controlled digested with AciI enzyme; producing distinctive RFLP patterns (83, 54, 44 & 23 bp). The enzyme digested assay was stable following extreme boiling, autoclaving, and microwaving treatments that break down DNA. The sensitivity was tested and validated in model meatballs and it was suitable for detecting 0.01% crocodile meatball matrices. The optimized RFLP assay was used to screen 3 commercial meatballs and 21 traditional medicines (TM). While no crocodile DNA was found in commercial chicken meatballs, 4/21 TM products were found correctly labelled to contain C. porosus DNA. The novel assay demonstrated sufficient merit to be used by regulatory agencies for any forensic and/or archaeological identification of C. porosus even under the state of decomposition.
Introduction
In 2016, the estimated global crimes on environment were USD 91–259 billion, threatening governments’ revenues, legitimate businesses, sustainable development, peace, and security.[Citation1] In this regard, the contribution from illegal wildlife trades was USD 7–23 billion, profusely risking global ecological balances.[Citation1] Usually, wild animals and their by-products are traded worldwide to feed the growing demands for exotic foods, clothing, decorative items, traditional medicines, and pets.[Citation2] While wildlife trades have been mostly illegal[Citation3] due to the protective measures by various conservation agencies,[Citation4] the increasing profit-making pursuit by selling cheaper meat and endangered animal products at higher prices have increasingly become a greater threat to the threatened species.[Citation5]
Tensen[Citation6] highlighted the four main reasons of increasing demand for wildlife: the uses of animal products in TM; superior taste and nutritive quality of wild meat as compared to captive breeds; unavailability and the growing exotic pet industry. In this regard, the increasing demand of some reptile meats, such as crocodiles, caimans, alligators, iguanas, and turtles has resulted in the development of national breeding programs, at least, in 30 countries including North, Central, and South America, Africa, Asia and Australia.[Citation7] While crocodiles are mostly reared for their valuable skins, its meat and blood are valuable by-products for human consumption in exotic foods,[Citation8] tonic medicines[Citation9] and cosmetics.[Citation10] Besides having antimicrobial, antioxidant and anti-inflammatory activities,[Citation11] certain crocodile products, such as blood, are consumed as asthma remedy.[Citation12] Additionally, crocodile products possess good anti-aging characteristics,[Citation10] reduce skin irritation, heal wounds[Citation13] and hinder human immunodeficiency virus (HIV-1) infection in-vitro.[Citation14] However, many reptiles such as crocodilians, lizards, snakes, and chelonians are a significant reservoir of Salmonella spp. and contribute to human non- typhoidal salmonellosis.[Citation15] Additionally, crocodiles are not permissible in Muslims halal[Citation16] and vegetarians[Citation17] consumers’ goods.
As authentication is concerned, six PCR assays[Citation17–Citation22] have been proposed for crocodile identification. Two of which are conventional PCR,[Citation18,Citation22] one PCR-RFLP[Citation19] and the other three are multiplex PCR assays.[Citation17,Citation20,Citation21] However, the documented methods are based on very long amplicon-lengths (373 bp–2000 bp) that are not so stable under extensive processing of food, pharmaceuticals, and cosmetics. Although the authors have claimed the stability of their assays in putrefied samples, numerous recently published reports[Citation23–Citation25] could not support the sustainability of so long amplicon-biomarkers, especially under the states of decomposition. So there is a call for a more stable, reliable and robust method for crocodile authentication in extensively processed samples such as traditional medicines. In our last report, we documented a double-gene targeting conventional multiplex PCR with short-amplicons but it was not tested in medicinal samples.[Citation26] Also, it was not self-authenticating, needing the sequencing help for authenticity verification.
In this regard, multiplex PCR-RFLP (mPCR- RFLP) assay, especially the double gene targeting one with short amplicon targets, would be specifically useful and trustworthy for the simultaneous detection of crocodile products in food and TM products. Because of the presence of more than one target for the same species, the detection of the missing target would be complemented by a second target because it is highly implausible that both targets would be broken down under compromised states.[Citation27] Recently, several double gene-based PCR-RFLP methods have been documented for squirrel, rat, and rabbit species;[Citation28] cattle, buffalo, and pork species[Citation27] and detection of pig, dog, cat, rat, and monkey species.[Citation29] However, such a valuable method has been missing for crocodile detection. To overcome the knowledge gap, for the first time, we developed and validated a double gene targeting duplex PCR-RFLP assay with very short and stable amplicon-markers (127 bp of cytb gene and 77 bp of atp6 gene) that yield distinctive RFLP patterns (44 bp and 83 bp for cytb gene and 23 bp and 54 bp for atp6 gene) for C. porosus in various processing conditions, meatball formulation and traditional medicine products.
Materials and methods
Collection of meat samples
Raw meat samples from chicken (Gallus gallus) and crocodile (C. porosus) were purchased in triplicates on three different dates from Pasar Besar Jalan Othman, Petaling Jaya, Selangor, Malaysia and Krocies Outlet, Old Klang Road, Kuala Lumpur, Malaysia, respectively. A total of 27 (3 x 3 x 3) commercial meatballs of 3 different brands were purchased from Malaysian outlets on three different dates. Purchase of TM is summarized in . All samples were stored at −20°C until further use to prevent enzymatic degradation of DNA.[Citation29]
Table 1. Analysis of traditional Chinese medicines with Crocodylus porosus PCR Assay.
DNA extraction
DNA was extracted from meat and meatball samples using Yeastern Genomic DNA Mini Kit (Yeastern Biotech Co., Ltd, Taipei, Taiwan).[Citation30] Briefly, 20 mg of muscle tissues was grounded and homogenised with a micropestle. Then, lysis buffer was added, followed by proteinase K. The mixture was incubated at 60°C to lyse cells and proteins. A spin column was used for the attachment of DNA to the glass fibre matrix under centrifugation. Potential contaminants were removed with ethanol containing wash buffer. The purified DNA was eluted in an elution buffer. DNA from plant species was extracted using the DNeasy Plant Mini Kit (QIAGEN GmgH, Hilden, Germany).[Citation27] DNA from TM was extracted using Yeastern Genomic DNA Mini Kit (Yeastern Biotech Co., Ltd, Taipei, Taiwan). Concentration and purity of the extracted DNA were determined using UV-VIS Spectrophotometer (NanoPhotometer Pearl, Implen GmbH, Germany) based on the absorbance at 260 nm and absorbance ratio at A260/A280, respectively[Citation31].
Design of species-specific primers
Two sets of oligonucleotide primers specific to the cytb and stp6 genes of C. porosus were designed following a standardized procedure published in our earlier report. The primers used were summarized in . The specificity of the designed primers was ensured by three different testing systems. Firstly, the theoretical specificity of the similar and distant species was confirmed by online Basic Local Alignment Tool (BLAST) in NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Secondly, the total mismatches between the target and non-target species were determined in-silico by aligning the primers against 37 animal and plant species that included 13 true crocodile species, 14 terrestrial meat providing animals, 2 aquatic species, and 3 plant species using ClustalW multiple sequence alignment program (http://www.genome. jp/tools/clustalw/). Finally, real specificity was confirmed through a PCR assay against the total DNA templates of 18species. The primers were synthesized by Integrated DNA Technologies (IDT), Singapore, and supplied by First BASE Laboratories Sdn. Bhd., Selangor, Malaysia. To confirm the authenticity, all PCR products product (127 bp of cytb gene and 77 bp of atp6 gene) were sequenced as explained in the previous report.[Citation26]
Table 2. Sequences of Primers Used in This Study
Multiplex PCR for double gene targeted authentication
Simplex PCR was performed in 25 µl reaction volume containing 0.125 µl GoTaq Flexi DNA Polymerase (Promega, Madison, USA), 5 µl of 5x GoTaq Flexi Buffer, 0.2 mM of each dNTP, 2 mM MgCl2, 2 µM, 1 µM of 0.5 µM (20 ng/µl) primers for the each of atp6, cytb and 18s rRNA genes. For DNA template, 1 µl (20 ng/µl) of total DNA was used and the reaction was run in ABI 96 Well verity thermal cycler (Applied Biosystems, Foster City, CA). The cycling parameters involved initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 45 s; elongation at 72°C for 40 s and final elongation at 72°C for 5 min. The amplified PCR products were visualized first in 2% agarose gel stained with Florosafe DNA stain (1st base Laboratories, Selangor, Malaysia) using a gel documentation system (AlphaImager HP, California, USA). Afterwards, duplex and triplex PCR systems were optimized under the same PCR conditions that were used for simplex PCR, but the annealing time was extended to 1 min. Poor resolution of agarose gel for small oligos required separation and visualisation of mPCR products by QIAxcel Advanced Capillary Electrophoresis System (QIAGEN Hilden, Germany) with QX Size Marker 25 bp-500 bp v2.0.[Citation26]
Preparation of crocodile and chicken model meatballs
Pure meatballs were prepared according to[Citation33] with a balanced amount of grinded chicken and crocodile meat with cooking salt, garlic and other ingredients as shown in . To obtain crocodile-spiked chicken meatball, 1%, 0.5%, 0.1%, and 0.01% crocodile meat was added with 100 g of chicken meat in the formulation. The meats with all other ingredients were mixed well by vigorous blending and the emulsified homogenous meat mixtures were shaped into balls. To simulate cooking and extensive food processing effect, prepared raw meatballs were subjected to cooking at 100°C for 90 min, autoclaving at 120°C under 45-psi pressure for 2.5 h and subjected to microwave cooking for 2.5 h. All samples were prepared on three different days in triplicates and were stored at −20°C for DNA extraction.[Citation34]
Table 3. Ingredients used in meatball preparation.
Test for model chicken meatball, commercial chicken meatball, and TM products
The sensitivity of the assay was determined by extracting the DNA of spiked model chicken meatball with crocodile meat (10%, 1%, 0.5%, 0.1%, and 0.01%) under raw and extreme autoclaving (120°C at 45 psi for 2.5 h). Then, model chicken meatball was subjected to stability test by boiling at 98°C for 90 min; autoclaving for 2.5 h and microwave oven cooking at 700 W for 30 min. A total of three different brands of each commercial chicken meatball were cross-tested with crocodile-specific primer in triplicates. For TM products, 30 mg sample was used for DNA extraction.[Citation35]
Enzymatic digestion and RFLP analysis
The sequences of the amplified PCR products were retrieved from NCBI. Publicly available NEBcutter version 2.0 software (http://tools.neb.com/NEBcutter) was used to select the specific and appropriate restriction endonucleases for all the PCR amplicons prior to test the mPCR- RFLP assay to ensure distinctive RFLP patterns for all targets.[Citation35] The restriction patterns of the PCR amplicons of crocodile mitochondrial cytb and atp6 genes are given in .
Table 4. Restriction Digests of the PCR Products target
RFLP analysis of crocodile PCR products
The simplex PCR products of crocodile cytb and atp6 genes were digested with AciI restriction endonucleases (New England Biolabs, Ipswich, MA, United States), respectively. The total volume of each digestion reaction was 25 μL, which was composed of 1 μg of unpurified PCR product, 1× digestion buffer (supplied with the enzyme), 1U of each enzyme, and a balanced amount of sterilized distilled water. The reaction mixtures were gently mixed and spun down and incubated at 37°C with AciI in a shaking water bath for 20 min. Finally, the digestion reaction was stopped by heating the reaction mixtures at 65°C for 20 min. The duplex PCR products were digested simultaneously in a 25 μL reaction mixture containing 20 μL of unpurified PCR product, 2.5 μL of digestion buffer and 1.5 μL of AciI. The reaction was mixed by gentle shaking, spun down, and incubated in a shaking water bath first at 37°C for 20 min and then at 65°C for 20 min to stop the enzymatic digestion. The digests were separated in an automated QIAxcel Advanced Capillary Electrophoresis System (QIAGEN GmbH, Hilden, Germany) using a QIAxcel DNA High- Resolution Kit (QIAGEN GmbH, Hilden, Germany).
Results and discussion
Quality and quantity of extracted DNA
Total genomic DNA was obtained from pure and admixed meat products under raw, boiled, autoclaved, and microwaved processed meat samples. DNA was also collected from model and commercial meatballs and TM. To determine concentration and purity of the extracted DNA samples, an absorbance ratio of 1.7–2.0 at 260/280-nm was taken as standard because many earlier reports have used it as a benchmark for good quality DNA.[Citation27]
As expected, DNA extraction from most of the TMs was quite challenging. Out of the 21 TMs, three crocodile herbal soup and a dried meat powder yielded 169–431 ng.µL−1 DNA but they were needed to be soaked and boiled in water for 3 h. On the other hand, four herbal jellies yielded 10–15 ng.µL−1 DNA and other 13 TMs yielded 20–35 ng.µL−1 DNA. These differences could be attributed to differential processing and contents of TMs (herbal soup, herbal jelly and dried powder). However, very poor DNA yield from herbal jellies was probably because of the extensive degradation of DNA during manufacturing. Furthermore, TM preparations often involve decoction method to consume the essences of the materials and it makes the release of DNA from background matrices very difficult.[Citation35,Citation36]
Optimization of multiplex PCR-RFLP: specificity and sensitivity issues
In this research, targeting short length primers of mitochondrial cytb (127 bp) and atp6 (77 bp) genes were used as reported by.[Citation26] Number of mismatch nucleotides, pairwise distance, and phylogenetic tree were prudently evaluated against 37 species. Only one mismatch was found between C. porosus and C. siamensis whereas other Crocodylus and animal species recorded 9–77 nucleotide (7.08–60.63%) mismatches in cytb and 6–57 nucleotide (7.79 –74.03%) in atp6 targets. Pairwise distances were also computed, and the minimum distance (0.01) was observed with C. siamensis for both targets. The maximum distance of 2.13 was found in wheat (Triticum aestivum) and pig (Sus scrofa) species; while 0.93 in wheat (Triticum aestivum) and onion (Allium cepa) for cytb and atp6 targets correspondingly. Additionally, all crocodile species were clustered in their respective domains when the amplicon sequences were subjected to construction of phylogenetic trees and so it was highly unlikely that they would be cross-amplified by PCR.[Citation26] After passing through the theoretical screening, simplex PCR assay was optimized for each of the target genes of C. porosus. Subsequently, duplex and triplex PCR systems were optimized and the products were separated and visualized using a QIAxcel Advanced Capillary Electrophoresis System (QIAGEN Hilden, Germany) with QX Size Marker 25 bp-500 bp v2.0. Both in-silico and PCR analyses confirmed adequate genetic distances among the target and non-target species and so no cross specificity was detected. The originality was further confirmed by sequencing as documented earlier.[Citation26]
Literature searched revealed three multiplex PCR assays involving crocodile identification are available.[Citation17,Citation18,Citation20] However, those reports are based on very long amplicon-lengths (373 bp–2000 bp) that are not so stable under extensive processing of food, pharmaceuticals, and cosmetics.[Citation37] Additionally, none of these documents included real food or TM samples and so the reliability of those assays for foods and TMs screening applications cannot be confirmed. This gap was partially addressed in our recently published report[Citation26] wherein C. porosus was detected in raw, processed, and commercial chicken meatballs and some TMs. However, this report was not confirmatory and needed help from sequencing. Moreover, the number of real-life TM samples was only three, which were insufficient for drawing a statistical conclusion.
Authentication of amplified PCR products definitely increases the reliability of the assay, and PCR-RFLP method based on lab-on-a-chip technology has become a useful technique for the authentication of meat species such as cattle, buffalo, porcine,[Citation27] or fish.[Citation38] The identification of game or exotic meats by this technique has been reported for species such as rat, rabbit, and squirrel[Citation28] and dog, cat, rat, monkey and porcine.[Citation29] Other methods such as probe hybridisation or DNA sequencing can be used but those are expensive, laborious and need high-quality DNA which is quite unlikely for heat/chemical-treated DNA extracted from processed meats or meat products.[Citation39] In this regard, PCR-RFLP has been proven to be a practical, highly repeatable, and reliable technique for meat species identification in food and meat industry.[Citation40]
Recently double gene targeting PCR-RFLP assays were documented for the differentiation of buffalo, bovine and porcine.[Citation41] This mPCR-RFLP assay was self-explanatory and can confirm product authenticity using simple instrumentation.[Citation40] Therefore, RFLP analysis was performed for the double-gene targeting mPCR assay for C. porosus under various matrices.
Table 5. Analysis of admixed commercial chicken meat ball products with Crocodylus porosus PCR assay
In this study, the multiplex PCR products of crocodiles were digested simultaneously with AciI, and clear fingerprints were obtained for each of the two different targets (). First, each target was digested separately to study its individual restriction patterns in order to eliminate any ambiguities that may arise from the final multiplex PCR products. Lane 2 and Lane 4 in demonstrate two fragments of length 23 and 54 bp; and 44 and 83 bp, which resulted from AciI digestion of the PCR products of crocodile atp6 and cytb genes in separate tubes, respectively. Finally, the multiplex PCR products (lane 5) were subjected to RE digestion with AciI and this generated molecular fingerprints for all targets (atp6 and cytb) composed of a total of four fragments (23, 44, 54, and 83 bp) (lane 6). After the optimization of mPCR-RFLP assay under pure states, it was evaluated for the screening of model chicken meatball under raw, boiled, and autoclaved states. The limit of detection for mPCR-RFLP assay was 0.5% adulteration level ( and ). Model chicken meatballs were deliberately adulterated with crocodile meat, and their restriction digestion patterns were studied (). The digest of all samples clearly presented the signature fingerprints of 4 fragments (lanes 4–9), reflecting that variations in food processing treatments cannot affect the stability of any of the biomarkers developed in this study. In other words, this novel mPCR-RFLP assay was sensitive, reliable, and robust for the discriminatory detection of crocodile in processed foods.[Citation19] has carried out PCR-RFLP to discriminate C. porosus, C. palustris and C. gangeticus in fresh blood samples, nonetheless, the assay was not tested under food processing conditions. Previously, successful PCR-RFLP assay has been carried out in various food products, namely frankfurter,[Citation27,Citation42] meatballs,[Citation34] burgers[Citation29,Citation41,Citation43] for the detection of rabbit, rat, squirrel, beef, buffalo, pig, cat, dog, and monkey.
Figure 1. RFLP analysis of simplex (lanes 1 and 3) and mPCR (lane 5) products before (lanes 1, 3 and 5) and after (lanes 2, 4, and 6) restriction digestion with Aci1. In the gel image (a), lanes 1 and 2: atp6; lanes 3 and 4: cytb; lanes 5 and 6 (atp6 and cytb) of C. porosus before (77 and 127 bp) and after (23,54 bp and 44,83 bp) RE digestion. Lane M: DNA ladder. Lane 7: negative control. Corresponding electropherograms are shown with labels (b-d).
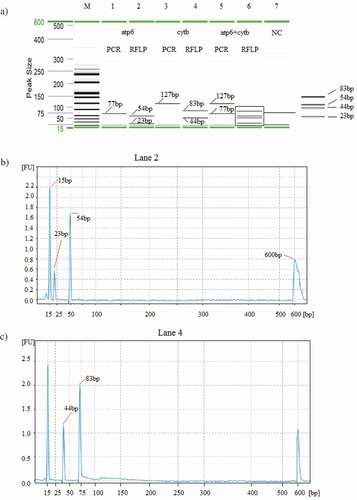
Figure 2. RFLP analysis of PCR products of crocodile-adulterated model chicken meatballs after restriction digestion with AciI. In the gel image a), lanes 1–3 are sensitivity test with 10% (Lane 1), 1% (Lane 2) and 0.5% (Lane 3). Lanes 4–9 are stability analysis of the crocodile-specific target DNA under boiling at 98°C for 90 min (Lanes 4–5), autoclaving at 121°C for 150 min under a pressure of 45 psi (lanes 6–7) and microwave cooking at 700 W for 30 min (Lanes 8–9). Lanes 4, 6, 8: Pure crocodile ball; Lanes 5, 7, 9: 10% crocodile meat spiked model chicken meatball. Lane M: DNA ladder. Lane 10 is negative control. Corresponding electropharograms are demonstrated with respective labels (b-c).
Earlier, we have scientifically proven that the stability of the PCR assay under extensive processing atmosphere largely depends on the amplicon sizes; longer targets break down before the shorter ones.[Citation26] This study carefully addressed this point and kept amplicon lengths of 77 bp and 127 bp; far shorter than previous amplicon sizes of 628 bp and 780 bp;[Citation18,Citation19] 373 bp, 486 bp, and 578 bp;[Citation20,Citation21] 1000 bp and 2000 bp[Citation17] and 600 bp and 690 bp.[Citation22] Moreover, double gene sites were used as targets for each species to complement a potential missing target. Therefore, this novel mPCR assay offered better reliability but equivalent or better sensitivity compared to those of other published reports.[Citation27]
Meatball analysis
In the food industry, the substitution of costly meats by a cheaper substitute is very common and it is frequently done to increase profit margin and survive in the competitive market.[Citation40] Therefore, model chicken meatballs were deliberately spiked with crocodile meat samples and were blindly screened using the mPCR assay, prior to the mPCR-RFLP assay described above ().
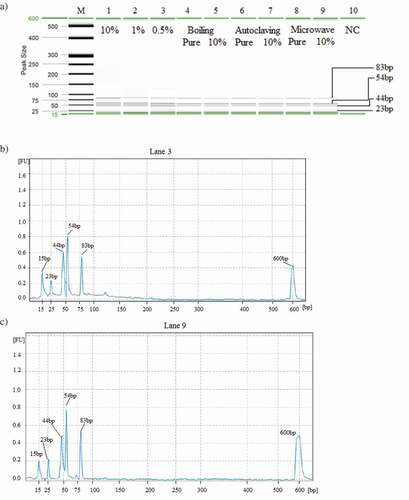
Figure 3. Sensitivity and stability analysis of model chicken meatball. In the gel image, lanes 1–5: Sensitivity analysis of PCR products from 10%, 1%, 0.5%, 0.1%, and 0.01% crocodile adulterated chicken meatball, respectively. Lanes 6–11: Stability analysis of the crocodile-specific target DNA under boiling at 98°C for 90 min (Lanes 6–7), autoclaving at 121°C for 150 min under a pressure of 45 psi (lane 8–9) and microwave cooking at 700 W for 30 min (Lanes 10–11). Lanes 6, 8, 10: Pure crocodile ball; Lanes 7, 9, 11: 10% crocodile meat spiked model chicken meatball. Lane M: DNA ladder. Lane 12: NC is negative control.
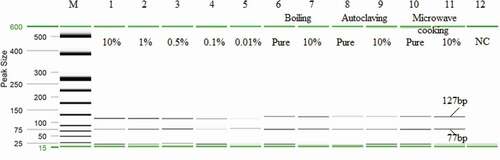
Three sets of model chicken meatball adulterated with crocodile were made following[Citation34] to test the sensitivity and to simulate the most likely forms of adulteration. This novel mPCR assay was first optimized and validated for sensitivity test in a very popular meat product, model chicken meatball, spiked with 10%, 1%, 0.5%, 0.1%, and 0.01% crocodile meat as shown in . Then, these model chicken meatball products were tested under raw and heat treated conditions to evaluate the reliability and accuracy of the method to demonstrate the common forms of adulteration in the commercial food industry.[Citation35]
Additionally, the 10% crocodile-adulterated model chicken meatball products were subjected to boiling at 98°C for 90 min, autoclaving for 120°C at 2.5 h under 45 psi and microwave oven cooking at 700 W for 30 min because these treatments were known to break down DNA (Asing et al., 2016; Hossain et al., 2016). To the best of our knowledge, no other studies have tested the double gene mPCR assay for crocodile under food processing conditions such as boiling, extreme autoclaving (2.5 h) and microwave cooking (700 W for 30 min). Previous research by Meganathan and co-workers[Citation21] reported sensitivity up to 10 pg in highly putrefied crocodile samples, tissues, and blood, but the sensitivity under the processed sample remained inconclusive. In this research, double gene targeted species in model meatballs were amplified at all levels of adulteration including the 10% autoclaved model chicken meatball samples () indicating a robust and reliable detection method.
Commercial meatball samples were also spiked with 0.1% crocodile meat and were used as positive control to simulate real-life matrices.[Citation30] A total of three different ‘halal’ branded chicken meatballs (A–C) were purchased in triplicates and tested on three different days (). While the crocodile meat-spiked model chicken meatballs PCR product was obtained from all positive controls, no commercial meatballs were found to be positive for crocodile DNA (result not shown), reflecting the absence of crocodile meat adulteration in chicken meatball formulations in Malaysia. The findings are significant in Malaysian prospect since the country is committed to develop a halal-hub industry and has been strictly monitoring the halal standard of marketed foods. Another reason might be the higher prices of crocodiles over chicken that makes the crocodile adulteration in chicken unlikely for profit-making purposes.
Analysis of traditional medicines
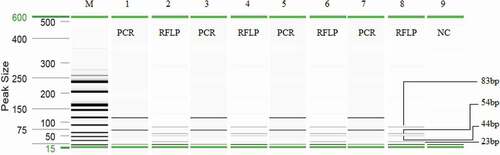
Over the recent years, the trades of the numerous TMs have drastically increased because of their purported multi-cure efficacies.[Citation44] The global alternative & complementary medicine market was valued at USD 40.32 billion in 2015.[Citation45] Since some TMs may be made from the parts of wild animals, it has brought a great threat to the existence of several wildlife species such as elephants, rhinos and tigers to pangolins, reptiles, fish, and rare birds and plants.[Citation1] The worldwide demands for TMs in increasing volumes, have put a great challenge in traffic control points such as airport officials in determining the biological origins and the consequent CITES status of animal ingredients contained in capsules, powders, liquids or tablets forms the final products; wherein their biological morphologies are drastically destroyed, bringing a failure to the currently available mass spectrometric, chromatographic, barcoding, and PCR techniques that rely on fragile long-length and unstable biological analytes.[Citation46] Therefore, we applied here the optimized multiplex PCR-RFLP method to screen DNA in 21 different TM products collected from various outlets across Malaysia including four C. porosus containing the product for validation purpose ().
The amplified PCR products from the crocodile positive samples were digested with AciI restriction endonuclease enzyme and distinctive RFLP fingerprints (23, 44, 54 and 83 bp) were obtained, identifying the C. porosus materials in TMs (). Recently,[Citation35] investigated 153 TM and found 40% (62/153) screened TMs contained undeclared Malayan box turtle. However, in the present case, all of four crocodile positive TM contained C. porosus and so no mislabelling was detected.
Figure 4. Analysis of traditional Chinese medicines. In the gel image, lanes 1, 3, 5, and 7 are PCR products from sample A, B, C, and D medicines before digestion and lanes 2, 4, 6 and 8 after digestion with AciI, respectively. Lane M: ladder DNA and lane 9: negative control.
Our main objective was to develop a convenient, economical, and applicable method for the authentication of C. porosus in food and traditional medicines. However, most often the forensic laboratories receive highly degraded samples for examination[Citation47] such as to determine animal ingredients contained in various forms of final products as described earlier in this report.[Citation46] Hence, the determination of species origin using these samples becomes a challenging task.[Citation48] Consequently, the current protocol proves efficient and economical without the further need of sequencing analysis, even in cases of highly degraded tissue samples.[Citation19]
Conclusion
Crocodylus porosus is one of 23 Crocodylus species, and despite being protected, it has been overexploited for its various organs, such as meat and skins, which are believed to have healing properties for uses in exotic food products and revitalizing TM. Hence, the population of this species has significantly declined, putting it at risk of extinction even though breeding programs are common nowadays due to laundering. Therefore, it is feared that continuous, high-volume exploitation might lead to a serious population decline in the wild, making the C. porosus extinct. The Malaysian government regulates the sale of this species in domestic or international markets, but the illegal trade is greatly suspected. Thus, there is a requirement for a dependable tracing method for C. porosus identification. This study has developed a multiplex PCR-RFLP assay having two different targets for the same species for the detection of crocodile under complex food matrices and TM. The targets were very short (77 bp and 127 bp) of mitochondrial cytb and atp6 origins and thus were very stable and available in multiple copies. The lower limit of detection (0.5%) was suitable for the analysis of processed foods such as meatballs and TMs. Although no mislabelling was detected when 21 TMs were screened, identification of the four products claimed to contain C. porosus DNA with distinctive AciI-restriction profiles, showed its real-life application in TM analysis. Thus, it might be practical and rationale to use this novel multiplex PCR-RFLP assay for any archaeological and forensic screening of C. porosus under any matrices with great reliability and confidence.
Acknowledgments
This study was supported by the University of Malaya Grant No. GC001A-14SBS and PG288-2016A to Md. Eaqub Ali. The authors would like to thank Wildlife and National Parks, Malaysia for the permission to carry out this research. All authors declare that they have contributed to this article and they do not have any conflict of interest to publish it in journal. The study did not involve any live animals and so ethical permission was not required.
Additional information
Funding
References
- Nellemann, C.; Henriksen, R.; Kreilhuber, A.; Stewart, D.; Kotsovou, M.; Raxter, P.; Mrema, E.; Barrat, S. The Rise of Environmental Crime – A Growing Threat to Natural Resources Peace, Development and Security. A UNEPINTERPOL Rapid Response Assessment. United Nations Environment Programme and RHIPTO Rapid Response–Norwegian Center for Global Analyses; 2016. www.rhipto.org
- Challander, D.W.S.; Harrop, S.R.; MacMillan, D.C. Towards Informed and Multi-Faceted Wildlife Trade Interventions. Global Ecology and Conservation 2015, 3, 129–148. DOI: 10.1016/j.gecco.2014.11.010.
- Rosen, G.E.; Smith, K.F. Summarizing the Evidence on the International Trade in Illegal Wildlife. EcoHealth 2010, 7, 24–32. DOI: 10.1007/s10393-010-0317-y.
- CITES. What Is CITES? https://cites.org/eng/disc/what.php ( accessed Feb 26th 2018)
- Brodmann, P.D.; Nicholas, G.; Schaltenbrand, P.; Ilg, E.C. Identifying Unknown Game Species: Experience with Nucleotide Sequencing of the Mitochondrial Cytochrome B Gene and a Sub- Sequent Basic Local Alignment Search Tool Search. European Food Research Technology 2001, 212(4), 491–496. DOI: 10.1007/s002170000284.
- Tensen, L. Under What Circumstances Can Wildlife Farming Benefit Species Conservation? Global Ecology and Conservation 2016, 6, 286–298. DOI: 10.1016/j.gecco.2016.03.007.
- Hutton, J.; Webb, G. Crocodiles: Legal Trade Snaps Back. In The Trade in Wildlife: Regulation for Conservation; Oldfield, S., Ed.; Earthscan Publications Ltd: London, 2003.
- Hoffman, L.C.; Cawthorn, D. Exotic Protein Sources to Meet All Needs. Meat Science 2013, 95(4), 764–771. DOI: 10.1016/j.meatsci.2013.04.027.
- Chaeychomsri, W.; Chaeychomsri, S.; Siruntawineti, J.; Hengsawadi, D.; Cuptapun, Y. Freeze-Dried Crocodile Blood Production as Food Supplement. Biologic Sciences Bioengineering 2009, 108(1), S22.
- Venter, T.; Fox, L.T.; Gerber, M.; Preez, J.L.; Van, Z.S.; Boneschans, B.; Plessis, J. Physical Stability and Clinical Efficacy of Crocodylus Niloticus Oil Lotion. Brazil Journal of Pharmacognosy 2016, 26(4), 521–529. DOI: 10.1016/j.bjp.2016.03.011.
- Phosri, S.; Mahakunakorn, P.; Lueangsakulthai, J.; Jangpromma, N.; Swatsitang, P.; Daduang, S.; Dhiravisit, A.; Thammasirirak, S. An Investigation of Antioxidant and Anti-Inflammatory Activities from Blood Components of Crocodile (Crocodylus Siamensis). The Protein Journal 2014, 33, 484–492. DOI: 10.1007/s10930-014-9581-y.
- Ziment, I.; Tashkin, D.P. Alternative Medicine for Allergy and Asthma Current Reviews of Allergy and Clinical Immunology. The Journal of Allergy and Clinical Immunology 2000, 106(4), 603–614. DOI: 10.1067/mai.2000.109432.
- Li, H.; Deng, Y.; Zhang, Z.; Fu, Q.; Zheng, Y.; Cao, X.; Nie, J.; Fu, L.; Chen, L.; Xiong, L.; Shen, D.; Chen, Q. Evaluation of Effectiveness in a Novel Wound Healing Ointment-Crocodile Oil Burn Ointment. African Journal of Traditional, Complementary, and Alternative Medicines 2017, 14(1), 62–72.
- Kozlowski, H.N.; Lai, E.T.L.; Havugimanac, P.C.; Whitec, C.; Emilic, A.; Sakac, D.; Binnington, B.; Neschadim, A.; McCarthy, S.D.S.; Brancha, D.R. Extracellular Histones Identified in Crocodile Blood Inhibit In-Vitro HIV-1 Infection. AIDS 2013, 30(13), 2043–2052. DOI: 10.1097/QAD.0000000000001159.
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guentherx, S.; Pasmansk, F.; Wagenaar, J.A.; Weese, J.S.; Wielerx, L.H.; Windahl, U.; Vanrompay, D.; Guardabassi, L. Bacterial Zoonoses Transmitted by Household Pets: State-of-the-Art and Future Perspectives for Targeted Research and Policy Actions. Journal Comparative Path 2016, 155, 27–40. DOI: 10.1016/j.jcpa.2015.03.004.
- MS 1500. Halal Food - Production, Preparation, Handling and Storage - General Guidelines (Second Revision). Standards Malaysia: Selangor; 2009.
- Unajak, S.; Meesawat, P.; Anyamaneeratch, K.; Anuwareepong, D.; Srikulnath, K.; Choowongkomon, K. Identification of Species (Meat and Blood Samples) Using nested-PCR Analysis of Mitochondrial DNA. African Journal of Biotechnology 2011, 10(29), 5670–5676.
- Meganathan, P.R.; Dubey, B.; Haque, I. Molecular Identification of Crocodile Species Using Novel Primers for Forensic Analysis. Conservation Genetics 2009a, 10(3), 767–770. DOI: 10.1007/s10592-008-9658-2.
- Meganathan, P.R.; Dubey, B.; Haque, I. Molecular Identification of Indian Crocodile Species: PCR-RFLP Method for Forensic Authentication. Journal of Forensic Sciences 2009b, 54, 1042–1045. DOI: 10.1111/j.1556-4029.2009.01119.x.
- Meganathan, P.R.; Dubey, B.; Jogayya, K.N.; Whitaker, N.; Haque, I. A Novel Multiplex PCR Assay for the Identification of Indian Crocodiles. Molecular Ecology Resources 2010, 10, 744–747. DOI: 10.1111/j.1755-0998.2009.02767.x.
- Meganathan, P.R.; Dubey, B.; Jogayya, K.N.; Haque, I. Validation of a Multiplex PCR Assay for the Forensic Identification of Indian Crocodiles. Journal of Forensic Sciences 2011, 56, 1241–1244. DOI: 10.1111/j.1556-4029.2011.01812.x.
- Jogayya, K.N.; Meganathan, P.R.; Dubey, B.; Haque, I. Mitochondrial 16S Ribosomal RNA Gene for Forensic Identification of Crocodile Species. Journal of Forensic and Legal Medicine 2013, 20, 334–338. DOI: 10.1016/j.jflm.2012.09.018.
- Mane, B.G.; Mendiratta, S.K.; Tiwari, A.K. Beef Specific Polymerase Chain Reaction Assay for Authentication of Meat and Meat Products. Food Control 2012, 28(2), 246–249. DOI: 10.1016/j.foodcont.2012.05.031.
- Herrero, B.; Royo, L.J.; Lago, F.C.; Vieites, J.M.; Espineira, M. Authentication of Male Beef by Multiplex Fast Real-Time PCR. Food Additives & Contaminants: Part A 2013, 30(2), 218–225. DOI: 10.1080/19440049.2012.740164.
- Girish, P.S.; Haunshi, S.; Vaithiyanathan, S.; Rajitha, R.; Ramakrishna, C. A Rapid Method for Authentication of Buffalo (Bubalus Bubalis) Meat by Alkaline Lysis Method of DNA Extraction and Species Specific Polymerase Chain Reaction. Journal of Food Science Technology 2013, 50(1), 141–146. DOI: 10.1007/s13197-011-0230-6.
- Nizar, N.N.A.; Ali, M.E.; Hossain, M.A.M.; Sultana, S.; Ahamad, M.N.U. Double Gene Targeting PCR Assay for the Detection of Crocodylus Porosus in Commercial Products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2018. DOI: 10.1080/19440049.2018.1440644.
- Hossain, M.A.M.; Ali, M.E.; Hamid, S.B.A.; Asing,; Mustafa, S.; Desa, M.N.M.; Zaidul, I.S.M. Targeting Double Genes in Multiplex PCR for Discriminating Bovine, Buffalo and Porcine Materials in Food Chain. Food Control Particle B 2016, 73, 175–184. DOI: 10.1016/j.foodcont.2016.08.008.
- Ali, M.E.; Ahamad, M.N.U.; Asing,; Hossain, M.A.M.; Sharmin, S. Multiplex Polymerase Chain Reaction-Restriction Fragment Length Polymorphism Assay Discriminates of Rabbit, Rat and Squirrel Meat in Frankfurter Products. Food Control 2018, 84, 148–158. DOI: 10.1016/j.foodcont.2017.07.030.
- Ali, M.E.; Amin, M.; Razzak, M.A.; Hamid, S.B.A.; Rahman, M.M.; Rashid, N.R.A.; Asing. Short Amplicon-Length PCR Assay Targeting Mitochondrial Cytochrome B Gene for the Detection of Feline Meats in Burger Formulation. Food Analytical Methods 2015, 9(3), 571–581. DOI: 10.1007/s12161-015-0237-0.
- Rashid, N.R.A.; Ali, M.E.; Hamid, S.B.A.; Rahman, M.M.; Razzak, M.A.; Asing. A Suitable Method for the Detection of A Potential Fraud of Bringing Macaque Monkey Meat into the Food Chain. Food Additives & Contaminants Part A: Chemistry, Analysis, Control, Exposure & Risk 2015, 32(7), 1013–1022. DOI: 10.1080/19440049.2015.1039073.
- Napolitano, F.; Annicchiarico, G.; Catillo, G.; Cris, A.; Grandoni, F.; Marchitelli, C. Identification of Ovis Aries Gelsolin Isoform B, a Candidate Gene for Milk Quality. Small Ruminant Research 2014, 116(1), 21–27. DOI: 10.1016/j.smallrumres.2013.10.007.
- Martín, I.; García, T.; Fajardo, V.; Rojas, M.; Pegels, N.; Hernández, P.E.; González, I.; Martín, R. SYBR-Green Real-Time PCR Approach for the Detection and Quantification of Pig DNA in Feedstuffs. Meat Science 2009, 2, 252–259. DOI: 10.1016/j.meatsci.2009.01.023.
- Rohman, A.; Sismindari,; Erwanto, Y.; Che Man, Y.B. Analysis of Pork Adulteration in Beef Meatball Using Fourier Transform Infrared (FTIR) Spectroscopy. Meat Science 2011, 88(1), 91–95. DOI: 10.1016/j.meatsci.2010.12.033.
- Rahman, M.M.; Ali, M.E.; Hamid, S.B.A.; Mustafa, S.; Hashim, U.; Hanapi, U.K. Polymerase Chain Reaction Assay Targeting Cytochrome B Gene for the Detection of Dog Meat Adulteration in Meatball Formulation. Meat Science 2014, 97, 404–409. DOI: 10.1016/j.meatsci.2014.03.011.
- Asing,; Ali, M.E.; Hamid, S.B.A.; Hossain, M.A.M.; Mustafa, S.; Kader, M.A.; Zaidul, I.S.M. Lab-on-a-Chip-Based PCR-RFLP Assay for the Detection of Malayan Box Turtle (Cuora Amboinensis) in the Food Chain and Traditional Chinese Medicines. PloS One 2016, 11(10), 1–27. DOI: 10.1371/journal.pone.0163436.
- Jiang, L.L.; Lo, Y.T.; Chen, W.T.; Shaw, P.C. DNA Authentication of Animal-Derived Concentrated Chinese Medicine Granules. Journal of Pharmaceutical and Biomedical Analysis 2016, 129, 398–404. DOI: 10.1016/j.jpba.2016.07.030.
- Focke, F.; Haase, I.; Fischer, M. DNA-based Identification of Spices: DNA Isolation, Whole Genome Amplification, and Polymerase Chain Reaction. Journal of Agricultural and Food Chemistry 2011, 59, 513−520. DOI: 10.1021/jf103702s.
- Sultana, S.; Ali, M.E.; Hossain, M.A.M.; Asing,; Naquiah, N.; Zaidul, I.S.M. Universal Mini COI Barcode for the Identification of Fish Species in Processed Products. Food Research International 2018, 105, 19–28. DOI: 10.1016/j.foodres.2017.10.065.
- Mafra, I.; Ferreira, I.M.P.L.V.O.; Beatriz, M.; Oliveira, P.P. Food Authentication by PCR-based Methods. European Food Researcher Technological 2008, 227, 649–665. DOI: 10.1007/s00217-007-0782-x.
- Haider, N.; Nabulsi, I.; Al-Safadi, B. Identification of Meat Species by PCR- RFLP of the Mitochondrial COI Gene. Meat Science 2012, 90, 490–493. DOI: 10.1016/j.meatsci.2011.09.013.
- Hossain, M.A.M.; Ali, M.E.; Hamid, S.B.A.; Hossain, S.M.A.; Asing,; Nizar, N.A.N.; Mohammad, N.U.; Ali, L.; Asaduzzamand, M.; Akanda, M.J.H. Tetraplex PCR Assay Involving Double Gene-Sites Discriminates Beef and Buffalo in Malaysian Meat Curry and Burger Products. Food Chemistry 2017, 224, 97–104. DOI: 10.1016/j.foodchem.2016.12.062.
- Ahamad, M.N.U.; Ali, M.E.; Hossain, M.A.M.; Asing,; Sultana, S.; Jahurul, M.H.A. Multiplex PCR Assay Discriminates Rabbit, Rat and Squirrel Meat in Food Chain. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2017, 34(12), 2043–2057. DOI: 10.1080/19440049.2017.1359752.
- Rahman, M.M.; Ali, M.E.; Hamid, S.B.A.; Bhassu, S.; Mustafa, S.; Al-Amin, M.; Abdur- Razzak, M. Lab-on-a-Chip PCR-RFLP Assay for the Detection of Canine DNA. Food Analysis Methods 2015, 8(6), 1598–1606. DOI: 10.1007/s12161-015-0090-1.
- Still, J. Use of Animal Products in Traditional Chinese Medicine: Environmental Impact and Health Hazards. Complementary Therapies in Medicine 2003, 11, 118–122. DOI: 10.1016/S0965-2299(03)00055-4.
- Grand View Research. Alternative and complementary medicine market analysis by intervention (Botanicals, acupuncture, mind, body and yoga, magnetic intervention), by distribution method, and segment forecasts, 2013–2025; 2017
- Coghlan, M.L.; Haile, J.; Houston, J.; Murray, D.C.; White, N.E.; Moolhuijzen, P.; Bellgard, M.I.; Bunce, M. Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns. PLoS Genetics 2012, 8(4), e1002657. DOI: 10.1371/journal.pgen.1002657.
- Bellis, C.; Ashton, K.J.; Freney, L.; Blair, B.; Griffiths, L.R. A Molecular Genetic Approach for Forensic Animal Species Identification. Forensic Science International 2003, 134, 99–108.
- Marshall, H.D.; Johnstone, K.A.; Carr, S.M. Species-Specific Oligonucleotide and Multiplex PCR for the Forensic Discrimination of Two Species of Scallops, Placopecten Magellanicus and Chlamys Islandica. Forensic Science International 2007, 167, 1–7. DOI: 10.1016/j.forsciint.2006.05.043.
