 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present work aimed to compare the main ergogenic attributes of two commercialized stages (young and mature) of coconut water (CW) obtained from four coconut varieties. The changes of electrolytes and sugars in CW upon maturation were quantified by inductively coupled plasma mass spectrophotometer and high-performance liquid chromatography, respectively. Based on the electrolyte profiling, potassium yielded the highest amount (ranging from 237.41 to 361.20 mg/100 mL) followed by sodium, magnesium, calcium, iron, manganese, copper, selenium, and zinc across all the maturity stages tested. For sugars, there were lower amounts of fructose and glucose, but a higher amount of sucrose with the maturation of the fruits. In conclusion, the amount of beneficial nutrients in the form of sugars and minerals was higher than that of young CW, and the ergogenic attributes of mature CW especially from MATAG variety (M-MATAG) were the best to be exploited further in the development of natural energy drinks.
Introduction
Coconut (Cocos nucifera L.) has been described as one of the most important crop commodities, especially in the tropical countries. The largest producers of coconut are Indonesia, Philippines, and India, which contribute up to 75% of the world’s coconut production. In Malaysia, coconut is the fourth most vital industrial crop based on the total planted area, and its plantation is one of the oldest agro-based industries in the country.[Citation1] Among the many varieties of coconut planted in Malaysia, the varieties such as Aromatic Dwarf (PDN), Malayan Yellow Dwarf (MYD), Malayan Yellow Dwarf × Tagnanan Tall (MATAG), and Malayan Yellow Dwarf × West African Tall (MAWA) are the most popular among the coconut planters as they possess better economic values. At present, the MATAG variety is the most commercialized variety planted in Malaysia followed by PDN and MAWA.[Citation2] Meanwhile, MYD is more favourable among smallholders or individual planters because of its shorter stature, easy to manage, and can be planted in smaller area.[Citation3]
Coconuts are commercialized at two maturity stages, namely, young and mature. The young coconuts aged from six to eight months following the formation of the endocarp are often harvested for beverage when the maximum volume of coconut water (CW) is reached.[Citation4] The CW from young coconuts is not only refreshing and delicious, but also contains many beneficial nutrients such as minerals, sugars, vitamins, and inorganic ions, which contribute to its therapeutic value.[Citation5,Citation6] The potential of young CW as an oral rehydration fluid has been studied for elderly people and children with gastroenteritis.[Citation7] It has also been reported that CW can be used by diarrhoea patients to substitute for the loss of fluid from the gastrointestinal tract. Moreover, the unique feature of CW, which resembles the intracellular fluid, makes the juice a suitable selection for a natural rehydration beverage.[Citation8–Citation10] On the other hand, mature coconuts are formed between 11 and 13 months following the formation of the endocarp. They are often harvested for their albumin or flesh which is usually further processed into coconut milk, coconut oil, coconut cake, and coconut flakes.[Citation11,Citation12] However, CW from mature coconuts is underutilized where in most cases the water is discarded. Nevertheless, the CW from mature coconuts is still drinkable and possesses many functional electrolytes and sugars, which can be beneficial to the consumers.[Citation6]
Ergogenic aids can be defined as anything that enhances the use of energy through energy production, efficiency, and control. Examples of ergogenic aids are sugars and essential minerals (i.e., potassium, sodium, calcium, chromium, zinc, magnesium, and selenium) which are involved in many metabolic reactions in the human body.[Citation9,Citation13,Citation14] Among the population groups, athletes are the ones who normally consume ergogenic aids to enhance their physical performances.[Citation15] In fulfilling such needs, majority of artificial sports drinks are added with vitamins, fructose, electrolytes such as potassium and sodium, amino acids, and caffeine,[Citation16] which is not preferred by consumers over the natural products. Nowadays, people are becoming more aware of the importance of natural-based ingredients incorporated into the sports drinks. Subsequently, many manufacturers are searching for natural substitutes in their sports drinks formulations, and CW is becoming a popular alternative. In addition to the high mineral contents, adequate total sugars and good taste, CW is preferred as an ergogenic aid because it is convenient as it can be easily obtained from tropical regions.[Citation5,Citation17,Citation18] The present work is therefore aimed to compare the ergogenic attributes of two commercialized stages (i.e., young and mature) of CW from popular coconut varieties (PDN, MYD, MATAG, and MAWA) grown in Malaysia.
Materials and methods
Sample collection
Coconut samples were provided by the Malaysian Agricultural Research and Development Institute (MARDI), Bagan Datuk Station, Malaysia. A total of 48 young and mature coconut samples from four varieties (PDN, MYD, MATAG, and MAWA) were used in the present work. The maturity of the coconut samples was indicated by the bunch number and confirmed by observing the flesh thickness. The counting of bunch number was made from top to bottom with the first bunch number selected during the anthesis stage of coconut maturation.[Citation19] The selection method was recommended by the researchers from MARDI in determining the correct maturity of coconut. The varieties, maturities, and bunch number of the selected coconuts are listed in .
Table 1. Selection of coconut varieties and maturities.
Chemicals and reagents
Standard buffers of pH 4, 7, and 9 (Metrohm Ltd.) used for calibration were obtained from Perkin Elmer (USA). Sugar standards used for high-performance liquid chromatography (HPLC), namely, D-(–)-fructose, D-(+)- glucose, and sucrose were purchased from Sigma (USA). Methanol (analytical grade), acetonitrile (HPLC grade), and nitric acid were purchased from Merck (Germany). Water was purified with Milli-Q water purification system (ELGA LabWater, USA).
Quality assessment of coconut water
Fresh CW was manually drained off the fruits by using a stainless steel cleaver. The CW was filtered through a sterilized muslin cloth to remove solid particles or contaminants. The CW was then subjected to the following measurements: (i) pH by using a pH meter (Jenway, UK), (ii) total soluble solids by using a digital refractometer (Atago®, USA), (iii) volume by using measuring cylinders, (iv) percentage volume by dividing weight of nuts (g) with volume (mL) × 100%, and (v) colour (L*, a*, b*) using a calorimeter (Hunter Lab Ultra Scan PRO, USA).
Determination of electrolytes by ICP-MS
The electrolytes content of CW were determined using inductively coupled plasma mass spectrophotometer (ICP-MS) according to the modified method by Marie and co-workers.[Citation20] All glassware and centrifuge tubes used were treated with 15% nitric aci, and oven-dried at 60°C (Memmert, USA) for 24 h prior to the analysis. The CW samples were diluted with Milli-Q water at a ratio of 1:2000 to determine sodium, potassium, calcium, and magnesium. The dilution of 1:200 was used to determine iron. Undiluted sample was used for manganese, copper, selenium, and zinc. Following dilution, samples were filtered through 0.45 µm nylon filter. Calibration curve for each element was prepared in the range of 0.01–0.20 µg/mL. The CW samples and standards were subjected to ICP-MS (Perkin Elmer, USA) for the quantification of electrolytes in CW with the following conditions: peak processing mode: average; signal profile processing mode: average; detector mode: dual; dead time (ns): 55; calibration type: external calibration; number of replicates; 3.
Quantification of sugars by HPLC
Sugars in CW samples were quantified using a normal-phase HPLC according to the modified method by Lee and Coates.[Citation21] All CW samples were filtered through 0.45 µm nylon filter. A total of 20 µL sample was injected into PhenoSpehere 5 µm NH2 80A LC Column 250 × 4.6 mm (Phenomenex, USA) attached to the Waters 600 HPLC instrument system (Waters, USA) using a refractive index (Waters 410 Differential Refractometer). The HPLC analysis was performed in an isocratic elution for 20 min in a mobile phase of acetonitrile:deionized water (80:20, v/v) with a constant flow rate at 1.5 mL/min. Sugar (fructose, glucose, sucrose) standards were prepared in concentrations ranging from 0.25% to 2.50% (w/v) after which a calibration curve for each sugar was plotted.
Statistical analysis
Analyses were performed using two independent duplications with triplicate readings for each analysis. Statistical analysis was performed using Minitab 16 with Tukey Test in which p < 0.05 was accepted as significant.
Results and discussion
Quality assessment of coconut water
During maturation, physical properties and composition of CW undergo certain changes. It is therefore crucial to monitor these changes as the information is useful to indicate the quality indicator of CW. tabulates the quality attributes of CW of different coconut varieties and maturities.
Table 2. Quality attributes of coconut water from different varieties and maturities.
One of the quality parameters in CW is pH, since changes in pH will not only affect the consumers’ acceptability of CW but also determine the spoilage potential of the CW. shows that the pHs of CW significantly increased (p < 0.05) with maturation of the fruits in all varieties tested except for MAWA. The highest increment was detected in MATAG (from pH 5.36 to 6.33). Meanwhile, Y-MAWA yielded the lowest pH (5.32 ± 0.05) among all samples. Overall, pHs of CW samples tested were slightly acidic which was in agreement with previous studies.[Citation10,Citation22]
Colour is another vital parameter in indicating the quality of CW since it is the physical property that is immediately perceived by consumers prior to purchase. In the present work, the samples were analysed for changes in lightness and colour spectrum (L*, a*, b*) during maturation. Across all the maturities and varieties tested, M-PDN yielded the highest clarity which was measured at 91.46 ± 0.18 as shown in with all CW samples exhibiting L* values (indicate clarity) of lower than 100. In contrast, the L* value of Indonesian CW has been reported to be greater than 100 due to its high clarity. It has been claimed that the high clarity and lightness in CW provides benefit for future storage.[Citation10] reveals that all CW samples tested exhibited negative a* and b* values which indicate that CW from all varieties possessed greenish and bluish colour. In another study by Kailaku and colleagues, Indonesian variety of Genjah Salak had bluish spectrum while Dalam Pangandaran and MAWA showed greenish spectrum.[Citation10] The slight differences in colour intensity detected in the present work as compared to the previous study might be due to the different coconut varieties used, apart from other factors such as different plantation location, agricultural practices, and rainfall distribution.[Citation10] In addition, the colour of CW might also vary depending on the maturity, storage conditions, and botanical origin.[Citation23]
Another important parameter for CW intended for commercialization is its volume. shows that Y-MATAG yielded the highest volume (435.00 ± 77.91 mL) followed by M-MATAG (391.67 ± 52.98 mL) and Y-PDN (324.83 ± 53.95 mL). As expected, samples of all tested varieties yielded higher CW volume at young stage as compared to mature stage. It is proposed that throughout maturation, the water in coconut will be used for the formation of flesh; therefore, the volume of CW will decrease as the coconut matures.[Citation24] Since all the young coconut samples were harvested at the stage when the coconut flesh was developing, a similar decreasing pattern of CW volume was observed in all samples. PDN was measured to have the highest volume decrement (−137.66 mL). Previously, Yong and co-workers[Citation6] discovered that the flesh of coconut started to form thin jelly inside the coconut endosperm during the fifth month of maturation. As the nuts developed, mature coconuts increased their water-holding capacity until a jelly was formed inside the fruit cavity by the kernel. As a result of this, the volume of water began to decrease as it was continually utilized by the fruit to form the kernel.[Citation25]
In a study by Kailaku and co-workers, volume of CW from mature Indonesian varieties ranged from 330.0 to 632.5 mL in which the Indonesian MAWA (330.0 mL) yielded higher volume than Y-MAWA (249.17 mL) measured in the present work which might be due to the drought season in Malaysia during the time when the coconut samples were harvested.[Citation10] This inference was supported by Solangi and Iqbal who claimed that the volume of CW could be significantly affected by drought.[Citation5] As shown in , the highest percent volume of CW was found in M-MATAG (24.59 ± 3.62%). It has been reported that CW accounted for 15–30% of the nut’s weight at full maturity.[Citation24]
Sweetness and sugars in coconut water
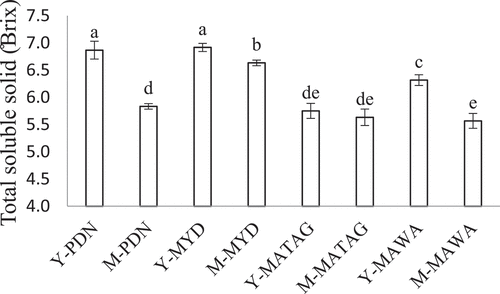
The natural sweetness of CW increases the consumers’ desire to voluntarily drink sufficient fluid.[Citation10] The sweetness level of CW can be indicated by the amount of total soluble solid (TSS). As shown in , the TSS of CW samples of all varieties (except for MAWA) significantly decreased (p < 0.05) with the maturation of the coconuts, which is in agreement with Jackson and his co-workers.[Citation25] In the present work, the highest TSS was measured in Y-MYD (6.92 ± 0.08°) and the lowest in M-MAWA (5.57 ± 0.14°). It has been reported that the level of natural sweetness in CW is mainly due to the presence of sugars which account for approximately 3–7% of the TSS.[Citation11,Citation22,Citation26]
Figure 1. Total soluble solid in coconut water samples from coconuts of different varieties and maturities. The data was expressed as mean ± SD (n = 6). Values that do not share the same superscript represent significant difference (p < 0.05).
Aside from contributing to the sweet taste of CW, sugars are also important as a source of ergogenic aid since it is the main source of energy for humans. Sugars are required for the immediate glycogen restoration and energy replenishment when people perform intense activities.[Citation10] In the present work, the sugar contents in CW samples were quantified using HPLC to determine the amount of glucose, fructose, and sucrose, and the results are depicted in .
Figure 2. Trend of sugars (A: fructose, B: glucose, C: sucrose) in coconut water samples from coconuts of different varieties and maturities. The data was expressed as mean ± SD (n = 6). Values that do not share the same superscript represent significant difference (p < 0.05).
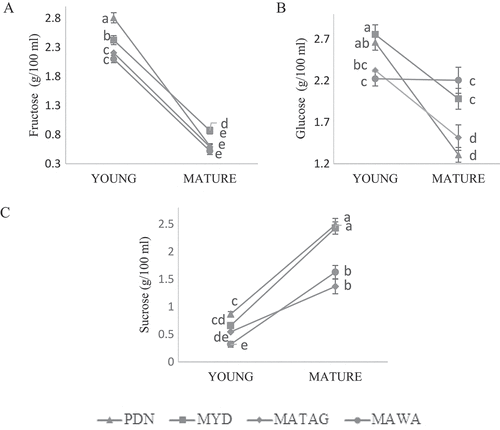
Result for fructose in CW from all varieties showed a significant (p < 0.05) decreasing trend with maturation of coconuts in which Y-MYD yielded the highest fructose (2.81 ± 0.09 g/100 mL) and M-MAWA the lowest (0.52 ± 0.06 g/100 mL). Similar to fructose, glucose was also measured to be significantly decreased (p < 0.05) in CW throughout the maturation of coconuts. The highest amount of glucose was measured in Y-PDN (2.76 ± 0.12 g/100 mL) and the lowest glucose was in M-MYD (1.31 ± 0.09 g/100 mL), respectively. In contrast with fructose and glucose, the amount of sucrose significantly increased (p < 0.05) in CW samples from all varieties with the maturation of coconuts (). From the results, M-MYD was measured to yield the highest sucrose (2.49 ± 0.11 g/100 mL) while Y-MATAG the lowest (0.54 ± 0.11 g/100 mL), respectively. In plants, a highly soluble disaccharide of sucrose functions as a carrier to provide a mobile energy source to the plant cells. Therefore, as the coconuts mature, the absorption and metabolism of sucrose have been reported to be faster than fructose and glucose in order to supply sufficient energy to the developing seedling within the fruit.[Citation27] In addition, invertase enzyme is high in young fruits to retain the osmotic pressure of cells by hydrolyzing sucrose into fructose and glucose to control sucrose accumulation during the fruit development.[Citation28] The present work is in agreement with previous studies were in agreement with the present work in which the increase in sucrose was accompanied by the decrease in glucose and fructose with the maturation of coconuts.[Citation5,Citation24,Citation25]
Ergogenic electrolytes in coconut water
Electrolytes are charged minerals required in small quantity (micronutrients) which play important functions in enhancing the energy production in humans. Since our body is unable to produce electrolytes, human obtain the essential electrolytes from foods. These electrolytes will eventually be lost through sweating and urinating.[Citation29] Aside from humans, plants also require micronutrients for growth.[Citation30] Minerals are absorbed by roots from soil in an ionic form and transferred via xylem to plant parts by respective transport proteins. The uptake of positive macronutrients into plants such as potassium, sodium, magnesium, and calcium are commonly needed for passive transport systems (ion channel).[Citation31] shows the amount of minerals in CW samples of different varieties and maturities.
Table 3. Electrolytes in coconut water from different varieties and maturities as detected by ICP-MS.
Based on , potassium was found to be the highest among the measured minerals in CW samples (216.8–361.2 mg/100 mL) with M-MATAG yielded the highest (361.2 mg/100 mL). As maturity increased, the amount of potassium was discovered to significantly increase (p < 0.05) as well in all varieties except for MYD. Sodium was the second highest mineral present in CW samples (7.31–58.59 mg/100 mL) with the highest was found in Y-PDN. High concentrations of potassium and sodium in CW samples at both maturity stages signify their hydration ability in replacing the major electrolytes within human extracellular fluid, which are mainly lost through sweat while performing intense exercise or activities.[Citation31,Citation32] In addition to potassium and sodium, other major minerals such as calcium and magnesium were also detected in CW samples. From , it is apparent that both calcium and magnesium significantly increased (p < 0.05) throughout the maturation of coconuts of all the varieties tested except for MYD. Calcium was measured to range between 1.48 and 40.64 mg/100 mL and magnesium between 0.09 and 21.96 mg/100 mL. In particular, the CW from Y-MYD yielded the highest amount of magnesium (21.96 ± 0.50 mg/100 mL) and calcium (40.64 mg/100 mL). The amount of magnesium found in the present work is in agreement with a study by Kailaku and co-workers at 2.4321.77 mg/100 mL.[Citation10]
In addition to macro minerals, the CW was also found to have ergogenic trace minerals such as manganese, iron, zinc, copper, and selenium (). As the coconuts matured, a significant (p < 0.05) increase in iron was observed in MAWA with M-MAWA yielded the highest amount (0.51 ± 0.02 mg/100 mL). Priya and Ramaswamy also reported the approximately similar value of iron in CW (0.5 mg/100 mL).[Citation24] In addition to iron, the amount of manganese also significantly increased (p < 0.05) in all varieties with the maturation of coconuts except for MATAG (). The highest amount of manganese was found in M-MAWA (565.80 ± 6.59 µg/100 mL) and the lowest in M-MYD (80.77 ± 0.39 μg/100 mL). In a study conducted by Kwiatkowski and co-authors (2008), they discovered that manganese in different maturities of Malaysian Green Dwarf (MGD) variety ranged between 20 and 197 µg/100 mL.[Citation33] They also discovered that as the coconut matured, the amount of manganese increased which is comparable to that of our findings.
Zinc is another important trace mineral found in CW. In general, the presence of zinc helps to maintain growth and is vital for metabolism in body including structural, regulatory and catalytic functions involved in the immune system.[Citation29] It is apparent from that the highest zinc was detected in Y-MYD (29.43 ± 0.84 µg/100 mL) while the lowest was in M-MAWA (9.37 ± 6.94 µg/100 mL). The amount of zinc reported by Kwiatskoski and authors[Citation33] in CW at different maturities of MGD ranged from 12 to 39 µg/100 mL and was slightly higher than that found in the present work (0.00–29.43 µg/100 mL). In the present work, copper significantly increased (p < 0.05) in CW samples from all varieties with the maturation of coconuts in which the highest amount was observed in M-MYD (28.93 ± 0.14 µg/100 mL). Chuku and Kalagbor (2014) however reported a lower range of copper in CW (0.2–1.3 µg/100 mL) from Nigerian coconut varieties.[Citation34] Selenium was detected to significantly increase (p < 0.05) only in MATAG and MAWA and significantly decreased (p < 0.05) in PDN as the coconuts matured (). The highest amount of selenium was found in M-MAWA (1.38 ± 0.02 µg/100 mL) and the lowest in M-MYD (0.46 ± 0.02 µg/100 mL). As compared to our results, CW from Brazilian coconut varieties yielded a slightly higher range of selenium (0.65–2.10 µg/100 mL).[Citation35]
In the present work, the highest potassium content in CW samples from all varieties and maturities is in agreement with that of previous studies.[Citation5,Citation7] Majority of the tested electrolytes showed an increasing trend (i.e., potassium, magnesium, calcium, iron, and copper) upon maturation, while others exhibited a decreasing trend (i.e., manganese and zinc). However, both sodium and selenium showed fluctuation throughout the maturation of coconuts. From the electrolytes result, CW from M-MATAG was chosen as the best sample for ergogenic properties due to the high amount of potassium and sodium in the juice. Sulaiman and his co-workers[Citation36] asserted that the amount of electrolytes in fruits is influenced by the fruit cultivars, and conditions of the plantation area such as climate, agricultural practices, soil, and water quality for irrigation. This is further supported by Maathius and Diatloff saying that minerals are naturally present in a small concentration in soil, and their quantity fluctuates depending on the environmental factors such as physicochemical properties (i.e., soil type, pH) and also climate (i.e., temperature, wind).[Citation37]
Comparison of coconut water with commercialized ergogenic drinks
The important criteria for the development of an ergogenic drink include water, sugars, and electrolyte content.[Citation38] Since the characteristics of CW tested in the present work is close to isotonic drink rather than energy drink based on the electrolyte content, a comparison of nutrients in CW with different brands of commercialized isotonic drinks was conducted. In addition, a comparison study was also performed with a current standard for oral rehydration therapy, namely, oral rehydration solution (ORS) and the data are presented in . The ORS is a standard for hydration drink developed by World Health Organization and United Nations Children’s Fund (UNICEF) for people to consume in treating dehydration. It is apparent that sodium in CW from M-MATAG (23.71 mg/100 mL) is comparable to that of commercialized isotonic drinks (40.0–69.1 mg/100 mL). Isotonic drink from Brand D contains calcium (17.3 mg/100 mL) and magnesium (7.5 mg/100 mL) which is very similar to CW from M-MATAG (calcium: 15.88 mg/100 mL; magnesium: 15.10 mg/100 mL). In addition, glucose content in CW from M-MATAG (1.51 mg/100 mL) is also close to ORS (2.0 mg/100 ml) as shown in . Overall, the amount of ergogenic related compounds in CW is comparable with the tested commercialized isotonic drinks and ORS.
Table 4. Comparison of electrolytes in young and mature CW, commercialized isotonic drinks, and ORS.
Conclusion
The present work evaluates the ergogenic attributes of CW from different varieties (PDN, MYD, MATAG, and MAWA) and maturities (young and mature) by comparing the physical attributes, functional electrolytes, and sugar contents. The physical results revealed that pH of CW increased while the TSS and volume of CW decreased as the coconut matured. The high amount of potassium makes CW an ideal natural hydration beverage which can be an excellent alternative for commercialized isotonic drinks. Furthermore, it has also been demonstrated that mature CW possessed higher ergogenic properties as compared to young CW due to its high content of functional electrolytes. Based on the comparison with other commercialized isotonic drinks, mature CW was found to contain comparable amounts of sodium, calcium, and magnesium, with the amount of glucose very similar to that of ORS. These findings suggest that mature CW, which is equipped with many functional electrolytes and adequate sugars, can be an excellent alternative for ergogenic drinks available in the markets. Overall, CW from M-MATAG would be the best candidate to be used in the development of a natural ergogenic drink due to its high content of potassium and sodium. The M-MATAG also possessed a high volume of water, which further added its economic value.
Acknowledgments
This research was financially supported by grants from the Malaysian Ministry of Agriculture (MOA) and Putra Grant (IPS: No. 9518300) from Universiti Putra Malaysia (UPM). The authors would like to thank MARDI (Bagan Datuk Station) for providing the coconut samples and information pertaining to their maturities and varieties.
Additional information
Funding
References
- Alyaqoubi, S.; Abdullah, A.; Samudi, M.; Abdullah, N.; Addai, Z.R.; Musa, K.H. Study of Antioxidant Activity and Physicochemical Properties of Coconut Milk (Pati Santan) in Malaysia. Journal of Chemical and Pharmaceutical Research. 2015, 7, 967–973.
- Taufik, A.M.; Akhir, H.M. Performance Evaluation of Coconut Dehusking Machine. Journal of Tropical Agriculture and Food Science. 2014, 42, 183–190.
- Said, I.; Abdul Rashid, S. Plant Material Booklet: Palms of Malaysia; Penerbit Universiti Teknologi Malaysia: Malaysia, 2001; pp 8.
- Yahya, S.; Zainal, I.M. Design and Performance of Young Coconut Shaping Machine. Journal of Tropical Agriculture and Food Science. 2014, 42, 19–28.
- Solangi, A.H.; Iqbal, M.Z. Chemical Composition of Meat (Kernel) and Nut Water of Major Coconut (Cocos Nucifera L.). Cultivars at Coastal Area of Pakistan. Pakistan Journal of Botany. 2011, 43, 357–363.
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos Nucifera L.). Water. Molecules. 2009, 14, 5144–5151. DOI: 10.3390/molecules14125144.
- Manjunatha, S.S.; Raju, P.S. Modelling the Rheological Behaviour of Tender Coconut (Cocos Nucifera L.). Water and Its Concentrates. International Food Research Journal. 2013, 20, 731–743.
- Khan, M.N.; Rehman, M.U.; Khan, K.W. A Study of Chemical Composition of Cocos Nucifera L. (Coconut) Water and Its Usefulness as Rehydration Fluid. Pakistan Journal of Botany. 2003, 35, 925–930.
- Campbell, B.I.; La Bounty, P.M.; Roberts, M. The Ergogenic Potential of Arginine. Journal of the International Society of Sports Nutrition. 2004, 1, 35–38. DOI: 10.1186/1550-2783-1-2-35.
- Kailaku, S.I.; Syah, A.N.A.; Risfaheri, R.; Setiawan, B.; Sulaeman, A. Carbohydrate-Electrolyte Characteristics of Coconut Water from Different Varieties and Its Potential as Natural Isotonic Drink. International Journal on Advanced Science, Engineering and Information Technology. 2015, 3, 174–177. DOI: 10.18517/ijaseit.5.3.515.
- Tan, T.C.; Cheng, L.H.; Bhat, R.; Rusul, G.; Easa, A.M. Composition, Physicochemical Properties and Thermal Inactivation Kinetics of Polyphenol Oxidase and Peroxidase from Coconut (Cocos Nucifera) Water Obtained from Immature, Mature and Overly-Mature Coconut. Food Chemistry. 2014, 142, 121–128. DOI: 10.1016/j.foodchem.2013.07.040.
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.P. Coconut Water Uses, Composition and Properties: A Review. Fruits. 2012, 67, 87–107. DOI: 10.1051/fruits/2012002.
- Volpe, S.L.;. Minerals as Ergogenic Aids. Current Sports Medicine Reports. 2008, 74, 224–229. DOI: 10.1249/JSR.0b013e31817ed0e2.
- Apostu, M.;. The Effect of Ergogenic Substances over Sports Performance. Procedia-Social and Behavioral Sciences. 2014, 117, 329–334. DOI: 10.1016/j.sbspro.2014.02.222.
- Silver, M.D.;. Use of Ergogenic Aids by Athletes. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2001, 9, 61–70. DOI: 10.5435/00124635-200101000-00007.
- Kalman, D.S.; Feldman, S.; Krieger, D.R.; Bloomer, R.J. Comparison of Coconut Water and a Carbohydrate-Electrolyte Sport Drink on Measures of Hydration and Physical Performance in Exercise-Trained Men. Journal of the International Society of Sports Nutrition. 2012, 9, 1–10. DOI: 10.1186/1550-2783-9-1.
- Medeiros, A.C.; de Paiva, V.D.F.L. Therapeutic Use of Coconut Water. Journal of Surgical and Clinical Research. 2013, 3, 83–91. DOI: 10.20398/jscr.v3i2.3570.
- Vigliar, R.; Sdepanian, V.L.; Fagundes-Neto, U. Biochemical Profile of Coconut Water from Coconut Palms Planted in an Inland Region. Jornal de Pediatria. 2006, 82, 308–312. DOI: 10.2223/JPED.1508.
- Jamadon, B.; Ahmad, N.; Nubaidillah, O. Buah kelapa untuk minuman segar. Teknologi Koko-Kelapa. 1987, 3, 31–38.
- Marie, N.; Verdier, C.; Le Bot, B.; Burgot, G. Analysis of Sodium and Potassium in Total Parenteral Nutrition Bags by ICP-MS and ICP-AES: Critical Influence of the Ingredients. American Journal of Analytical Chemistry. 2011, 2, 573–581. DOI: 10.4236/ajac.2011.25065.
- Lee, H.S.; Coates, G.A. Quantitative Study of Free Sugars and Myo-Inositol in Citrus Juices by HPLC and a Literature Compilation. Journal of Liquid Chromatography and Related Technologies. 2000, 23, 2123–2141. DOI: 10.1081/JLC-100100476.
- Prado, F.C.; Lindner, J.D.D.; Inaba, J.; Thomaz-Soccol, V.; Brar, S.K.; Soccol, C.R. Development and Evaluation of a Fermented Coconut Water Beverage with Potential Health Benefits. Journal of Functional Foods. 2015, 12, 489–497. DOI: 10.1016/j.jff.2014.12.020.
- Belay, A.; Haki, G.D.; Birringer, M.; Borck, H.; Lee, Y.C.; Cho, C.W.; Kim, K.T.; Bayissa, B.; Baye, K.; Melaku, S. Sugar Profile and Physicochemical Properties of Ethiopian Monofloral Honey. International Journal of Food Properties. 2017, 20, 1–12. DOI: 10.1080/10942912.2016.1255898.
- Priya, S.R.; Ramaswamy, L. Tender Coconut Water–Nature’s Elixir to Mankind. International Journal of Recent Scientific Research. 2014, 5, 1485–1490.
- Jackson, J.C.; Gordon, A.; Wizzard, G.; Mc Cook, K.; Rolle, R. Changes in Chemical Composition of Coconut (Cocos Nucifera) Water during Maturation of the Fruit. Journal of the Science of Food and Agriculture. 2004, 84, 1049–1052. DOI: 10.1002/(ISSN)1097-0010.
- Mahayothee, B.; Koomyart, I.; Khuwijitjaru, P.; Siriwongwilaichat, P.; Nagle, M.; Müller, J. Phenolic Compounds, Antioxidant Activity, and Medium Chain Fatty Acids Profiles of Coconut Water and Meat at Different Maturity Stages. International Journal of Food Properties. 2016, 19, 2041–2051. DOI: 10.1080/10942912.2015.1099042.
- Schaefer, E.J.; Gleason, J.A.; Dansinger, M.L. Dietary Fructose and Glucose Differentially Affect Lipid and Glucose Homeostasis. The Journal of Nutrition. 2009, 139, 1257–1262. DOI: 10.3945/jn.108.098186.
- Villanueva, M.J.; Tenorio, M.D.; Esteban, M.A.; Mendoza, M.C. Compositional Changes during Ripening of Two Cultivars of Muskmelon Fruits. Food Chemistry. 2004, 87, 179–185. DOI: 10.1016/j.foodchem.2003.11.009.
- Rana, B.; Kaushik, R.; Kaushal, K.; Arora, S.; Kaushal, A.; Gupta, S.; Upadhyay, N.; Rani, P.; Kaushik, P. Physicochemical and Electrochemical Properties of Zinc Fortified Milk. Food Bioscience. 2017, 21, 117–124. DOI: 10.1016/j.fbio.2017.12.008.
- Dias, M.I.; Morales, P.; Barreira, J.C.; Oliveira, M.B.P.; Sánchez-Mata, M.C.; Ferreira, I.C. Minerals and Vitamin B9 in Dried Plants Vs. Infusions: Assessing Absorption Dynamics of Minerals by Membrane Dialysis Tandem in Vitro Digestion. Food Bioscience. 2016, 13, 9–14. DOI: 10.1016/j.fbio.2015.11.004.
- Tai, C.Y.; Joy, J.M.; Falcone, P.H.; Carson, L.R.; Mosman, M.M.; Straight, J.L.; Oury, S.L.; Mendez, C.; Loveridge, N.J.; Kim, M.P.;; et al. An Amino Acid-Electrolyte Beverage May Increase Cellular Rehydration Relative to Carbohydrate-Electrolyte and Flavored Water Beverages. Nutrition Journal. 2014, 13, 1. DOI: 10.1186/1475-2891-13-47.
- Halim, H.H.; Dek, M.S.P.; Hamid, A.A.; Jaafar, A.H. Fatigue Onset through Oxidative Stress, Dehydration and Lactic Acid Accumulation and Its in Vivo Study Using Experimental Animals. Journal of Advanced Review on Scientific Research. 2017, 35, 1–12.
- Kwiatkowski, A.; Clemente, E.; Scarcelli, A.; Vida, J.B. Quality of Coconut Water ‘In Natura’ Belonging to Green Dwarf Fruit Variety in Different Stages of Development, in Plantation on the Northwest Area of Parana, Brazil. Journal of Food, Agriculture and Environment. 2008, 6, 102–105.
- Chuku, L.C.; Kalagbor, G.I. Protein and Mineral Element Content of Coconut (Cocos Nucifera) Water from Different Species. Open Journal of Advanced Drug Delivery. 2014, 2, 451–453.
- Aleixo, P.C.; Nóbrega, J.A.; Dário, S.J.; Regina, M. Determinação direta de selênio em água de coco e em leite de coco utilizando espectrometria de absorção atômica com atomização eletrotérmica em forno de grafite. Química Nova. 2000, 23, 310–312. DOI: 10.1590/S0100-40422000000300005.
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between Total Phenolic and Mineral Contents with Antioxidant Activity of Eight Malaysian Bananas (Musa Sp.). Journal of Food Composition and Analysis. 2011, 24, 1–10. DOI: 10.1016/j.jfca.2010.04.005.
- Maathuis, F.J.M.; Chapter, D.E. 1: Roles and Functions of Plant Mineral Nutrients. In Plant Mineral Nutrients: Methods and Protocols; Maathuis, F.J.M.;, Ed.; Humana Press: New Jersey, 2013; pp 1–21.
- Maughan, R.J.;. Sports Beverages for Optimising Physical Performance. In Functional and Speciality Beverage Technology; Wilson, T.; Temple, N.J., Eds.; Humana Press: New Jersey, 2009; pp 346–369.
