?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to use atomic force microscopy to compare effects of ultrasound bath, injecting calcium chloride solution and sodium chloride solution on the ultrastructure of muscle fibers and choose the preferred meat tenderizing method. The muscles fibers were tenderized and then scanned by atomic force microscopy. When meat was treated by ultrasound bath (50 W, 100 W, 250 W), CaCl2 injection (400 mM, 500 mM) and NaCl injection (100, 200, 300, 400, 500 mM), the sarcomere length was longer as compared with the control (P < 0.05). In consideration of the consumer demand and other palatability and quality traits of meat, the ultrasound bath could be applied to improve meat tenderness without detrimental effects.
Introduction
Among all the attributes of the quality of meat, tenderness is considered as the main characteristics which affect consumer satisfaction.[Citation1,Citation2] It is very important to produce tender meat products. There are various methods to tenderize meats chemically and physically. Several studies reported that sodium chloride (NaCl) was used to improve tenderness and overall palatability[Citation3–Citation6], meanwhile the treatment of calcium chloride has been a popular method for meat tenderization.[Citation7–Citation10] In addition, ultrasound is mainly used as a nondestructive processing technology in food industry.[Citation11–Citation15] Therefore, finding an appropriate concentration of chemical substances and suitable ultrasound intensity is demanded.
In addition, a great deal of emphasis is placed on the predictors of meat tenderness. Many technologies were applied to measure tenderness, such as Warner-Bratzler shear force (WBSF), myofibrillar fragmentation index (MFI) and the weakening of Z-disks measured by transmission electron microscopy (TEM). Many studies have pointed out the relationship between the length of sarcomere and tenderness using TEM.[Citation16–Citation18] However, tedious processes for the TEM examination could cause much difficulty in measuring sarcomere length accurately.[Citation10] It is necessary to develop a new method to measure sarcomere length instead of TEM.
Atomic force microscopy (AFM) is a novel method to detect biological surface ultrastructure because of its high resolution, simple sample preparation, data collection, and image analysis.[Citation19–Citation21] Applications of AFM in food science are reported, including complicated or quantitative structure analysis, surface topography, molecular manipulation, and nanofood characterization. Therefore, the objective of this study was to use AFM to investigate the effects of ultrasonic processing, CaCl2 and NaCl injection on the ultrastructure of muscle fibers to choose the preferred meat tenderizing method. These experimental data could provide references for the meat industry.
Materials and methods
Preparation of meat samples from qinchuan bulls
Qinchuan bulls (n = 6), which were supplied by National Beef Cattle Improvement Center at Northwest A&F University, were 18 months old (approximately 500 kg body weight) and slaughtered at the university slaughter facilities. These carcasses were hung in the room at 4℃. The right longissimus lumborum (LL) muscles were removed from each animal at 24 h after slaughter. These samples were wrapped and placed in plastic zipper storage bags on chilly bins containing ice. These samples were transported to the laboratory within 40 min after being removedand stored at −20℃ until further analysis.
Treatment of muscle samples by ultrasound, cacl2, and NaCl
Some portions (n = 18) were removed from the same muscle., Every portion was approximately 0.06 ± 0.005 g. These samples were randomly assigned to three groups subjected to an ultrasonic processor at a frequency of 40 kHz (Scientz, SB-5200 DTD), CaCl2 and NaCl injection. The selected intensity and the concentrations of CaCl2 and NaCl were as follows. Vacuum-pack bags were used in order to protect the sample from water, which was supposed to affect the ultrasound treatment. During the treatment for 5 min, ice and chilled water was put into the ultrasonic water bath to make the temperature at 4℃. These powers were 0 (control), 50, 100, 150, 200 and 250 W separately in 40 kHz. Samples from the CaCl2 treatment group and NaCl treatment group were injected at 3% (wt/wt) with a micro-syringe at 4℃ for 24 h, while the untreated samples were injected in deionized water as the control. The concentrations were 100, 200, 300, 400 and 500 mM, respectively.
Preparation of muscle fibers
The methods below was performed according to that had described by Gerelt et al.[Citation7] with some modifications. Each sample was diced with an operating scissor and then added to pre-chilled 1 mL homogenizing buffer (50 mM Tris-HCl, 5 mM EGTA,100 mM KCl, pH 7.6). The mixture was homogenized (T10 B S52, IKA, Germany) for 15 s at 9,000 g. The homogenate was centrifuged for 5 min, at 3000 g. This process was repeated three times. After each centrifugation, the supernatant was removed and the precipitate was re-suspended by 1 mL Tris-HCl buffer. The fiber solutions were ready for AFM analysis.
AFM imaging of muscle fibers
For each sample, at least five AFM images were obtained with multimode atomic force microscope (Multimode-8, Bruker Co., US). Triangular silicon tip on nitride AFM cantilevers with spring constant of 0.4 N/m was used in the AFM experiments at the ScanAsyst mode in the Air on 0.997 Hz. The fiber solutions were dropped onto the new cleaved mica sheet (Φ12 mm) and dried at room temperature for about 2 h. The sheet was fixed onto the AFM metal disc with double side tape.[Citation21] The scan rate was 0.997 Hz with 512 × 512 pixels. The AFM scanning size was 50 × 50 µm2. Height and error images were collected simultaneously, flattened, erased, and analyzed using AFM image processing offline software NanoScope Analysis V1.80 (Bruker Co., Santa Barbara, CA).[Citation22]
Data analysis
The qualitative data of sarcomere length were shown as mean ± SE (standard error). These data were subjected to one-way analysis of variance (ANOVA) using the SPSS statistical package (version 19.0; IBM Corporation, Chicago, IL, USA). The significance level was set at 5%. A Least Significant Difference (LSD) test was applied for post hoc comparisons.
Results and discussion
Sarcomere length of the longissimus lumborum muscle fibers
The structure of the sarcomere is vital for the striated appearance of the muscle fiber. The striations are periodic. These striations arise from dense A-bands and less dense I-bands in the myofibril. Bisecting the I-bands are dark lines known as Z-lines. The distance between adjacent Z-lines is the sarcomere length.[Citation2] In the previous study, Smulder et al. found that the muscle sarcomere length had a positive relationship with meat tenderness.[Citation17] Ishihara et al. also proved the sarcomere length analysis was more accurate than sensory analysis and shear force.[Citation23]
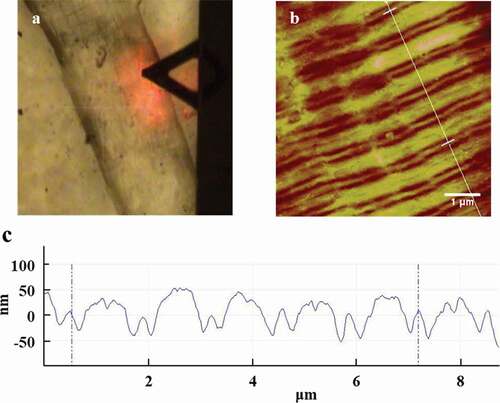
Two-dimentional images of muscle fibers were obtained with AFM in . The fiber direction was clearly shown with the optical microscopy in . In addition, sarcomeres run along the direction of the muscle fibers, which had the repeatable units. The direction of these fibers was the direction of the straight line in . Scanning size was 10.0 × 10.0 μm2 and five repeatable sarcomeres could be collected in .
Effects of ultrasonic processing on the sarcomere length of muscle fibers
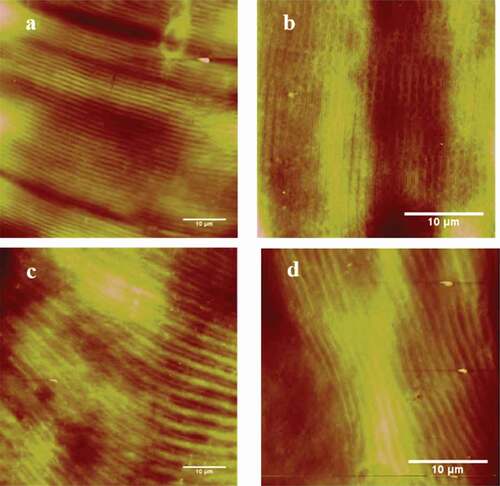
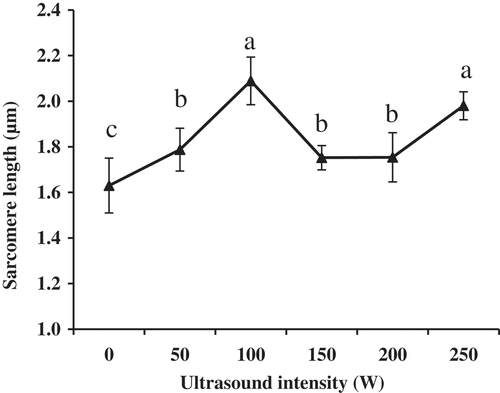
Typical AFM height images of muscle fibers treated by ultrasound are shown in and the corresponding changes in the sarcomere length of muscle fibers are shown in . With the intensity increasing to 100 W, the sarcomere length became longer (P < 0.05). The result may be caused by the ultrasonic capability of improving the activity of μ-calpain which accelerated the degradation of myofibrillar proteins.[Citation12–Citation14] However, the sarcomere length significantly decreased when the intensity was 150 W (P < 0.05). There are no significant differences in the sarcomere length in relation to the values obtained for the 150 W and 200 W intensities (P > 0.05). This result was consistent with the report of Gao et al.[Citation11], who stated that this may be related to the inhibit mechanization of too high-intensity ultrasound on the cathepsins from lysosomes. When the intensity of ultrasound was 250 W, the sarcomere length was significantly longer than when the intensity was 0 W, 50 W, 150 W and 200 W (P < 0.05). However, differences in the sarcomere length were not significant for the intensity of 100 W (P > 0.05). When ultrasound with the intensity of 250 W could cause drastic topographical changes, it induced perimysium to break down (,), which made the muscle fiber apart. Therefore, these dispersed muscle fibers changed more easily and the sarcomere length increased again.[Citation11]
Figure 2. AFM 2D images of the longissimus lumborum (LL) muscle fibers. (a) and (b) Control at 5 min. (c) and (d) Treated with 100 W ultrasound bath for 5 min.
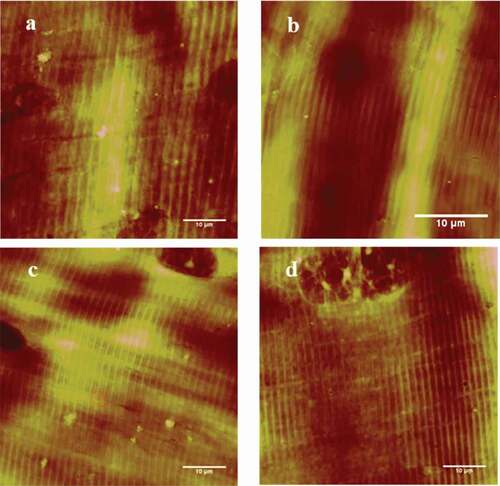
Figure 3. Changes of sarcomere length of the longissimus lumborum (LL) muscle fibers treated with the ultrasound bath. Values with different letters are significantly different among the intensities (P < 0.05).
Some studies found that ultrasound had no significant effect on tenderness.[Citation24,Citation25] Jayasooriya et al. stated that the high-power ultrasound (24 kHz, 12 W/cm2) and low-frequency (25.9 kHz) could improve the tenderness of bovine semitendinosus and longissimus lumborum muscles when the samples were treated for 4 minutes.[Citation12] The results could be caused by different experimental conditions. The samples used in our study weighed 0.06 g, while about 50 g of meat was used in Jayasooriya’s study. In addition, the frequency was set 40 kHz and the intensity was 100 W (0.1233 W/cm2 based on the size of ultrasonic cleaner: 318 mm × 255 mm × 300 mm), which was much lower than that in previous studies.
Effects of cacl2 on the sarcomere length of muscle fibers
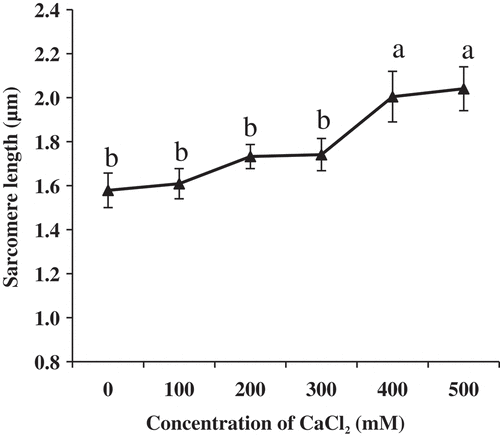
After CaCl2 injection for 24 h, AFM height images of muscle fibers are shown in . Changes in the sarcomere length of muscle fiber are shown in . As the concentration of CaCl2 increased to 300 mM, changes in sarcomere length were not obvious (P > 0.05). When treated in 400 and 500 mM CaCl2, the sarcomere length was significantly longer compared with the control group (P < 0.05). However, there were no significant differences between the two concentrations (P > 0.05). The result in the present study was inconsistent with a previous report.[Citation8] The effect was due to the change in enzymes of the calpain system, which was considered to be primarily responsible for the postmortem proteolytic tenderization of meat.[Citation26] In addition, the activity of μ-calpain was directly influenced by the concentration of Ca2+ in sarcoplasm.[Citation27] Proper the concentration of Ca2+ could introduce the activity of μ-calpain, which might improve the tenderness.
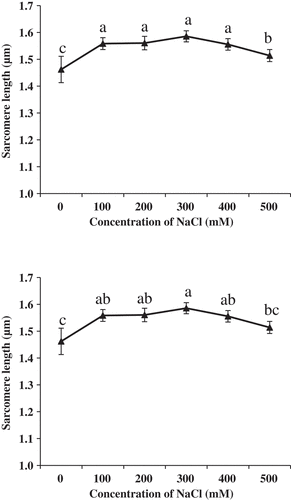
Effects of NaCl to the sarcomere length of muscle fibers
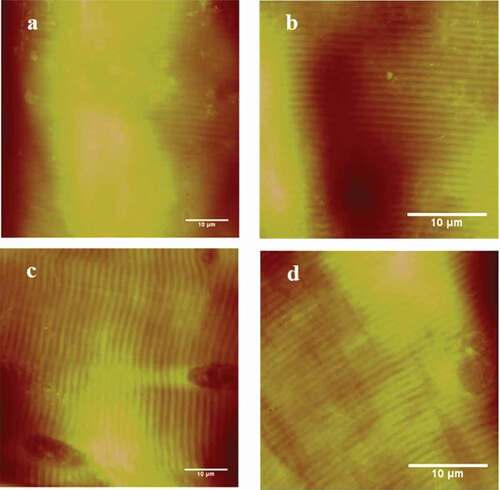
AFM height images of muscle fibers treated by NaCl are shown in and changes in the sarcomere length of muscle fibers are shown in . The sarcomere length treated by NaCl with the concentration of 100 mM, 200 mM, 300 mM 400 and 500 mM was longer than that of the control group (, P < 0.05). These results were consistent with the study of Boles and Swan[Citation3], who reported that meat treated with brine had significantly lower peak shear force values than the water injected control (P < 0.05). However, when the concentration was 500 mM, the sarcomere length was shorter than that when the concentration was 100, 200, 300 and 400 mM (P < 0.05). The mechanism for the meat tenderness treated by NaCl was not clear. Boles and Swan reported that NaCl solution decreased postmortem pH decline and water binding which influenced meat tenderness.[Citation3]
Comparison of effects of ultrasound, cacl2, and NaCl injection on the muscle fibers
Curve fitting analysis had been conducted for comparison of tendency of sarcomere length treated by the ultrasound, CaCl2, and NaCl injection. Fitting functions of were as follows:
In these equations above, where x is the intensity or the concentration, y is the corresponding sarcomere length. The analysis indicated that the tendencies of sarcomere length treated by CaCl2 and NaCl injection were similar, which was different from the ultrasound bath.
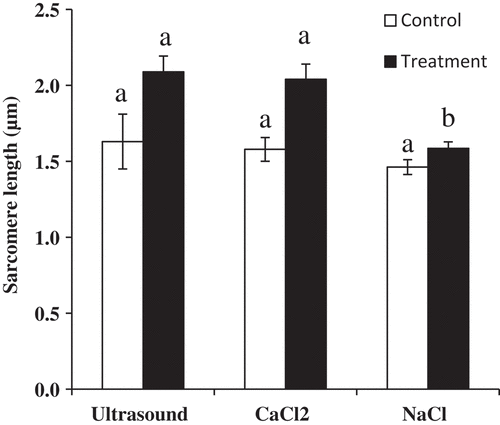
In addition, comparison of effects of three methods on the muscle fibers is shown in . Before the treatment, there were no significant differences among three methods (P > 0.05). When muscle fibers were treated by the 100 W ultrasound bath, 500 mM CaCl2 and 300mM NaCl injection, the sarcomere length treated by the ultrasound bath and CaCl2 injection was longer than that treated by NaCl injection (P < 0.05). However, 400 mM CaCl2 and 100 mM NaCl were chosen as the proper concentration in the present study. The reason was that too high concentrations of CaCl2 might influence the flavor of meat. In the previous study, panelists characterized 300 mM CaCl2 injected beef as being more metallic, bitter, and livery in taste.[Citation10] The demand for salted products was also changing towards products with a lower content of salt.[Citation4]
Conclusion
The present study may present an effective technique toward assessment for meat tenderness by observing ultrastructure of muscle fiber using atomic force microscope. The 100 W ultrasound baths, 400 mM CaCl2 and 100 mM NaCl in this study can be used as a basis for optimization of tenderizing beef. The ultrasound bath of 100 W was chosen as the preferred tenderization method without side effects. However, these findings are still some controversies. This was due to the fact that these results had been built and validated with experimental data in lab scale. In addition, further studies will have to be conducted to investigate the effect of this procedure on other important meat characteristics like flavor and microbial considerations.
Acknowledgments
We would like to thank the staff of the Laboratory of Agricultural and Food Biomechanics for help. We also thank Raheel Suleman at CAAS for his careful revising on this manuscript. The authors declare no conflict of interest.
Additional information
Funding
References
- Chen, L. J.; Li, X.; Ni, N.; Liu, Y.; Chen, L.; Wang, Z. Y.; Zhang, D. Q. Phosphorylation of Myofibrillar Proteins in Post-Mortem Ovine Muscle with Different Tenderness. J. Sci. Food Agric. 2016, 96, 1474–1483. DOI: 10.1002/jsfa.7244.
- Huff-Lonergan, E.; Zhang, E.; Lonergan, S. M. Biochemistry of Post-Mortem muscle-Lessons on Mechanisms of Meat Tenderization. Meat Sci. 2010, 86, 184–195. DOI: 10.1016/j.meatsci.2010.05.004.
- Boles, J. A.; Swan, J. E. Effects of Brine Ingredients and Temperature on Cook Yields and Tenderness of Pre-Rigor Processed Roast Beef. Meat Sci. 1997, 45, 87–97.
- Desmond, E. Reducing Salt: A Challenge for the Meat Industry. Meat Sci. 2006, 74, 188–196. DOI: 10.1016/j.meatsci.2006.04.014.
- Vote, D. J.; Platter, W. J.; Tatum, J. D.; Schmidt, G. R.; Belk, K. E.; Smith, G. C.; Speer, N. C. Injection of Beef Strip Loins with Solutions Containing Sodium Tripolyphosphate, Sodium Lactate, and Sodium Chloride to Enhance Palatability. J. Anim. Sci. 2000, 78, 952–957.
- Pszczola, D. E. Tackling Meat Issues in a Lean Economy. Food Technol. 2010, 64, 49–58.
- Gerelt, B.; Ikeuchi, Y.; Nishiumi, T.; Suzuki, A. Meat Tenderization by Calcium Chloride after Osmotic Dehydration. Meat Sci. 2002, 60, 237–244.
- Koohmaraie, M.; Shackelford, S. D. Effect of Calcium Chloride Infusion on the Tenderness of Lambs Fed a Beta-Adrenergic Agonist. J. Anim. Sci. 1991, 69, 2463–2471.
- Whipple, G.; Koohmaraie, M. Calcium Chloride Marination Effect on Beef Steak Tenderness and Calpain Proteolytic Activity. Meat Sci. 1993, 33, 265–275. DOI: 10.1016/0309-1740(93)90064-O.
- Morgan, J. B.; Miller, R. K.; Mendez, F. M.; Hale, D. S.; Savell, J. W. Using Calcium Chloride Injection to Improve Tenderness of Beef from Mature Cows. J. Anim. Sci. 1991, 69, 4469–4476.
- Gao, J.-P.; Wang, Y.; Liu, L.; Li, K.-Y.; Zhang, S.-Q.; Zhu, J. Effects of Ultrasound, CaCl2 and STPP on the Ultrastructure of the Milk Goat Longissimus Muscle Fiber Observed with Atomic Force Microscopy. Scanning. 2016, 38, 545–553. DOI: 10.1002/sca.21298.
- Jayasooriya, S. D.; Bhandari, B. R.; Torley, P.; D’Arcy, B. R. Effect of High Power Ultrasound Waves on Properties of Meat: A Review. Int. J. Food Prop. 2004, 7, 301–319. DOI: 10.1081/JFP-120030039.
- Reynolds, J. B.; Anderson, D. B.; Schmidt, G. R.; Theno, D. M.; Siegel, D. G. Effects of Ultrasonic Treatment on Binding Strength in Cured Ham Rolls. J. Food Sci. 1978, 43, 866–869. DOI: 10.1111/jfds.1978.43.issue-3.
- Zayas, J. F.;. Effect of Ultrasound Treatment on the Extraction of Insulin. Biotechnol. Bioeng. 1985, 27, 1223–1228. DOI: 10.1002/bit.260270818.
- Stadnik, J.; Dolatowski, Z. J.; Baranowska, H. M. Effect of Ultrasound Treatment on Water Holding Properties and Microstructure of Beef (M. Semimembranosus) during Ageing. LWT-Food Sci. Technol. 2008, 41, 2151–2158. DOI: 10.1016/j.lwt.2007.12.003.
- Rhee, M.; Wheeler, T.; Shackelford, S.; Koohmaraie, M. Variation in Palatability and Biochemical Traits within and among Eleven Beef Muscles. J. Anim. Sci. 2004, 82, 534–550. DOI: 10.2527/2004.822534x.
- Smulder, F. J. M.; Marsh, B. B.; Swartz, R. L.; Russell, R. L.; Honenecke, M. E. Beef Tenderness and Sarcomere Length. Meat Sci. 1990, 28, 349–363. DOI: 10.1016/0309-1740(90)90048-B.
- Takahashi, K.; Hattori, A.; Kuroyanagi, H. Relationship between Translocation of Paratropomyosin and Restoration of Rigor-Shortened Sarcomeres during Post-Mortem Ageing of Meat—A Molecular Mechanism of Meat Tenderization. Meat Sci. 1995, 40, 413–423.
- Haaheim, J.; Nafday, O. A. Dip Pen Nanolithography®: A “Desktop Nanofab™” Approach Using High-Throughput Flexible Nanopatterning. Scanning. 2008, 30, 137–150. DOI: 10.1002/sca.20098.
- Zhu, J.; Guo, L. H.; Zhang, B.; Hu, J.; Wang, G. D. Microscale Biomechanics Measurements with Atomic Force Microscopy and the Concerned Techniques. Life Sci. Instrum. 2007, 5, 3–9.
- Zhu, J.; Sabharwal, T.; Guo, L.-H.; Kalyanasundaram, A.; Wang, G.-D. Gloss Phenomena and Image Analysis of Atomic Force Microscopy in Molecular and Cell Biology. Scanning. 2009, 31, 49–58. DOI: 10.1002/sca.20133.
- Song, X.; Wang, Z.-W.; Tao, S.-Y.; Li, G.-X.; Zhu, J. Observing Effects of Calcium/Magnesium Ions and pH Value on the Self-Assembly of Extracted Swine Tendon Collagen by Atomic Force Microscopy. J. Food Qual. 2017, 2017, 1–8. DOI: 10.1155/2017/9257060.
- Ishihara, Y.; Moreira, R.; Souza, G.-D.; Salviano, A.; Madruga, M. Study of the Warner-Bratzler Shear Force, Sensory Analysis and Sarcomere Length as Indicators of the Tenderness of Sun-Dried Beef. Molecules. 2013, 18, 0432–9440. DOI: 10.3390/molecules18089432.
- Lyng, J. G.; Allen, P.; McKenna, B. The Effects of Pre- and Post- Rigor High-Intensity Ultrasound Treatment on Aspects of Lamb Tenderness. LWT-Food Sci. Technol. 1998, 31, 334–338. DOI: 10.1006/fstl.1997.0361.
- Pohlman, F. W.; Dikeman, M. E.; Zayas, J. F. The Effect of Low-Intensity Ultrasound Treatment on Shear Properties, Colour Stability and Shelf Life of Vacuum-Packaged Beef Semitendinosus and Biceps Femoris Muscles. Meat Sci. 1997, 45, 329–337.
- Pomponio, L.; Ertbjerg, P. The Effect of Temperature on the Activity of μ- and M-Calpain and Calpastatin during Post-Mortem Storage of Porcine Longissimus Muscle. Meat Sci. 2012, 91, 50–55. DOI: 10.1016/j.meatsci.2011.12.005.
- Goll, D. E.; Thompson, V. F.; Li, H. Q.; Wei, W.; Cong, J. Y. The Calpain System. Physiol. Rev. 2003, 83, 731–801. DOI: 10.1152/physrev.00029.2002.