?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the physicochemical, taste, and functional changes in low-salt Sufu paste were investigated during fermentation as enhanced by a mixed starter (Pichia fermentans, Kodamaea ohmeri, and Lactococcus lactis subsp. lactis) and α-ketoglutarate. The total free amino acids increased from 0.02 mg/g dry matter to 12.63 mg/g dry matter, and monosodium glutamate-like was the major free amino acid group (3.89 mg/g dry matter), followed by bitter according to the taste characteristics. Functional components such as γ-aminobutyric acid, riboflavin (VB2), and puerarin significantly increased to 30.96, 4.97, and 12.03 mg/g dry matter, respectively, at the end of post-ripening (p < 0.05). Protease, lipase, peptidase, α-amylase, aromatic amino acid aminotransferase, and branched-chain amino acid aminotransferase activities increased and were significantly correlated (p < 0.05) with most physicochemical and functional components, indicating that enzymes may play an important role in the fermentation process of Sufu paste. Additionally, the color of the Sufu paste changed from pale yellow to yellowish brown, whereas the Sufu paste fermented without the mixed starter and α-ketoglutarate changed to gray, the unique color of gray Sufu. The preliminary results indicated that the mixed starter and α-ketoglutarate are beneficial for taste quality and color and that fermentation may be an effective method to enhance the functional components in Sufu paste. This information would be useful for future improvements in the manufacturing process and quality of Sufu paste.
Introduction
Sufu, an oriental fermented food, is a highly flavored appetizer made from cubes of soybean curd. It has been consumed widely for more than 1,000 years in China and is becoming increasingly popular in western countries. The estimated annual production of Sufu is over 300,000 tons in China alone.[Citation1] The fermentation process used in producing Sufu not only improves its digestibility and bioavailability but also eliminates its flatulence component, beany flavor, and bitter taste of soybean. Sufu is rich in high-quality vegetal proteins, unsaturated fatty acids, isoflavones, γ-aminobutyric acid (GABA), and phytosterol.[Citation2] Compared with animal proteins, Sufu is cholesterol-free. It has an impact on preventing and treating chronic diseases, which is supported by epidemiological studies.[Citation3] In addition, it contains a sufficient quantity of various amino acids to meet dietary needs. Its composition of essential amino acids (EAAs) is comparable to that of egg and milk proteins.[Citation4] Therefore, Chinese people consider Sufu to be a healthy food.
Although a pure culture method for preparing Sufu has been developed, there are still some challenges in producing uniformly high-quality products. Currently, the post-ripening time is shorter than the traditional process which can take longer than 6 months. However, modern processes can still take about 2–3 months,[Citation5,Citation6] and the lengthy post-ripening period is considered to be a waste of space, time, and energy. In addition, the traditional brine process usually leads to a high concentration of salt in Sufu, ranging from 10% to 30%. A high salt level is considered to retard the hydrolysis of protein and lipid and prevent the isoflavone glucosides from being converted into aglycones. Furthermore, the high salt content in food could lead to an increase in dietary sodium intake and thereby limit human consumption.[Citation5] Therefore, a low-salt Sufu may be beneficial in shortening the post-ripening period and preferable from the viewpoint of public nutritional health.Citation[2]
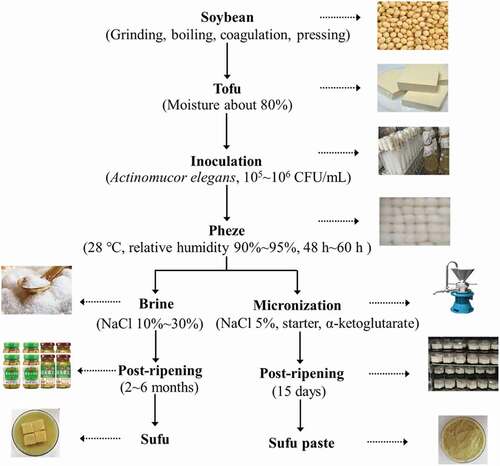
Flavor is one of the most important sensories that contributes to the quality and acceptability of fermented food, and it is mainly generated from complex metabolic pathways including amino acid metabolism, esterification, Strecker degradation, Maillard reaction, and so on.[Citation7] For example, among these metabolic pathways, the transamination reaction is a major process in the formation of cheese flavor, and the α-keto acid required for transamination is the limiting factor in the amino acid degradation.[Citation8,Citation9] Therefore, the addition of exogenous α-ketoglutarate to cheese curd has been proposed to increase amino acid degradation during ripening. It has been clearly shown that the intensity of the aroma in a 6-week (or 12-week) cheddar containing α-ketoglutarate was equivalent to the intensity of aroma in a 6-month cheddar.[Citation10] The addition of strains capable of producing large quantities of the enzymes involved in flavor formation is also an alternative to accelerate ripening.[Citation11] Sufu is often called “Chinese cheese” because of the similarities in the principle of their production. Inspired by the enhancements that have been achieved in cheese aroma, the mixed starter, comprised of positive flavor-producing microbiota, namely Pichia fermentans (CICC 33120), Kodamaea ohmeri (CICC 32993), and Lactococcus lactis subsp. lactis (CICC 21030), was optimized on the basis of enzyme activities in the previous studies (unpublished data). As a fermented soybean food, Sufu paste may be considered as a novel Sufu product. The differences and similarities between the low-salt Sufu paste and the Sufu produced by the normal manufacturing method as well as the salt content are listed in . The processes used for tofu preparation, pehtze fermentation, and the technical principle used to produce Sufu paste are very similar to those used to produce Sufu. Compared to traditional Sufu, the post-ripening period for Sufu paste is much shorter (15 days) and has a low salt content (5%) due to the micronization and fermentation that are enhanced by the mixed starter and α-ketoglutarate.
Although numerous papers related to Sufu have been published,[Citation12–Citation14] few reports concerning Sufu paste are currently available. To provide information essential for improvements in quality control and the manufacturing process of Sufu paste, this study investigated the physicochemical, taste, and functional components of the Sufu paste during fermentation. Furthermore, the correlations between enzyme activities and physicochemical and functional components were also analyzed by the Pearson’s correlation coefficients (r).
Materials and methods
Strains and chemicals
Actinomucor elegans (CICC 41043), P. fermentans (CICC 33120), K. ohmeri (CICC 32993), and L. lactis subsp. lactis (CICC 21030) were purchased from the China Center of Industrial Culture Collection (CICC). α-ketoglutarate (food grade) was purchased from Huao Co. Ltd, China. Puerarin, daidzin, genistein, daidzein, GABA, β-sitosterol, stigmasterol, riboflavin (VB2), amino acid standard solutions, and pyridoxal 5ʹ-phosphate (PLP) were purchased from Sigma–Aldrich Co. Ltd, USA. Acetonitrile, methanol, acetic acid, and trifluoroacetic acid were of high-performance liquid chromatography (HPLC) grade. All other chemicals were of analytical grade.
Preparation of Sufu paste
Preparation of the inoculum suspension
A. elegans was used as the starter for the pehtze preparation. It was activated by culturing for two generations in potato dextrose agar medium. After incubation at 28°C for 60 h, the medium and its biomass were harvested and homogenized to obtain an inoculum suspension containing approximately 105–106 colony-forming units/mL.
Preparation of the mixed starter
On the basis of the previous studies, P. fermentans and K. ohmeri were activated by culturing in 5 °Bé malt extract agar for two generations. Subsequently, they were cultured individually in optimized wheat flour medium (wheat flour:water = 1:20, w/v), supplemented with 2% (w/v) glucose and 0.5% (w/v) NaCl at 28°C and 160 rpm for 48 h. Similarly, L. lactis subsp. lactis was activated by de Man, Rogosa, Sharpe medium for two generations and then cultured in optimized soybean flour medium (soybean flour:water = 1:20, w/v), supplemented with 1% (w/v) glucose, 0.5% (w/v) NaCl, and 10% (w/v) tomato juice, at 37°C and 160 rpm for 24 h. Late exponential phase P. fermentans was mixed with its optimized protectants including sorbitol (25%), sodium glutamate (15%), and pectin (20%). K. ohmeri was mixed with its optimized protectants including sorbitol (25%), sodium glutamate (10%), and sucrose (20%). L. lactis subsp. lactis was mixed with its optimized protectants pectin (10%), sodium glutamate (5%), and sucrose (15%). After prefreezing, all the late exponential phase strains were freeze-dried in an IEC Lyoprep 3000 freeze drier (Lyoprep, Dunstable, UK) at −60°C for approximately 65 h. The mixed starter, comprising P. fermentans, K. ohmeri, and L. lactis subsp. lactis at an optimized ratio of 2:2:1 (w/w/w), was prepared just before use.
Preparation of Sufu paste
A schematic diagram detailing the procedure used to produce Sufu paste is shown in . Briefly, tofu (moisture about 80%) was obtained after soaking, grinding, sieving, acid coagulation, and pressing and was used as the raw material for Sufu paste. It was cut into rectangular pieces (2.0 cm × 2.0 cm × 1.5 cm) and inoculated with A. elegans by spraying the inoculum suspension onto the surface. The inoculated tofu pieces were then placed in an incubation room at a controlled temperature (28°C), a controlled relative humidity (90–95%), and adequate air circulation to ensure appropriate levels of aeration. Fresh pehtze was obtained when tofu appeared a slightly yellowish white color. The total incubation time was 48–60 h. The pehtze was micronized at 2,800 rpm using a colloid mill (JML-50, Shanghai Zhuheng Ltd., China). Following this, 0.1% (w/w) mixed starter, 5% (w/w) NaCl, and 0.4% (w/w) α-ketoglutarate were added to the micronized pehtze and mixed well. About 150 g fresh weight of pheze was then placed in individual wide-mouthed glass bottles with a total capacity of 280 mL. Post-ripening was performed at 28°C for 15 days, and a sample referred to as “Sufu paste fermentation enhanced with a mixed starter and α-ketoglutarate” (SPSK) was obtained. To obtain “Sufu paste enhanced with a mixed starter” (SPS), the Sufu paste was fermented without α-ketoglutarate, and all other conditions were consistent with SPSK. To abtain SP (Sufu paste), the Sufu paste was fermented without the mixed starter or the α-ketoglutarate, and all other conditions were consistent with SPSK.
Determination of proximate composition
The moisture, fat, protein, starch, total acid (TA), and amino acid nitrogen (AAN) contents were measured according to the Association of Official Analytical Chemists.[Citation15]
Determination of free amino acid
To determine free amino acids (FAAs), freeze-dried samples (0.20 g) were first mixed with 40 mL HCl (0.01 mol/L) and sonicated for 30 min at 30°C. Following this, the filtered sample solution was diluted with 0.01 mol/L HCl to a final volume of 50 mL. Subsequently, 5 mL of the sample solution was mixed with 5 mL of sulfosalicylic acid (8%, w/v) and placed in the refrigerator for 12 h at 4°C. After centrifugation (8,000 g, 10 min), the supernatant was collected and filtered through a 0.22-μm filter. The FAAs were analyzed using an L-8800 amino acid analyzer (Hitachi Ltd., Japan).
Determination of functional components
The HPLC equipment (Agilent 1260, Agilent Ltd., USA) was equipped with a Phecda C18 column (250 × 4.6 mm, 5 μm). Briefly, an ultraviolet spectrophotometer at a wavelength of 254 nm was used to detect isoflavone.[Citation12] The linear HPLC gradient consisted of (A) acetonitrile and (B) deionized water containing 1% (v/v) acetic acid. After the injection of 20 μL sample solution into the column, solvent A increased from 30% to 50% within 15 min and then from 50% to 70% over another 5 min, where it was held for the next 8 min. Finally, solvent A decreased to 30% over 2 min. The levels of isoflavones were calculated from standard curves of the area responses for isoflavone standards. For the analysis of phytosterols, the mobile phase was methanol containing 0.1% (v/v) acetic acid. The solvent flow rate was 1.0 mL/min, and the detection wavelength was 210 nm. Quantitative data for β-sitosterol and stigmasterol were obtained by comparison with known standards. GABA was measured by determining the absorption at 254 nm. The mobile phase consisted of methanol (A) and deionized water (B). Solvent A increased from 20% to 100% over 20 min and then held for the 15 min. Finally, solvent A decreased to 20% over the next 5 min. VB2 was analyzed using a fluorescence detector. Sodium acetate solution (0.05 mol/L) and methanol (65:35, v/v) were used as the mobile phase. The solvent flow rate was 1.0 mL/min. The excitation and emission wavelengths were 462 and 522 nm, respectively.
Determination of color
Indices of color were determined using a portable Minolta Chroma Meter CR-200 (Minolta Camera Co. Ltd, Osaka, Japan) as described by Dalkyoung et al.[Citation16] in terms of L* (lightness), a* (redness and greenness), and b* (yellowness and blueness). Three measurements were made at different locations on each of the triplicate samples. The color difference (ΔE) between the different Sufu paste samples was calculated as
Determination of enzyme activities
Protease activity was determined according to the method described by Leonard and Wildi,[Citation17] using 2% casein as substrate. Briefly, 1.0 mL of enzyme solution was added to 1.0 mL of substrate solution (dissolved in phosphate buffer at pH 7.2) and incubated at 40°C for 10 min along with the respective controls. The reaction was stopped by adding 2.0 mL of trichloroacetic acid (TCA) (0.4 mo1/L) and the reaction mixture was then centrifuged at 9,000 × g for 10 min at 4°C. The precipitate was removed, and 1.0 mL of the supernatant was added to 5.0 mL of Na2CO3 solution (0.4 mo1/L). Then, 1.0 mL of Folin reagent was immediately added to each tube and incubated at 40°C for 20 min. The protease activity was expressed as the difference in absorbance at 660 nm between the control and the test sample. One unit of protease activity is defined as the amount of enzyme that liberates 1 μg of tyrosine per minute.
Lipase activity was assessed by potentiometric titration using polyvinyl alcohol and pure olive oil emulsion as substrates. Briefly, 1.0 mL of enzyme solution, 4.0 mL of a freshly prepared polyvinyl alcohol and pure olive oil emulsion, and 5.0 mL of phosphate buffer (pH 7.5) were mixed and incubated in a water bath at 37°C for 1 h. The reaction was stopped by adding 15 mL of 95% ethanol. The titration end point (pH 10.3) was determined using a 0.05 mol/L NaOH solution. One unit of lipase activity is defined as the amount of enzyme that liberates 1 μmol of oleic acid per minute during the incubation.
α-Amylase activity was assayed as described by Inatsu et al.[Citation18] with some modifications. Briefly, 0.1 mL of enzyme solution and 0.5 mL of soluble starch solution (1 g/100 mL) were mixed and incubated in a water bath at 37°C for 10 min. Subsequently, 0.5 mL of iodine solution (iodine 0.1 g/L, potassium iodide (KI) 40 g/L), 0.1 mL of HCl (0.1 mol/L), and 3.0 mL of double distilled water were added, and the absorbance was recorded at 660 nm. One unit of α-amylase activity is defined as the amount of enzyme that catalyzes the hydrolysis of 10 mg of soluble starch per 30 min under the assay conditions.
Peptidase activity was routinely determined using soybean peptide as a substrate with some modifications.[Citation19] Briefly, 1.0 mL of enzyme solution, 1.0 mL of substrate solution (2 g/100 mL), and 3.0 mL of Tris-HCl buffer (0.1 mol/L, pH 7.5) were mixed and incubated in a water bath at 37°C for 12 h. Then, the reaction mixture was centrifuged at 4,500 × g for 10 min, and 1 mL of supernatant was added to 2 mL of cadmium-indene solution (1.0 g of cadmium chloride and 0.8 g of ninhydrin dissolved in 90 mL of ethyl alcohol and 10 mL of glacial acetic acid). After heating in a water bath at 85°C for 15 min, the absorbance at 507 nm was measured. Free amino groups were quantified with leucine as the standard.
Aromatic amino acid aminotransferase (ARAT) was measured according to the method described by Yvon et al.[Citation20] with some modifications. Briefly, the reaction mixture contained 10 mL of enzyme solution and 10 mL of substrate solution (20 mg/mL sodium phenylpyruvate, 50 mg/mL L-glutamic acid, 0.167 mmol/L PLP, pH 8.0). The reaction was performed at 37°C for 1 h and stopped by incubation in an ice bath for 10 min. To develop the color, 5 mL of the reaction mixture and 2 mL of chromogenic solution (0.050 g of ferric chloride dissolved in 60 mL of dimethylsulfoxide and 2 mL of glacial acetic acid, and finally diluted with double-distilled water to 100 mL) were measured at 640 nm. One unit of ARAT activity is defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of phenylpyruvic acid per hour under the assay conditions. Branched-chain amino acid aminotransferase (BCAT) was monitored as previously described, except that the amino acid substrate used was leucine instead of phenylalanine.
Statistical analysis
All statistical analyses were subjected to one-way analysis of variance (ANOVA) using Origin 8.0 software. The means and standard deviations were calculated from the data obtained from three separate experiments. Significant differences were determined via an ANOVA. Means in the same column with different letters were significantly different according to Duncan’s multiple range test (p < 0.05). In addition, the correlations between enzyme activities and physicochemical and functional components were characterized by the Pearson’s correlation coefficients (r).
Results and discussion
Dynamic changes of conventional physicochemical components
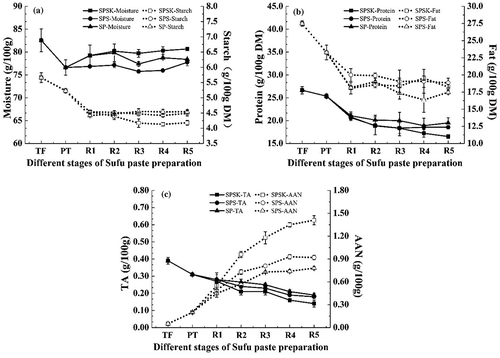
The dynamic changes of conventional physicochemical components during Sufu paste fermentation are shown in . In SPSK, SPS, and SP, the contents of moisture, fat, protein, starch, and total acid (TA) all decreased significantly (p < 0.05), whereas AAN increased significantly (p < 0.05). The moisture contents ranged from 75.77 to 82.57 g/100 g and accounted for the largest proportion during Sufu paste fermentation (). In addition to water, fat, protein, and starch were the major components that affected the texture, taste, and flavor, and the levels of all three of these components decreased continuously, regardless of the types of Sufu paste (). These decreases may be associated with the activities of lipase, protease, peptidase, and α-amylase secreted by the complex microbial systems. It is generally accepted that the protease and peptidase catalyze the degradation of protein into smaller molecules that contribute to the particular flavor and texture.[Citation21] Soybean lipids are known to be hydrolyzed into fatty acids and form esters, thus providing a pleasant odor to the product.[Citation5] The added salt, starter culture, and α-ketoglutarate also had great impacts on the composition of the dry matter. Both AAN and TA are important indices of Sufu quality, which indicate the degree of ripening during fermentation.[Citation13] During the fermentation process, the levels of AAN rose significantly from 0.05 to 1.41 g/100 g after post-ripening (p < 0.05), whereas TA levels decreased significantly from 0.39 to 0.15 g/100 g in SPSK (p < 0.05), superior to SPS and SP (). It is very likely that this was due to the addition of the mixed starter and α-ketoglutarate. For instance, the mixed P. fermentans, K. ohmeri, and L. lactis subsp. lactis starter is known to accelerate the proteolysis[Citation22,Citation23] and thus is likely to have an important influence on AAN levels. A typical reaction that can reduce TA levels is esterification, which transforms the carboxylic acids or oxygen-containing inorganic acids and alcohols into esters. Particularly, after post-ripening, the AAN and TA in SPSK were better than the Sufu standard (AAN≥1.30 g/100 g; TA≤0.35 g/100 g).[Citation24] Apart from the abovementioned two reasons, micronization was also crucial in shortening the post-ripening time of Sufu paste.
Figure 2. Dynamic changes of conventional physicochemical components during Sufu paste fermentation. (a) Moisture and Starch; (b) Fat and Protein; (c) TA and AAN. SPSK: Sufu paste fermented with starter and α-ketoglutarate; SPS: Sufu paste fermented with starter but without α-ketoglutarate; SP: Sufu paste fermented without mixed starter or α-ketoglutarate; TF: Tofu to make pehtze; PT: pehtze fermented for 48–60 h; R1–R5: Sufu paste post-ripening for 3, 6, 9, 12, and 15 days, respectively. The p-values were calculated from one-way analysis of variance (ANOVA) using origin 8.0 software. Error bars indicate standard deviation.
Dynamic changes of free amino acid profiles
FAAs, released either by the starter culture or through endogenous proteases, are considered to be important in creating the pleasant and palatable taste of fermented foods and indirectly contribute to the development of their typical aroma since they are precursors of numerous volatile compounds.[Citation25] The FAA profiles are shown in . A total of 15 kinds of FAAs were identified during fermentation. In the ripened Sufu paste, Glu was the most abundant, followed by Ala and Leu. Glu is generally considered to be one of the most important contributors towards the flavor of oriental fermented soybean products. Glutaminase, produced by microorganisms such as A. elegans and L. lactis subs, can convert glutamine into Glu. Notably, α-ketoglutarate can be transformed into glutamate through amino acid transamination.[Citation26] This may explain the phenomenon that a greater increase of Glu content was noted in SPSK than in SPS. Generally, the contents of total free amino acids (TFAA) and most of the individual FAAs increased at the end of the post-ripening period. These results are consistent with those observed in the Sufu prepared with mold-fermented processCitation[2] and other oriental fermented products, such as Kinema, sausage, and cheese.[Citation26,Citation27] The contents of TFAA and EAAs of TF were only 0.02 mg/g dry matter (DM) and 0.15 mg/g DM, respectively. The TFAA contents increased significantly (p < 0.05) in PT and Sufu paste, presumably due to the proteolytic enzymes secreted by microorganisms. After post-ripening, comparing the three types Sufu paste, the TFAA (12.63 mg/g DM) of SPSK was the highest, while the EAA (4.89 mg/g DM) of SPS was the highest. These results indicate that the addition of the mixed starter and α-ketoglutarate, as well as fermentation time, have important influences on the FAA levels and their relative proportions.
Table 1. Dynamic changes of free amino acid (FAA) in SPSK, SPS, and SP during different fermentation stages.a
As one of the important quality indicators, taste perception is a high priority in the food industry. On the basis of taste characteristics, FAAs can be grouped as monosodium glutamate (MSG)-like, sweet, bitter, and tasteless according to the report by Tseng et al.[Citation28] While the TFAA contents increased rapidly during fermentation, the relative proportion of each amino acid remained essentially constant. After post-ripening, all of MSG-like, sweet, bitter, and tasteless FAAs in SPSK, SPS, and SP increased significantly compared to those of TF and PT (p < 0.05). MSG-like was the major FAA group in SPSK (3.89 mg/g DM), whereas the majority of FAAs in SPS and SP were bitter with contents of 5.03 mg/g DM and 4.49 mg/g DM, respectively (). Similar to the taste characteristics of Sufu paste, bitter was also the most abundant taste group in douchiba[Citation29] and thua nao.[Citation30] The bitter taste may arise due to the unbalanced proteolysis of alkaline-fermented soybean. Although a bitter taste limits the acceptance and marketing of fermented foods, a limited level of bitterness may be desirable. For instance, two of the so-called bitter amino acids, namely Phe and Tyr, have been shown to have a significant umami-enhancing effect on the umami taste of MSG/NaCl mixtures at subthreshold concentrations.[Citation31]
Figure 3. Taste characteristics of FAAs during Sufu paste fermentation. SPSK: Sufu paste fermented with starter and α-ketoglutarate; SPS: Sufu paste fermented with starter but without α-ketoglutarate; SP: Sufu paste fermented without mixed starter or α-ketoglutarate; TF: Tofu to make pehtze; PT: pehtze fermented for 48–60 h; R1–R5: Sufu paste post-ripening for 3, 6, 9, 12 and 15 days, respectively. After post-ripening for 15 days, all of MSG-like, sweet, bitter, and tasteless amino acids in SPSK, SPS, and SP increased significantly compared to those of TF and PT (p < 0.05). The p-values were calculated from ANOVA using origin 8.0 software.
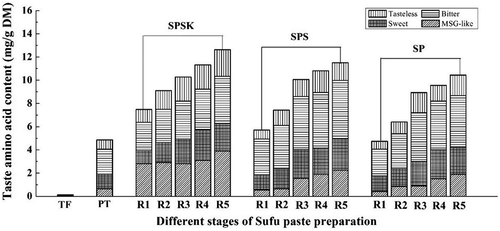
As a basic taste typified by the Glu and Asp, the MSG-like taste can impart a savory, brothy, rich, or meaty taste sensation. A more delicious and hedonic taste was obtained in SPSK due to the larger proportion of MSG-like amino acids compared to SPS and SP. It has been reported that there is a synergistic effect in the interaction between taste-active amino acids. For instance, when Cys, His, Met, Pro, or Val was added to the mixture of MSG and 5ʹ-ribonucleotide, the umami taste was more intense than that of MSG or 5ʹ-ribonucleotide alone.[Citation32] In terms of the sweet taste, the quantities of sweet FAAs, displayed in , were relatively low. Gly and Ala elicit a strong sweet taste due to the ability of these molecules to bind to sweet substance receptors. It should be noted that although tasteless amino acids are not taste active, they can enhance the taste intensity of other compounds by modulating the signal transduction from the taste receptors to the brain.
Dynamic changes of functional components
The dynamic changes of functional components, including isoflavones, GABA, and phytosterols, are shown in . Isoflavones have been linked to the prevention of cancer, hypercholesterolemia, cardiovascular disease, osteoporosis, and the relief of menopausal symptoms in certain women.[Citation33] The best natural source of isoflavones is soybeans, which have been a major part of the traditional diet for eastern Asian populations for centuries.[Citation34] Isoflavones occur in the form of aglycones (daidzein and genistein) and the corresponding glucosidic conjugates such as daidzin. Isoflavone aglycones have higher biological activities than the corresponding glucosidic conjugates. Our results showed that puerarin levels increased rapidly during fermentation (especially in PT), whereas daidzin levels decreased slightly (). Daidzein and genistein increased significantly (p < 0.05) during TF and PT preparation, and only increased slightly thereafter, with the highest contents of 0.443 mg/g DM and 0.816 mg/g DM, respectively (). Similar trends, in terms of isoflavone content and composition, have been observed during Sufu manufacturing.Citation[2] These results may be attributed to the hydrolysis of the corresponding glucosidic conjugates of isoflavone to aglycones by β-glucosidase.[Citation13] In short, our study suggested that Sufu paste was rich in isoflavone aglycones, and fermentation might be beneficial for the enhancement of its physiological effects.
Figure 4. Dynamic changes of functional components during Sufu paste fermentation. (a) Puerarin and daidzin; (b) daidzein and genistein; (c) GABA and VB2; (d) β-sitosterol and stigmasterol. SPSK: Sufu paste fermented with starter and α-ketoglutarate; SPS: Sufu paste fermented with starter but without α-ketoglutarate; SP: Sufu paste fermented without mixed starter or α-ketoglutarate; SB: Soybean to make tofu; TF: Tofu to make pehtze; PT: pehtze fermented for 48–60 h; R1–R5: Sufu paste post-ripening for 3, 6, 9, 12, and 15 days, respectively. The p-values were calculated from ANOVA using origin 8.0 software. Error bars indicate standard deviation.
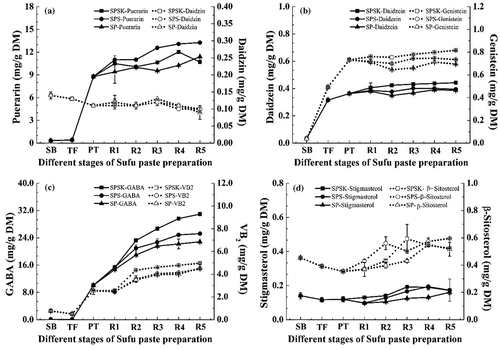
Another functional component that can be generated by fermentation is GABA, a non-protein amino acid that acts as an inhibitory neurotransmitter, and exhibits hypotensive effects.[Citation35] The GABA contents of SB and TF were only 0.04 and 0.02 mg/g DM, respectively (). Statistical analysis indicated that GABA increased significantly (p < 0.05) during fermentation, being up to 30.96 mg/g DM in SPSK, indicating that fermentation is an effective method for GABA enrichment. Significantly, the GABA content of SPSK was higher than that in SPS (25.23 mg/g DM) or in SP (22.85 mg/g DM). It is widely accepted that some bacteria, such as L. lactis subsp. and Bacillus licheniformis, can accumulate GABA under strict anaerobic conditions.[Citation36,Citation37] Sufu paste post-ripening is generally confined to an anaerobic environment. Therefore, the effective accumulation of GABA might be caused by an abundant GABA-producing bacterium. After post-fermentation, VB2 increased to 4.97 mg/g DM, being approximately eightfold higher than in SB and TF (p < 0.05), suggesting that fermentation is also an effective method to enhance VB2 levels ().
As the most commonly occurring phytosterols, β-sitosterol and stigmasterol are structurally and functionally analogous to cholesterol. The β-sitosterol and stigmasterol contents in SB, TF, and PT were almost the same as that in the Sufu paste after post-ripening, suggesting that fermentation did not appreciably change the phytosterol levels (). The β-sitosterol (0.453 mg/g DM) and stigmasterol (0.141 mg/g DM) contents were consistent with a previous study, in which β-sitosterol ranged from 0.10 to 0.51 mg/g and stigmasterol ranged from 34 to 177 mg/g.[Citation38] In summary, most of the functional components were improved during the fermentation of Sufu paste, especially in SPSK.
Dynamic changes of color
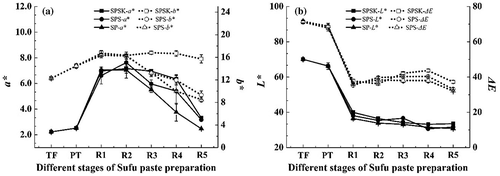
Color is an important factor in Sufu paste, influencing consumer acceptance. The changes in color a*, b*, L*, and ΔE values during the Sufu paste preparation are shown in . The values of a* and b* generally increased at the TF, PT, and R1 stages and subsequently decreased after 6 days post-ripening (R2) (). However, there was a noticeable trend that both the L* and ΔE values decreased with increasing fermentation time. The highest L* and ΔE values were observed in TF, being 70.06 and 71.16, respectively (). At the end of the post-ripening period, the color of both SPSK and SPS changed from pale yellow to yellowish brown, whereas the color of SP changed to gray, which is the characteristic color of gray Sufu. These color changes might be caused by both the enzymatic and non-enzymatic browning reactions that occurred in the ripening period. For instance, the oxidation of flavonoids to hydroxyl compounds could reduce the degree of yellow color observed.[Citation39] The unique changes in the color of SP are probably related to the unique microbial composition, which is affected remarkably by the addition of the mixed starter and α-ketoglutarate.
Figure 5. Changes of color during different stages of Sufu paste preparation. SPSK: Sufu paste fermented with starter and α-ketoglutarate; SPS: Sufu paste fermented with starter but without α-ketoglutarate; SP: Sufu paste fermented without mixed starter or α-ketoglutarate; TF: Tofu to make pehtze; PT: pehtze fermented for 48–60 h; R1–R5: Sufu paste post-ripening for 3, 6, 9, 12, and 15 days, respectively. The p-values were calculated from ANOVA using origin 8.0 software. Error bars indicate standard deviation.
Dynamic changes of enzyme activities
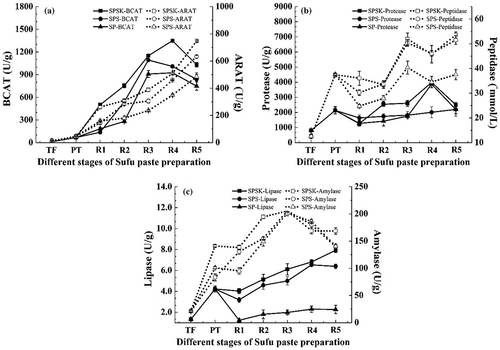
Enzymes, including protease, lipase, peptidase, α-amylase, ARAT, and BCAT, are important for the production of the characteristic flavor, color, and texture of traditional oriental fermented soybean foods. The enzyme activities detected at the different stages of Sufu paste preparation are shown in . Generally, compared with TF, the protease, lipase, peptidase, α-amylase, ARAT, and BCAT activities increased significantly (p < 0.05) during the Sufu paste preparation process. Most enzymes are secreted by the complex microbiota along with microbial growth and metabolism. The microbial changes during the production of Sufu may support the view.[Citation40] During the post-ripening period, the highest enzyme activities of protease (3987.75 U/g), lipase (7.9 U/g), peptidase (53.54 mmol/L), α-amylase (205.44 U/g), ARAT (747.52 U/g), and BCAT (1349.15 U/g) were detected in SPSK, indicating that the mixed starter had an important contribution to enzymatic activity. In addition, the protease, lipase, peptidase, and α-amylase activities in PT were higher than those in R1, demonstrating that NaCl can retard or inactivate the action of hydrolytic enzymes and lead to a decline in their enzymatic activity.
Figure 6. Dynamic changes of enzyme activity during different stages of Sufu paste preparation. (a) Branched-chain amino acid aminotransferase (BCAT) and aromatic amino acid aminotransferase (ARAT); (b) protease and peptidase; (c) lipase and α-amylase. SPSK: Sufu paste fermented with starter and α-ketoglutarate; SPS: Sufu paste fermented with starter but without α-ketoglutarate; SP: Sufu paste fermented without mixed starter or α-ketoglutarate; TF: Tofu to make pehtze; PT: pehtze fermented for 48–60 h; R1–R5: Sufu paste post-ripening for 3, 6, 9, 12, and 15 days, respectively. The p-values were calculated from ANOVA using origin 8.0 software. Error bars indicate standard deviation.
Correlations between enzyme activities and physicochemical and functional components
As shown in , strong correlations were found between enzyme activities and most physicochemical components. Specifically, the protease activity was significantly correlated with protein (r = −1.000, p < 0.01), TA (r = −0.991, p < 0.01), and AAN (r = 1.000, p < 0.01) in SPSK. The correlations between enzyme activities of peptidase, BCAT, and ARAT and physicochemical components including protein, TA, and AAN were also significant (p < 0.01). Protease could cleave peptide bonds at internal positions within proteins/peptides to produce peptide fragments, which can then be hydrolyzed by peptidases to produce amino acids deeply. BCAT and ARAT are two important enzymes in the transamination reaction which can help convert amino acids into aromatic components.[Citation8] Here, the protein and TA decreased continuously, while the AAN increased during the different fermentation stages. Lipase and α-amylase were negatively correlated with fat (r = −0.964, p < 0.01) and starch (r = −0.996, p < 0.01), respectively. This phenomenon could be explained by the fact that lipids are degraded to fatty acids and glycerol by lipases, whereas starches are cleaved by α-amylase to produce glucose and oligosaccharides.[Citation41]
Table 2. Correlation (r) between enzyme activities and physicochemical components during different fermentation stages.
The enzyme activities were also correlated with functional components and color indexes (). Specifically, positive correlations were observed between puerarin, daidzin, daidzein, genistein, stigmasterol, β-sitosterol, GABA, and VB2 and enzyme activities. For instance, puerarin was positively correlated with protease (r = 0.929, p < 0.01), lipase (r = 0.929, p < 0.01), peptidase (r = 0.893, p < 0.01), α-amylase (r = 0.536, p ≥ 0.05), BCAT (r = 0.964, p < 0.01), and ARAT (r = 0.929, p < 0.01) in SPSK. The phenomenon may be attributed to the fact that enzymes catalyze the protein, fat, starch, and polypeptide into lower-molecular-weight components including amino acids, fatty acids, glycerol, glucose, oligosaccharides, and flavors (alcohols, acids, esters, aldehydes, and ketones). The enzymatic reactions damaged the cell integrity and network within the particles, which was influenced by different anions (Ca2+, Mg2+, Na+, and H+) and ionic strengths in coagulator.[Citation42] As a result, the bound puerarin was released from the gels. The color indexes a*, b* were positively correlated with enzyme activities, while L* was negatively correlated with enzyme activities, including protease (r = −0.964, p < 0.01), lipase (r = −0.964, p < 0.01), peptidase (r = −0.857, p < 0.05), α-amylase (r = −0.679, p ≥ 0.05), BCAT (r = −0.929, p < 0.01), and ARAT (r = −0.964, p < 0.01). In conclusion, these results indicated that the changes of physicochemical and functional components may be attributed to the extremely abundant enzymes secreted by the complex microbiota during the fermentation process. Thus, enzymes play an important role in the characteristic taste, color, and texture of Sufu paste.
Conclusion
This is the first scientific report concerning the physicochemical, taste, and functional component changes of Sufu paste, which was enhanced by the mixed starter and α-ketoglutarate during the post-ripening period. The results showed that the mixed starter and α-ketoglutarate are beneficial in shortening the post-ripening time since the AAN and TA in SPSK were superior to SPS and SP. Most of the functional components were improved during the fermentation of the Sufu paste, indicating that fermentation may be an effective method to enhance the functional components of fermented soybean food. FAAs (especially Glu) increased significantly at the end of post-ripening, suggesting that post-ripening is important for promoting taste quality. Enzyme activities were significantly correlated with most physicochemical and functional components. Thus, enzymes may play an important role in the characteristic taste, color, and texture of fermented soybean food. Hopefully, our results will be useful for future improvements in the manufacturing process and the quality of Sufu paste. Furthermore, these results will also provide a new insight to improve the quality of traditional fermented soybean foods.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (31760458) and the Science and Technology Program of Guizhou Province ([2017]5788).
Additional information
Funding
References
- Moy, Y.; Chou, C. Changes in the Contents of Sugars and Organic Acids during the Ripening and Storage of Sufu, a Traditional Oriental Fermented Product of Soybean Cubes. J. Agric. Food Chem. 2010, 58, 12790–12793. DOI: 10.1021/jf1033653.
- Yin, L.; Li, L.; Li, Z.; Eizo, T.; Masayoshi, S. Changes in Isoflavone Contents and Composition of Sufu (Fermented Tofu) during Manufacturing. Food Chem. 2004, 87, 587–592. DOI: 10.1016/j.foodchem.2004.01.011.
- Wang, J.; Lin, Q.; Wang, Y.; Chen, X. Research on Soybean Curd Coagulated by Lactic Acid Bacteria. SpringerPlus. 2013, 2, 250–260. DOI: 10.1186/2193-1801-2-250.
- Han, B.; Rombouts, F. M.; Nout, M. J. R. Amino Acid Profiles of Sufu, a Chinese Fermented Soybean Food. J. Food Compost. Anal. 2004, 17, 689–698. DOI: 10.1016/j.jfca.2003.09.012.
- Han, B. Z.; Rombouts, F. M.; Nout, M. J. A Chinese Fermented Soybean Food. Int. J. Food Microbiol. 2001, 65, 1–10. DOI: 10.1016/S0168-1605(00)00523-7.
- Han, B.; Wang, J. H.; Rombouts, F. M.; Nout, M. J. R. Effect of NaCl on Textural Changes and Protein and Lipid Degradation during the Ripening Stage of Sufu, a Chinese Fermented Soybean Food. J. Sci. Food Agric. 2003, 83, 899–904. DOI: 10.1002/jsfa.1425.
- Moy, Y. S.; Lu, T. J.; Chou, C. C. Volatile Components of the Enzyme-Ripened Sufu, a Chinese Traditional Fermented Product of Soy Bean. J. Biosci. Bioeng. 2012, 113, 196–201. DOI: 10.1016/j.jbiosc.2011.09.021.
- Yvon, M.; Rijnen, L. Cheese Flavour Formation by Amino Acid Catabolism. Int. Dairy J. 2001, 11, 185–201. DOI: 10.1016/S0958-6946(01)00049-8.
- Ardö, Y. Flavour Formation by Amino Acid Catabolism. Biotechnol. Adv. 2006, 24, 238–242. DOI: 10.1016/j.biotechadv.2005.11.005.
- Banks, J. M.; Yvonb, M.; Griponb, J. C.; Fuentea, M. A. D. L.; Brechanya, E. Y.; Williams, A. G.; Muir, D. D. Enhancement of Amino Acid Catabolism in Cheddar Cheese Using α-ketoglutarate: Amino Acid Degradation in Relation to Volatile Compounds and Aroma Character. Int. Dairy J. 2001, 11, 235–243. DOI: 10.1016/S0958-6946(01)00053-X.
- Rijnen, L.; Courtin, P.; Gripon, J. C.; Yvon, M. Expression of a Heterologous Glutamate Dehydrogenase Gene in Lactococcus Lactis Highly Improves the Conversion of Amino Acids to Aroma Compounds. Appl. Environ. Microbiol. 2000, 66, 1354–1359. DOI: 10.1128/AEM.66.4.1354-1359.2000.
- Cai, R. C.; Li, L.; Yang, M.; Cheung, H. Y.; Fu, L. Changes in Bioactive Compounds and Their Relationship to Antioxidant Activity in White Sufu during Manufacturing. Int. J. Food Sci. Technol. 2016, 51, 1721–1730. DOI: 10.1111/ijfs.13149.
- Xia, X.; Li, G.; Zheng, J.; Ran, C.; Kan, J. Biochemical, Textural and Microstructural Changes in Whole-Soya Bean Cotyledon Sufu during Fermentation. Int. J. Food Sci. Technol. 2014, 49, 1834–1841. DOI: 10.1111/ijfs.12492.
- Feng, Z.; Chen, H.; Lv, X. T.; Deng, H. L.; Chen, X.; Li, J. J.; Guo, L. Accelerated Ripening of Kedong Sufu with Autochthonous Starter Cultures Kocuria Rosea KDF3 and Its Protease KP3 as Adjuncts. J. Appl. Microbiol. 2014, 116, 877–889. DOI: 10.1111/jam.12433.
- AOAC. Official Methods of Analysis of the AOAC, 18th ed.; Association of Official Analytical Chemists: Washington D.C., USA, 2006.
- Dalkyoung, Y.; Hongkyoon, N.; Prinyawiwatkul, W. Physicochemical and Functional Properties of Chitosans Affected by Sun Drying Time during Decoloration. LWT - Food Sci. Technol. 2009, 42, 1553–1556. DOI: 10.1016/j.lwt.2009.05.004.
- Leonard, K.; Wildi, B. S. Proteases of the Genus Bacillus. I. Neutral Proteases. Biotechnol. Bioeng. 1970, 12, 179. DOI: 10.1002/bit.260120205.
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus Subtilis Strains in Thua Nao, a Traditional Fermented Soybean Food in Northern Thailand. Lett Appl. Microbiol. 2010, 43, 237–242. DOI: 10.1111/j.1472-765X.2006.01966.x.
- Boutrou, R.; Sepulchre, A.; Gripon, J. C.; Monnet, V. Simple Tests for Predicting the Lytic Behavior and Proteolytic Activity of Lactococcal Strains in Cheese. J. Dairy Sci. 1998, 81, 2321–2328. DOI: 10.3168/jds.S0022-0302(98)70121-3.
- Yvon, M.; Thirouin, S.; Rijnen, L.; Fromentier, D.; Gripon, J. C. An Aminotransferase from Lactococcus Lactis Initiates Conversion of Amino Acids to Cheese Flavor Compounds. Appl. Environ. Microbiol. 1997, 63, 414–419.
- Li, Y.; Yu, R.; Chou, C. Some Biochemical and Physical Changes during the Preparation of the Enzyme-Ripening Sufu, a Fermented Product of Soybean Curd. J. Agric. Food Chem. 2010, 58, 4888–4893. DOI: 10.1021/jf904600a.
- Cardoso, V. M.; Borelli, B. M.; Lara, C. A.; Soares, M. A.; Pataro, C.; Bodevan, E. C.; Rosa, C. A. The Influence of Seasons and Ripening Time on Yeast Communities of a Traditional Brazilian Cheese. Food Res. Int. 2015, 69, 331–340. DOI: 10.1016/j.foodres.2014.12.040.
- Borla, O. P.; Davidovich, L.; Roura, S. Isolation and Characterization of Proteolytic Microorganisms from Fresh and Fermented Cabbage. LWT - Food Sci. Technol. 2010, 43, 298–301. DOI: 10.1016/j.lwt.2009.07.006.
- Trade standard SB/T 10170. Fermented Bean Curd (Sufu); Ministry of Commerce of the People’s Republic of China: Beijing, 2007.
- Herranz, B.; Fernández, M.; Hierro, E.; Bruna, J. M.; Ordóñez, J. A.; De, L. H. L. Use of Lactococcus Lactis Subsp. Cremoris NCDO 763 and α-ketoglutarate to Improve the Sensory Quality of Dry Fermented Sausages. Meat Sci. 2004, 66, 151–163. DOI: 10.1016/S0309-1740(03)00079-2.
- Yvon, M.; Berthelot, S.; Gripon, J. C. Adding Alpha-Ketoglutarate to Semi-Hard Cheese Curd Highly Enhances the Conversion of Amino Acids to Aroma Compounds. Int. Dairy J. 1998, 8, 889–898. DOI: 10.1016/S0958-6946(99)00011-4.
- Tjener, K.; Stahnke, L. H.; Andersen, L.; Martinussen, J. Addition of α-ketoglutarate Enhances Formation of Volatiles by Staphylococcus carnosus during Sausage Fermentation. Meat Sci. 2004, 67, 711–719. DOI: 10.1016/j.meatsci.2004.02.003.
- Tseng, Y. H.; Lee, Y. L.; Li, R. C.; Mau, J. L. Non-Volatile Flavour Components of Ganoderma tsugae. Food Chem. 2005, 90, 409–415. DOI: 10.1016/j.foodchem.2004.03.054.
- Qin, L.; Ding, X. Evolution of Proteolytic Tasty Components during Preparation of Douchiba, a Traditional Chinese Soy-Fermented Appetizer. Food Technol. Biotechnol. 2007, 45, 85–90.
- Dajanta, K.; Apichartsrangkoon, A.; Chukeatirote, E.; Frazier, R. A. Free-Amino Acid Profiles of Thua Nao, a Thai Fermented Soybean. Food Chem. 2011, 125, 342–347. DOI: 10.1016/j.foodchem.2010.09.002.
- Lioe, H. N.; Apriyantono, A.; Takara, K.; Wada, K.; Yasuda, M. Umami Taste Enhancement of MSG/NaCl Mixtures by Subthreshold L‐α‐aromatic Amino Acids. J. Food Sci. 2005, 70, s401–s405. DOI: 10.1111/j.1365-2621.2005.tb11483.x.
- Zhao, C. J.; Schieber, A.; Gänzle, M. G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations – A Review. Food Res. Int. 2016, 89, 39–47. DOI: 10.1016/j.foodres.2016.08.042.
- Faraj, A.; Vasanthan, T. Soybean Isoflavones: Effects of Processing and Health Benefits. Food Rev. Int. 2004, 20, 51–75. DOI: 10.1081/FRI-120028830.
- Zhang, X.; Qu, Y.; Ma, Q.; Zhang, Z.; Li, D.; Wang, J.; Shen, W.; Shen, E.; Zhou, J. Illumina MiSeq Sequencing Reveals Diverse Microbial Communities of Activated Sludge Systems Stimulated by Different Aromatics for Indigo Biosynthesis from Indole. PLoS One. 2015, 10, e125732. DOI: 10.1371/journal.pone.0125732.
- Dhakal, R.; Bajpai, V. K.; Baek, K. H. Production of GABA (γ-Aminobutyric Acid) by Microorganisms: A Review. Braz. J. Microbiol. 2012, 43, 1230–1241. DOI: 10.1590/S1517-83822012000400001.
- Zhao, C.; Zhang, Y.; Wei, X.; Hu, Z.; Zhu, F.; Xu, L.; Luo, M.; Liu, H. Production of Ultra-High Molecular Weight Poly-γ-glutamic Acid with Bacillus licheniformis P-104 and Characterization of Its Flocculation Froperties. Appl. Biochem. Biotechnol. 2013, 170, 562–572. DOI: 10.1007/s12010-013-0214-2.
- Wu, Q.; Shah, N. P. High γ-aminobutyric Acid Production from Lactic Acid Bacteria: Emphasis on Lactobacillus brevis as a Functional Dairy Starter. Crit. Rev. Food Sci. Nutr. 2016. DOI: 10.1080/10408398.2016.1147418.
- Yamaya, A.; Endo, Y.; Fujimoto, K.; Kitamura, K. Effects of Genetic Variability and Planting Location on the Phytosterol Content and Composition in Soybean Seeds. Food Chem. 2007, 102, 1071–1075. DOI: 10.1016/j.foodchem.2006.07.001.
- Ma, Y.; Wang, J.; Cheng, Y.; Yin, L.; Li, L. Some Biochemical and Physical Changes during Manufacturing of Grey Sufu, a Traditional Chinese Fermented Soybean Curd. Int. J. Food Eng. 2013, 9, 45–54. DOI: 10.1515/ijfe-2012-0204.
- Han, B. Z.; Cao, C. F.; Rombouts, F. M.; Nout, M. J. R. Microbial Changes during the Production of Sufu-A Chinese Fermented Soybean Food. Food Control. 2004, 15, 265–270. DOI: 10.1016/S0956-7135(03)00066-5.
- Yasuda, M.; Tachibana, S.; Kuba-Miyara, M. Biochemical Aspects of Red Koji and Tofuyo Prepared Using Monascus Fungi. Appl. Microbiol. Biotechnol. 2012, 96, 49–60. DOI: 10.1007/s00253-012-4300-0.
- Li, L.; Wang, J. Comparative Study of Chemical Composition and Texture Profile Analysis between Camembert Cheese and Chinese Sufu. Biotechnol. Front. 2012, 1, 1–8.