ABSTRACT
Different pretreatments can affect the quality of Hypsizygus marmoreus (HM). In this study, the effects of thermal treatment or combined with color protection and hardening on color, texture, microstructure, polyphenol oxidase (PPO) and Peroxidase (POD) C-1: Explain the first time? activities, and the volatile components of HM were investigated. The results showed compared with the control group, both heating group (H group) and color protection, hardening and heating (CHH group) significantly enhanced the color difference, and reduced all texture parameters as well as PPO and POD activities. Additionally, in two treatment groups, the structure of HM became loose, the amounts of alcohols, ketones, and aldehydes were changed significantly. Compared with the H group, the color difference and PPO activities in CHH group were significantly decreased, while all texture parameters were increased significantly, the fibers of HM showed regular arrangement. In conclusion, the combination of CHH performed better effects on hardness, chewiness, microstructure, and enzymes activities related to enzymatic browning.
Introduction
Mushroom, as a kind of food, has been consumed by many countries in the world for centuries.[Citation1]Edible mushrooms are considered to be the valuable health foods which are rich in essential amino acids, dietary fibers, vitamins, and trace minerals, and are low in fat and calories.[Citation2] Besides, they also contain diverse bioactive compounds such as polysaccharides, phenolics, polyphenolics, and lectins which present significant benefits to human health.[Citation1] Hypsizigus marmoreus (HM), also known as hon-shimeji, is a kind of popular edible mushroom in China, Korea, and Japan.[Citation3] The production of HM is continuously increased in these countries during recent years, due to not only its nutritional values but also the desirable flavor and unique texture.[Citation4] Additionally, the medicinal properties of the biological active compounds extracted from HM have drawn many attentions of the researchers. There are accumulated investigations which have reported the antitumor and antioxidant effects of polysaccharide[Citation5] the antihypertensive effect of peptides[Citation6] and antiallergic effects of ethanolic extracts in HM.[Citation7]
The quality losses including the crown browning, the water loss, and body perishing induced by the long postharvest time, reduce the market value of HM. Therefore, the processing or preservation of this species gets more attention to increase its flavor, nutritional benefit, and consumption.[Citation8] Previous studies have investigated different methods to retain the freshness of mushrooms, like packaging, refrigeration, coating, and special treatments.[Citation9] Xing et al. found that the postharvest quality loss was significantly reduced in HM wrapped with the biaxially oriented polypropylene film.[Citation10] Moreover, Chen et al. reported that the treatment of hydrogen-rich water suppressed the rot incidence by enhancing the antioxidant defense system of HM.[Citation11] The morphological ripeness and texture properties are generally used to judge the quality of edible mushrooms.[Citation12] Notably, the volatile compounds can also be the valuable indexes to reflect the flavor quality.[Citation13] It was demonstrated that the concentration of volatile compounds decreased considerably with the prolonged storage time.[Citation14] However, there are seldom studies on the effects of heating treatment on the alterations in volatile compounds of HM.
Therefore, the profiles of the volatile compounds, texture properties, ultrastructure, and the activities of enzymes related to enzymatic browning in HM pretreated with color protection, hardening, and heating were investigated in the present study.
Materials and methods
Sample preparation
The freshly harvested HM were friendly provided by Shanxi Dingchang Agricultural Science and Technology Co., LTD. The mushrooms were washed thoroughly and the samples with a diameter of 1.0 cm to 1.2 cm and without hollow and body lesions were selected for next treatments. With fresh HM as the control samples, the HM in the heating group (H group) was treated with 80°C for 10 min in hot water. In group with the combination of color protection, hardening, and heating (CHH group), HM was soaked with the mixed liquor (it is prepared according to the concentration of citric acid, NaCl2 and CaCl2 in the solution 0.1%, 2%, and 0.1%, respectively) for 5-min then was heat treated with 85°C for 10-min, and then cooled rapidly with cold water.
Color difference
The color of HM samples was measured by using Chromameter. Ten pieces of HM were bundled together after treatment. The rope avoided 5 cm from the handle under the mushroom cover. The probe of the colorimeter was placed 5 cm from the handle to the mushroom cover to ensure that the detected part would not be translucent. The Mushroom handle was measured at 6 cm under the mushroom cover. There were three bundles of the mushroom stalk, each bundle was calculated five times, and the average value was calculated to get the total color difference for each sample.
Instrumental texture assessment
The texture of non-processed and treated HM was detected by a texture analyzer. The HM sample was punctured under the model of TPA with a 6 mm diameter cylinder probe, 0.3 N primary stress, and 5 s interval time, at a rate of 90 mm/min. The speeds before or after puncture test were 200 mm/min with the data acquisition rate of 200 times per second. The values of hardness, springiness, cohesiveness, and chewiness were determined for different HM samples.
Microstructure analysis
The HM samples from different groups were cut into small pieces about 3 mm3 and then fixed in 4% glutaraldehyde overnight. After dehydration in a graded series of ethanol, samples were immersed into isoamyl acetate, and finally coated with gold. The microstructure of HM samples was observed by the scanning electron microscopy (S-4800, Hitachi, Ltd., Japan).
PPO and POD activities assay
The activities of PPO and POD in HM were measured with the reagent kits purchased from the Tianjin Jiancheng Bioengineering Institute. The total proteins were extracted according to the manufacturer’s instructions. PPO and POD activities were assessed using the spectrophotometer at 410 nm and 470 nm, respectively.
Analysis of volatile components
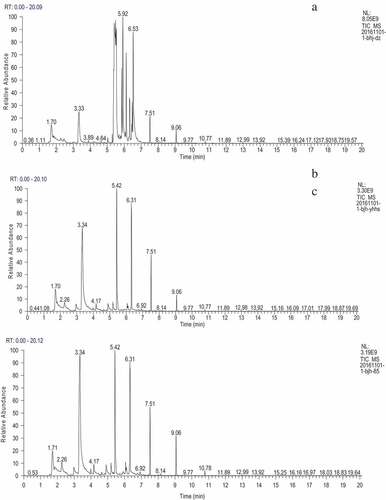
The different groups of HM were mashed with plant smashing machine and stored at −18 C for storage. Samples were extracted in a sealed headspace bottle of 5.0 g at 15 mL for 30-min by solid phase microextraction (SPME) fiber DVB/CAR/PDMS 15 mL (aged at 250°C for 3 h before use). then sampling after 3 min desorption at 230°C. The gas chromatography/mass spectrometry (GC/MS) analysis was applied to analyze the volatile components in fresh and treated HM samples. Chromatographic column used in this study was 30 m × 0.25 mm. The GC condition was programmed as follows: initial 50°C for 3 min followed by the first temperature increase up to 150°C with a speed of 50°C/min and the second increase up to 230°C with a speed of 10°C/min. The inlet temperature was 230°C. Carrier gas was helium at a flow rate of 1 mL/min. MS was conducted in the EI mode with the ionization energy of 70 eV and a mass range of 35–335 amu/sec in full-scan mode.
Statistical analysis
Experimental data were analyzed by one-way ANOVA using the GraphPad Prism. The results were expressed as mean ± standard deviation. The asterisk means the significant difference between control and treatment groups (* P < 0.05 and ** P < 0.01). The trilateral means the significant difference between H and CHH groups (▽ P < 0.05 and ▽▽ P < 0.01).
Results and discussion
Color changes
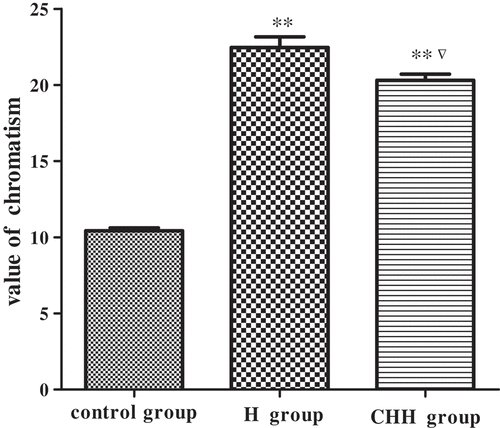
The changes in the color of HM treated with heating, hardening, and color protection are shown in . Compared to the control group, the value of charomatism is significantly enhanced in HM receiving thermal treatment alone and the combination of all the three pretreatments, indicating a very noticeable color change. The similar effect of temperature on color difference was also observed in potato.[Citation15] The increasing value of charomatism could be due to the low-enzymatic browning, and the alteration in heat-sensitive components.[Citation16]
Figure 1. Effect of different pretreatment on the color of Hypsizygus marmoreus. ** P < 0.01 indicates a statistical difference between control and treatment groups. ▽ P < 0.05 indicates a statistical difference between H and CHH groups. Values are mean ± SD. H group: heating group; CHH group: color protection, hardening, and heating group
Texture
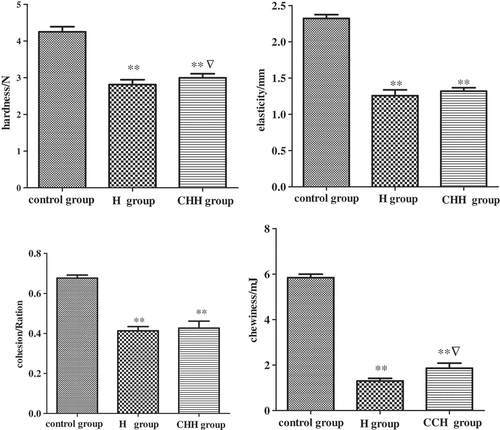
The textures of HM under different treatment conditions are presented in . The results showed that the thermal treatment alone or combined treatments had significant effects on hardness, springiness, cohesiveness, and chewiness in HM. Compared to the fresh HM in control group, the hardness of samples in all treatment groups decreased significantly, which could be induced by the water loss. This phenomenon is repeatedly observed in other plant.[Citation17] Meanwhile, with the comparison to the control group, the springiness, cohesiveness, and chewiness were all decreased under heating and combined treatment groups. Notably, the hardness and chewiness are significantly higher in the combined treatment group than those in the thermal group, indicating that the pretreatments of color protection and hardness together with heating improved the textures of HM.
Figure 2. Effect of different pretreatment on the texture of Hypsizygus marmoreus ** P < 0.01 indicates a statistical difference between control and treatment groups. ▽ P < 0.05 indicates a statistical difference between H and CHH groups. Values are mean ± SD. H group: heating group; CHH group: color protection, hardening, and heating group
Microstructure
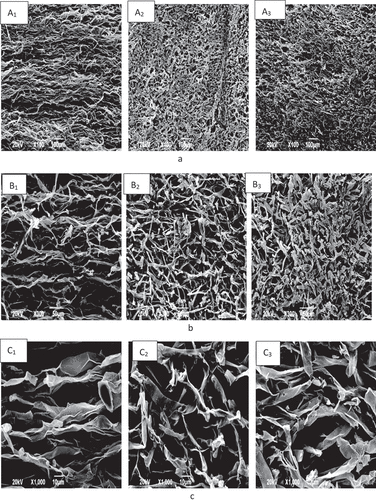
The representative microstructure photographs of control and treated HM are showed in with three magnifications. There are apparent differences among HM samples with different treatments. In the control group, the fibers of the HM body showed regular and conferted arrangement with distinct fibril structure (A1 and A2). After heating, the fibers in mushrooms become shrunken and arranged disparately (B1 and B2). When the HM samples are subjected to heating combined with color protection and harden treatments, the arrangement of fibers shows regular but loosening (C1 and C2). Under the high power, the fibers present integrated structure (A3), while in the thermal treatment group, the vertexes of fibers are broken and the fibers become deformity (B3). The reason for this phenomenon could be that the thermal treatment increased the friability of fiber, resulting in the disintegrated fiber structure. Notably, the fibers in HM receiving combined treatments remain relatively intact structure without obvious deformities, indicating that the hardening treatment may play a certain role in the protection of fibril microstructure, although a few vertex broken exist which is due to the heating (C3).
Figure 3. Microstructure analysis of Hypsizygus marmoreus using scanning electron microscopy. A1, B1, and C1 are from control group; A2, B2, and C2 are from heating group; A3, B3, and C3 are from color protection, hardening, and heating group. A1, A2, and A3 × 100; B1, B2, and B3 × 300; C1, C2, and C3 × 1000
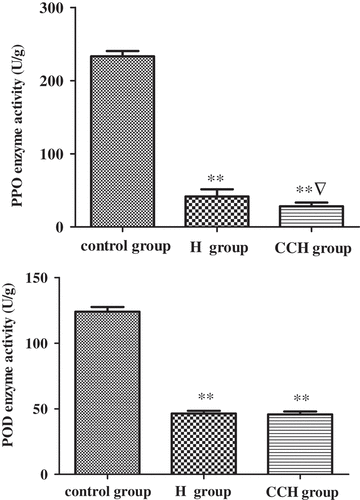
Activities of PPO and POD
The activity of PPO in HM receiving thermal, hardening, and color protection treatments is presented in . PPO, as an enzyme commonly found in vegetables and fruits, is considered to contribute to enzymatic browning.[Citation15] PPO can catalyze the oxidation of o-dihydroxy compounds to the corresponding quinines which later forms the dark-colored pigments through polymerization reactions.[Citation18] Therefore, the inhibition of enzymatic browning is crucial important in the food industry. In this study, compared with the control group, the activities of PPO in the two pretreatment groups are significantly decreased, and the PPO activity in the combined treatment group is also significantly lower when compared to the heating group, which suggests that the combined treatment shows better protection role than heating alone. POD functions in eliminating the cellular peroxides and protect the membrane structure against the attack from peroxides.[Citation19] It also plays important roles in suppressing browning reaction.[Citation20] In the current study, the activities of POD reduced significantly in pretreatment groups, with the comparison to the control group, suggesting that both the combined treatment and the heating could inhibit the browning of HM.
Figure 4. Effect of different pretreatment on brown-related enzyme activity of Hypsizygus marmoreus. ** P < 0.01 indicates a statistical difference between control and treatment groups. ▽ P < 0.05 indicates a statistical difference between H and CHH groups. Values are mean ± SD. H group: heating group; CHH group: color protection, hardening, and heating group
Volatile aroma compounds
The volatile compounds of HM samples from different groups are presented in . Results showed that the compounds in fresh HM included three aldehydes, nine alcohols, five esters, two ketones, three alkanes, and one acid. The GC-MS analysis of the fresh HM samples is shown in . According to the aroma volatile profile of fresh HM in this study, the most prominent compounds are alcohols with 55.36%, followed by ketones with 25.44%. Aldehydes are somewhat less amount with 10.14%, whereas alkanes, esters, acid account for low percentages of 0.26%, 0.22%, and 0.01%, respectively, indicating that the prominent aroma compounds in HM are alcohols, ketones, and aldehydes. In HM treated with heating, there are 44.74% of alcohols, 1.07% of aldehydes, 1.09% of alkanes, 0.34% of esters, and 0.04% of acid, while in the combination treatment group, the most prominent compound is alcohols with 42.72%, followed by 0.88% of aldehydes, 0.28% of alkanes, and 0.08% of esters. Compared to the control group, the accounts of ethyl alcohol, isobutanol, 2-methylbutanol, hexanal in pretreatment groups are higher than those in control HM, whereas, (E)-2-Octenal, nonanal, 3-octanone, and 1-octene are decreased in pretreatment HM. Results from other studies also showed that alcohols, ketones, and aldehydes are the main volatile compounds which contribute to the desirable flavor of mushroom.[Citation13,Citation21] The changes and decreases in alcohols, ketones, and aldehydes induced by pretreatments may partly result from the interaction of macromolecular compounds, like protein, polysaccharide, and lipids, with aroma and taste compounds.[Citation22] Another reason for the loss of volatile compounds is the high temperature during the thermal treatment. However, the detailed mechanism underlying the loss of volatile compounds by combined pretreatments of color protection, hardening, and heating requires further investigations.
Table 1. The effect of different treatments on volatile components (H group: heating group; CHH group: color protection, hardening, and heating group)
Conclusion
In this study, the pretreatment induced changes in color, texture, microstructure, activities of PPO and POD, and the contents of volatile compounds in HM are observed. Compared to the thermal treatment alone, the combination of color protection, hardening, and heating preserve better qualities of HM such as hardness, chewiness, microstructure, and PPO activities. It still needs further studies to efficiently preserve the flavor substances of HM during the process of pretreatments.
Additional information
Funding
References
- Roncero-Ramos, I.; Delgado-Andrade, C. The Beneficial Role of Edible Mushrooms in Human Health. Curr. Opin. Food Sci. 2017, 14, 122–128. DOI: 10.1016/j.cofs.2017.04.002.
- Thatoi, H.; Singdevsachan, S. K. Diversity, Nutritional Composition and Medicinal Potential of Indian Mushrooms: A Review. Afr. J. Biotechnol. 2014, 13(4), 523–545. DOI: 10.5897/AJB2013.13446.
- Zhang, J.; Shi, L.; Chen, H.; Sun, Y.; Zhao, M.; Ren, A.; Chen, M.; Wang, H.; Feng, Z. An Efficient Agrobacterium-Mediated Transformation Method for the Edible Mushroom Hypsizygus Marmoreus. Microbiol. Res. 2014, 169(9–10), 741–748. DOI: 10.1016/j.micres.2014.01.004.
- Tsai, P. F.; Ma, C. Y.; Wu, J. S. A Novel Glycoprotein from Mushroom Hypsizygus Marmoreus (Peck) Bigelow with Growth Inhibitory Effect against Human Leukemic U937 Cells. Food Chem. 2013, 141(2), 1252–1258. DOI: 10.1016/j.foodchem.2013.04.024.
- Akavia, E.; Beharav, A.; Wasser, S. P.; Nevo, E. Disposal of Agro-Industrial By-Products by Organic Cultivation of the Culinary and Medicinal Mushroom Hypsizygus Marmoreus. Waste Manag. 2009, 29(5), 1622–1627. DOI: 10.1016/j.wasman.2008.10.024.
- Kang, M. G.; Kim, Y. H.; Bolormaa, Z.; Kim, M. K.; Seo, G. S.; Lee, J. S. Characterization of an Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from the Edible Mushroom Hypsizygus Marmoreus. Biomed Res. Int. 2013, (2013), 283964.
- Sano, M.; Yoshino, K.; Matsuzawa, T.; Ikekawa, T. Inhibitory Effects of Edible Higher Basidiomycetes Mushroom Extracts on Mouse Type IV Allergy. Int. J. Med. Mushrooms. 2002, 4(1), 5–41. DOI: 10.1615/IntJMedMushr.v4.i1.
- Singhal, S.; Rasane, P.; Kaur, S.; Garba, U.; Singh, J.; Raj, N.; Gupta, N. Mushroom Cultivation, Processing and Value Added Products: A Patent Based Review. Recent Pat Food Nutr. Agric. 2018, 10. Epub ahead of print. DOI:10.2174/2212798410666180604101353.
- Chen, H.; Zhang, J.; Hao, H.; Feng, Z.; Chen, M.; Wang, H.; Ye, M. Hydrogen-Rich Water Increases Postharvest Quality by Enhancing Antioxidant Capacity in Hypsizygus Marmoreus. AMB Express. 2017, 7(1), 221. DOI: 10.1186/s13568-017-0496-9.
- Xing, Z.; Wang, Y.; Feng, Z.; Tan, Q. Effect of Different Packaging Films on Postharvest Quality and Selected Enzyme Activities of Hypsizygus Marmoreus Mushrooms. J. Agric. Food Chem. 2008, 56(24), 11838–11844. DOI: 10.1021/jf8024387.
- Taghizadeh, M.; Gowen, A.; Ward, P.; O’Donnell, C. P. Use of Hyperspectral Imaging for Evaluation of the Shelf-Life of Fresh White Button Mushrooms (Agaricus Bisporus) Stored in Different Packaging Films. Innovative Food Sci. Emerg. Technol. 2010, 11(3), 423–431. DOI: 10.1016/j.ifset.2010.01.016.
- Harada, A.; Yoneyama, S.; Doi, S.; Aoyama, M. Changes in Contents of Free Amino Acids and Soluble Carbohydrates during Fruit-Body Development of Hypsizygus Marmoreus. Food Chem. 2003, 83(3), 343–347. DOI: 10.1016/S0308-8146(03)00093-1.
- Donglu, F.; Wenjian, Y.; Kimatu, B. M.; Liyan, Z.; Xinxin, A.; Qiuhui, H. Comparison of Flavour Qualities of Mushrooms (Flammulina Velutipes) Packed with Different Packaging Materials. Food Chem. 2017, 232, 1–9. DOI: 10.1016/j.foodchem.2017.03.161.
- Jl, M.; Beelman, R. B.; Ziegler, G. R.; Royse, D. J. Effect of Nutrient Supplementation on Flavor, Quality, and Shelf Life of the Cultivated Mushroom, Agaricus Bisporus. Mycologia. 1991, 83(2), 142–149. DOI: 10.1080/00275514.1991.12025990.
- Zhang, Z.; Wang, J.; Zhang, X.; Shi, Q.; Xin, L.; Fu, H.; Wang, Y. Effects of Radio Frequency Assisted Blanching on Polyphenol Oxidase, Weight Loss, Texture, Color and Microstructure of Potato. Food Chem. 2018, 248, 173–182. DOI: 10.1016/j.foodchem.2017.12.065.
- Mz, D.; Rahman, R. A.; Tan, C. P.; Bakar, J.; Olusegun, L. Effect of Blanching on Enzyme Activity, Color Changes, Anthocyanin Stability and Extractability of Mangosteen Pericarp: A Kinetic Study. J. Food Eng. 2016, 178, 12–19. DOI: 10.1016/j.jfoodeng.2016.01.001.
- Rocculi, P.; Romani, S.; Galindo, F. G.; Rosa, M. D. Effect of Minimal Processing on Physiology and Quality of Fresh-Cut Potatoes: A Review. Global Sci. Books Food. 2009, 3(SI1), 18–30.
- Aal, T.; de CastroLeite Júnior, B. R.; Oliveira, M. M.; Cristianini, M. High Pressure Processing of Cocoyam, Peruvian Carrot and Sweet Potato: Effect on Oxidative Enzymes and Impact in the Tuber Color. Innovative Food Sci. Emerg. Technol. 2016, 34, 302–309. DOI: 10.1016/j.ifset.2016.02.010.
- Ba, K.; Singh, S. P. Enzyme Stability, Thermodynamics and Secondary Structures of Alpha-Amylase as Probed by the CD Spectroscopy. Int. J. Biol. Macromol. 2015, 81, 450–460. DOI: 10.1016/j.ijbiomac.2015.08.032.
- Zhang, L.; Yu, Z.; Jiang, L.; Jiang, J.; Luo, H.; Fu, L. Effect of Post-Harvest Heat Treatment on Proteome Change of Peach Fruit during Ripening. J. Proteomics. 2011, 74(7), 1135–1149. DOI: 10.1016/j.jprot.2011.04.012.
- Yang, W.; Yu, J.; Pei, F.; Mariga, A. M.; Ma, N.; Fang, Y.; Hu, Q. Effect of Hot Air Drying on Volatile Compounds of Flammulina Velutipes Detected by HS-SPME-GC-MS and Electronic Nose. Food Chem. 2016, 196, 860–866. DOI: 10.1016/j.foodchem.2015.09.097.
- Saha, B.; Bucknall, M. P.; Arcot, J.; Driscoll, R. Profile Changes in Banana Flavour Volatiles during Low Temperature Drying. Food Res. Int. 2018, 106, 992–998. DOI: 10.1016/j.foodres.2018.01.047.