?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Water holding capacity, texture characteristics, and qualitative structural properties had been mainly focused on heat-induced gelation of myofibrillar proteins; however, the quantitative analysis to ultrastructure of the proteins and heat-induced gel was rarely reported in the previous study. The objective of this study was to investigate quantitatively the effects of different pH values and ion concentrations on the ultrastructure of myofibrillar proteins and thermal-induced gel from swine longissimus dorsi using atomic force microscopy. Protein and gel groups in different conditions were set up, respectively, for the comparative study. The proteins of the gel group were treated first by water and 0.1 M, 0.3 M, 0.6 M NaCl solutions separately at pH 5.5, 6.5, 7.5, and then heated in the water bath. However, protein group were treated without the water bath but directly dried in the air at room temperature. Image roughness Rq were used as an index for quantitative analysis. Atomic force microscopy and related offline analysis software were used to collect the height images and determine the roughness of the myofibrillar proteins and heat-induced gel, respectively. It had been indicated that pH values and ionic concentrations had shown a significant effect on the roughness of heat-induced gel and myofibrillar proteins according to the atomic force microscopy (AFM) height images. The roughness of the gel is minimal while the myofibrillar proteins were treated by 0.6 M NaCl at pH 7.5. It is suggested that roughness of AFM height images could be used as an index to determine the gelling conditions.
Introduction
Meat is animal flesh which is eaten as one kind of food. Animals hunting, domestication, breeding, improvement had been used gradually for meat and meat production since prehistoric times. Meat is edible raw but is normally eaten after cooked and seasoned or processed in a variety of ways. Meat consists of a variety of proteins with the specific structures and functions. Due to the functional properties, which contribute to meat, the meat proteins are divided into three general classifications, i.e. myofibrillar, stromal, and sarcoplasmic proteins. Myofibrillar proteins are the collective proteins that comprise the myofibril including actin, myosin and several other structural proteins in muscle fibers. In which, actin and myosin are the most important components of myofibrillar proteins for muscle fiber structure, and are the most abundant proteins in muscle which are directly involved in the muscle contraction and relaxation. Myofibrillar proteins are salt-soluble proteins, which are soluble in high ionic strength solution, i.e. 0.3 M or higher. Stromal proteins such as collagen, elastin, and reticulin are the main components in the connective tissues and extracellular matrix. Stromal proteins as insoluble proteins cannot be dissolved in high ionic strength of the salt solution. The sarcoplasmic proteins such as hemoglobin, myoglobin pigments, and a wide variety of enzymes help to contribute to the red color of muscle, and continue to function in meat aging. Sarcoplasmic proteins can be dissolved in low ionic strength solution. Among the three kinds of proteins, the myofibrillar proteins account for roughly half of meat proteins.[Citation1] The heat-induced gels refer to the aggregation between the denaturation or non-denaturation of protein molecules, and orderly process of 3D network structure. The forming force is mainly the interaction between proteins and water molecules, and the forces of attraction and repulsion among neighboring peptides to reach phase equilibrium between the adjacent peptides.[Citation2,Citation3] The gelation characteristics of myofibrillar proteins can reflect the quality of meat products and its performance during gelation, which has far-reaching significance to maintain quality and develop processed meat products.[Citation4]
It is a complex dynamic process that the secondary structure and the structural features of the myofibrillar proteins changed significantly in the process of heating.[Citation5] Heat-induced gelation properties based on interactions among myofibrillar proteins are dependent on many factors, such as pH, ionic strength, protein concentration, type of muscle as well as the heat-induced temperature and so forth,[Citation6,Citation7] especially pH and ionic strength.[Citation1,Citation8,Citation9] The previous studies had mainly focused on water-holding capacity, texture, and the qualitative microstructure;[Citation10–Citation15] however, quantitative ultrastructural studies are rarely executed in details. The aim of this study was to investigate the effect of different sodium ionic strength, pH value on the ultrastructure of myofibrillar proteins, and their heat-induced gels from swine longissimus dorsi muscle. The surface roughness of the height images of proteins and gels that observed and collected with by atomic force microscopy was analyzed to identify the specific effects of pH values and ionic strength on the ultrastructural properties as a geometric parameter index.
Materials and methods
Extraction of myofibrillar proteins
Myofibrillar proteins were prepared from swine longissimus dorsi, which bought from the Trust-Mart in Yangling at Shaanxi according to the method of Wu[Citation16] with minor changes, and stored at −20℃ until extraction. The pork is the production from Yurun Company and slaughtered on 6 months old and always kept in 4℃ refrigerator after slaughtering. The swine longissimus dorsi was thawed at 4℃ for 24 h before it was chopped and mixed with five times volume of precooled isolation buffer (Buffer A: 0.1 mol/L NaCl, 10 mmol/L Na2HPO4, 2 mmol/L MgCl2, 1 mmol/L EGTA, pH7.0). After discarding the upper red liquid, the pellet below was resuspended in the five times volume of Buffer A, and then homogenated for 30 s at high speed. Homogenate was filtrated through single layer gauze and homogenized in Buffer A again. The mixture was centrifuged at 500 g for 10 min, and then the sediment was resuspended, homogenized with 5 volume of Buffer A, and centrifuged twice in the same conditions. The meat sediment was resuspended, homogenized in 5 volumes of 0.1 mol/L NaCl solution, and then centrifuged at 500 g for 10 min. The steps above were repeated twice. The myofibrillar proteins collected were washed with 5 volumes of distilled water and centrifuged at 5,000 g for 10 min. The protein extracts were dried by vacuum freeze-drying and then stored at 4℃ until further using in proteins gelling and other experiments, respectively.
Preparation of proteins and gels for AFM imaging
The myofibrillar protein concentration was firstly mixed and adjusted to 40 mg/ml with three different concentrations of NaCl solutions (0.1, 0.3, and 0.6 M) at pH value of 5.5, 6.5, and 7.5 individually. Then, 40 μL of the myofibrillar protein solution was deposited onto freshly cleaved mica sheets with micro pipette. The protein gel groups were heated from 30℃ to 70℃ in a water bath gradually and kept at 70℃ for 30 min. The proteins and gels samples on the mica sheets were kept in petri dishes and dried at 4℃ refrigerator. The dried samples were washed with distilled water by three times to remove sodium salt crystal, and then dried in the air at room temperature for AFM imaging.
AFM imaging of proteins and gels
The mica sheets with proteins and gels were fixed onto metal sheets with double-sided adhesive, and then fixed onto the scanner top with magnetic attraction. All samples were scanned with the NanoScope V Multimode-8 AFM at ScanAsyst intelligence mode produced by Bruker Company (Santa Barbara, CA, USA). AFM probes adopted were SCANASYST-AIR type with a triangle cantilever (T = 650 nm, L = 115 μm, W = 25 μm, f0 = 70 kHz, k = 0.4 N/m) made from Silicon Nitride by Bruker Company (Camarillo, CA, USA). An x–y stage positioner and a 30 magnification CCD video, combined with the AFM scanner, were used to determine where the tip could be placed above the sample and adjust the reflect position of Laser on the back of cantilever. When the tip approaches and scans the sample surface, the AFM would collect the height images automatically in 512 pixels with a scan rate of 1 Hz.
Statistical analysis
Typical height images were collected and then statistically analyzed by using the offline software Nanoscope Analysis V1.10 (Bruker Co., Santa Barbara, USA). AFM images (n ≥ 5) were analyzed for each sample to ensure that the results valid and reliable after a second-order flatten, and noisy line erasing. The quantitative data of roughness Rms was recorded as Mean±SD, which was calculated by the function as follows:
where Zi is the random height value, Zave is the average of the height values among the reference markers, and N is the number of points among the reference markers. Data analysis was carried out using Excel (Microsoft Co., USA) and SPSS V.17.0 (IBM Co., USA). All data were analyzed by one-way analysis of variance (ANOVA) for significant differences, with minimum significance set at the 5% level (P < 0.05), followed by Duncan test to find differences.
Results and discussion
Effects of pH values and ion concentration on roughness of proteins
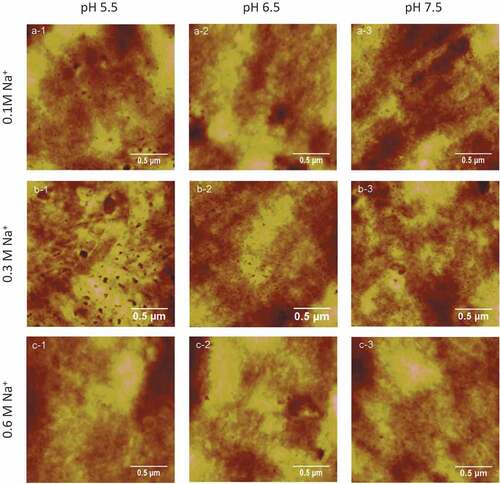
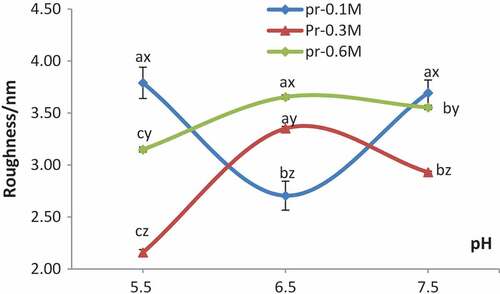
Typical AFM height images of proteins in 0.1, 0.3, 0.6 M sodium chloride at pH5.5, 6.5, 7.5 individually are shown in . Roughness Rms of AFM height images of proteins in different conditions were collected with commercial offline software, as shown in . As presented in , the myofibrillar proteins at pH 6.5 had shown the dramatic increases of roughness Rms with increasing concentration of sodium ions up to 0.6 M. In contrast, the roughness Rms of proteins at pH 5.5 and 7.5, showed great decreases in NaCl solution from 0.1 M to 0.3 M, and dramatic increases from 0.3 M to 0.6 M. Besides, the roughness Rms decreased drastically in 0.1 M NaCl but increased dramatically in 0.3 M and 0.6 M NaCl when pH value varied from 5.5 to 6.5. On the contrary, the roughness increased drastically in 0.1 M NaCl but decreased greatly in 0.3 M and 0.6 M NaCl while pH value changed from 6.5 to 7.5. The roughness Rms of myofibrillar proteins suspended in 0.3 M and 0.6 M NaCl solutions was extremely high at pH 5.5–6.5 and decreased dramatically with increase of the pH value to 7.5. However, the roughness Rms of myofibrillar proteins in 0.1 M NaCl solution showed an opposite tendency compared with that in 0.3 M and 0.6 M NaCl solutions. Shang and his colleagues had found that the solubility of myofibrillar proteins increased with the increment of NaCl concentration, and the solubility was maximum in 0.6 M NaCl solution, besides the solubility of myofibrillar protein in this concentration increased with the pH values which increase from 5.5 to 6.5.[Citation17] In 0.1 M NaCl solutions, the solubility of myofibrillar protein was low, and the protein particles in solution were dispersed more evenly, whereas the solubility increased with increase of the ion concentration to 0.3 M in which proteins were dissolved only partly, so the distribution of proteins was non-uniform and resulted in uneven network structure.[Citation18]
Effects of pH values and ions concentration on the roughness of proteins gels
The typical height images of protein gels treated in three different ions concentrations (0.1, 0.3, 0.6 M) at three different pH values (5.5, 6.5, and 7.5) are shown in , and the effects of pH and ions concentrations on roughness Rms are shown in which were collected from the section analysis and roughness analysis with the height images. As presented in , the heating of myofibrillar proteins at pH 6.5 from 30℃ to 70℃ gradually had resulted in decreases of roughness Rms with increasing concentrations of NaCl solutions up to 0.6 M. On the contrast, similar heating process at pH 5.5 and 7.5 had caused a minor change of roughness Rms when the concentration of NaCl varied from 0.1 M to 0.3 M. Besides, the roughness decreased drastically when the pH values changed from 5.5 to 7.5 in 0.1 M NaCl solution. The roughness Rms of heat-induced gels in 0.6 M NaCl showed the lowest value compared with the other two sodium concentrations, i.e. 0.1 M and 0.3 M. It is to be noted that roughness of heat-induced gels of myofibrillar proteins was shown to be independent of pH and salt concentration. The roughness Rms of the heat-induced gels was minimum in 0.6 M at pH 7.5, which indicated that the surfaces of the heat-induced gels formed under this condition were more even, and the fluctuation of the sample surface is small. It was determined that smaller roughness value was related to better quality of film organization and structure, which is consistent with the study of Guo’s previous studies.[Citation1,Citation19] Fei and his colleagues had studied the effect of pH on the microstructure of the myofibrillar protein gels, and they also discovered that the gel microstructure showed a lower homogeneity and much more aggregation properties under the condition of close to the isoelectric point compared with the neutral condition.[Citation20] It may be that the rate of protein aggregation was greater than the rate of protein degeneration near the isoelectric point, thus producinga larger number of proteins aggregation, and then forming the structure disordered, resulting in a larger roughness Rms.[Citation19] However, with the increase of pH values, the aggregation velocity of proteins was close to that of protein denaturation probably; therefore, the surface morphology of the gel formed could be uniform. Moreover, the quantity and distribution of electrostatic charges on the surface of proteins had been affected by pH values which influenced the folding state of proteins and the interaction among the protein molecules, resulting in an impact on the three-dimensional network structure of heat-induced gels.[Citation21] Electrostatic interaction, hydrophobic interaction, disulfide bonds, and hydrogen bonds played major roles in the formation and maintenance of the gel for the interaction balance of repulsion and attraction between denatured protein molecules.[Citation22] The roughness Rms of the gels were significantly large and the structures of proteins gels were a disorder with a larger block mass at pH 5.5. However, protein is relatively dispersed. Therefore, structure form was affected by pH value in which pH 5.5 was relatively close to the isoelectric point of myofibrillar proteins (PI = 5.0); the repulsion among proteins was weak and easy to aggregate. And the distribution would be more even along with the increases of pH values, Ionic bonds, and the reduction of aggregation degree. Furthermore, the morphology and surface roughness of the protein’s gels in 0.6 M NaCl was better and smaller than that in 0.1 M and 0.3 M NaCl solutions. The roughness of gels in 0.3 M NaCl is greater than that in 0.1 M NaCl except at pH 6.5. The result is consistent with the study from Yang and his colleagues in which myofibrillar protein molecules aggregated roughly and irregularly in low salt buffer.[Citation23] Although there were no good gels formed in 0.1 M NaCl, however the network structure and the distribution of proteins were more even than that in 0.3 M NaCl solution. At the same time, effective electrostatic interactions between protein molecules were also affected by the ionic strength of solution around;[Citation24] charges on the surface of the proteins were neutralized by sodium ions Na+, which resulted in the attraction among the denatured protein molecules and strengthen polymerization to form relatively uniform gels. However, myofibrillar proteins cannot be dissolved well in a low concentration of NaCl solutions, i.e. 0.1 M and 0.3 M, which would cause an uneven interaction among the proteins, so the surface structure of gel formed was rough and clutter finally.
Figure 3. Typical AFM height images of gels in different concentrations of NaCl at various pH values
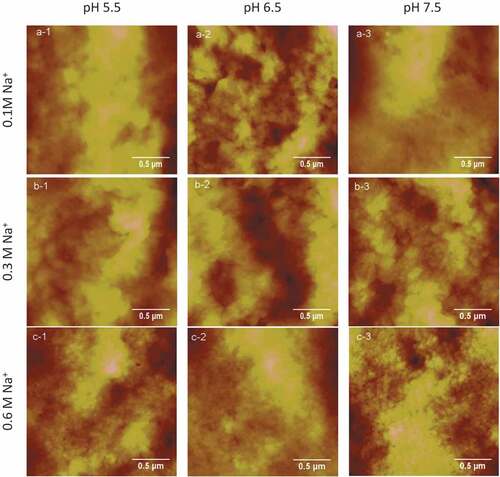
Figure 4. Roughness of gels in different concentrations of NaCl at various pH values
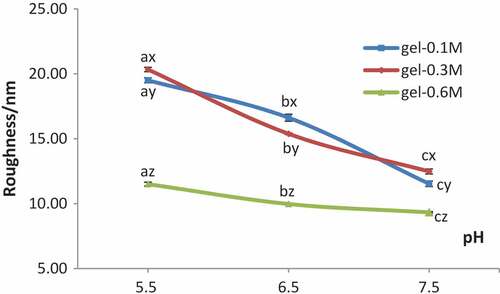
In addition, the roughness Rms of the gels was greater than that of the protein dried naturally. The surface structure of myofibrillar proteins changed visibly in the heating process. Protein degenerated and stretched for heating treatment,[Citation25] which made its amino acid side chains such as internal hydrophobic groups and sulfhydryl exposure and interact together; thus, proteins cross-linked to form stable three-dimensional network structure. Furthermore, heating rate might be one of the important factors. The heating rate adopted in this study was 3℃/min, which means proteins degeneration was rapid and the polymerization time was short in a fast heating, so no uniform gel structure could be formed. The impact of heating rate on gels formation and the surface roughness need a further study.
Conclusion
Myofibrillar proteins are of extreme importance to the functional properties of meat. The data collected above supported the relationship between sodium ion concentration and pH value, which affected the roughness Rms of porcine myofibrillar protein and gels. The surface roughness had a significant difference between proteins and the heat-induced gels in different ion concentrations at pH values. The roughness of the gels treated with 0.6 M NaCl at pH 7.5 was minimal, which suggested better homogeneity would support the related application in meatballs, sausages, and other grounded meat production.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Guo, S. L.; Zhao, G. M.; Wang, Y. F.; Zhang, C. H.; Hao, H. T. Effects of Ionic Strength and pH Values on the Characteristics of Heat-Induced Gelation of Myofibrillar Proteins. Food Sci. Technol. 2008, 33(1), 84–87.
- Dai, J. J.; Han, M. Y.; Xu, X. L.; Zhou, G. H. Effects of Heating Methods on Gel Properties of Chicken Muscle Homogenate. Food Sci. 2014, 35(5), 18–22.
- Wang, P.;. Study on Characteristics of Blood Protein Gelation and Its Influence on Formation of Myofibrillar Protein Gel; Nanjing Agricultural University: Nanjing, 2010.
- Westphalen, A. D.; Briggs, J. L.; Lonergan, S. M. Influence of Muscle Type on Rheological Properties of Porcine Myofibrillar Protein during Heat-Induced Gelation. Meat Sci. 2006, 72(4), 697–703. DOI: 10.1016/j.meatsci.2005.09.021.
- Xu, X. L.; Han, M. Y.; Fei, Y.; Zhou, G. H. Raman Spectroscopic Study of Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Textural Characteristic. Meat Sci. 2011, 87(3), 159–164. DOI: 10.1016/j.meatsci.2010.10.001.
- Florence, L.; Benoit, F.; Ahmed, O.; Culioli, J. Thermal Gelation of Brown Trout Myofibrils from White and Red Muscles: Effect of pH and Ionic Strength. J. Sci. Food Agric. 2002, 82, 452–463. DOI: 10.1002/jsfa.1057.
- Peng, Z. Q.;. Study on Solubility and Gel Functionalities of Salt-Soluble Proteins from Muscles; Nanjing Agricultural University: NanJing, 2005.
- Lavelle, C. L.; Foegeding, E. A. Gelation of Turkey Breast and Thigh Myofibrils: Effects of pH, Salt and Temperature. J. Food Sci. 1993, 58(4), 727–730. DOI: 10.1111/jfds.1993.58.issue-4.
- Lefevre, F.; Culioli, J.; Joandel-Monier, S.; Qulioli, J. Muscle Polymorphism and Gelling Properties of Myofibrillar Proteins from Poultry, Mammals, and Fish. In Quality Attributes of Muscle Foods; Xiong, Y. L., Ho, C. T., Shahidi, F., Eds.; Kulmer Academic/Plenum Publishers: New York, 1999, 365–391.
- Li, Y.; Li, X.; Zhang, C. H.; Sun, H. M.; Dong, X. B. Oxidation and Decrease of Gelling Properties for Meat Myofibrillar Protein Induced by Hydroxyl Radical. Trans. Chin. Soc. Agric. Eng. 2013, 29(12), 286–292.
- Miao, J.; Zou, Y. F.; Wang, P.; Li, C. B.; Xu, X. L. Effects of Washing Treatment and pH Adjustment on Heat-Induced Gelling Properties of Minced Chicken with Low-Salt. Trans. Chin. Soc. Agric. Eng. 2012, 28(24), 287–292.
- Bai, Y.; Zhong, G. L.; Zhou, G. H.; Peng, Z. Q.; Xu, X. L. Effect of Protein Concentration on Heat-Induced Gel Properties of Myosin from Rabbit Psoas Major Muscles. Food Sci. 2009, 30(21), 83–86.
- Xu, X. L.; Wang, X.; Zhou, G. H.; Lin, L. J.; Huang, H. B. Effect of Phosphates on Heat-Induced Gelation Properties of Myosin from Rabbit Skeletal Muscles. Food Sci. 2005, 26(03), 42–46.
- Kang, C. Y.; Zhao, Q. C. Study on WHC and Ultrastructure of Heat-Induced Gelation of CB Salt-Soluble Protein. Food Sci. 2007, 28(01), 50–53.
- Jin, H. G.; Peng, Z. Q.; Zhou, G. H. Heat-Induced Gelation Properties of Salt-Soluble Proteins from Beef Muscle. Food Sci. 2008, 29(08), 95–99.
- Wu, M. G.; Xiong, Y. L.; Chen, J. Rheology and Microstructure of Myofibrillar Protein-Plant Lipid Composite Gels: Effect of Emulsion Droplet Size and Membrane Type. J. Food Eng. 2011, 106(4), 318–324. DOI: 10.1016/j.jfoodeng.2011.05.022.
- Shang, Y. B.; Li, H. J.; Xia, Y. Y.; Chen, Z. D. Effect of Solution Conditions on Solubility and Heat-Induced Gel Strength of PSE Porcine Myofibrillar Proteins. Food Sci. 2010, 31(05), 35–39.
- Li, Y. N.;. Study on the Extraction of Myofibrillar Proteins of the Duck and Gel Properties; Tianjin University of Commerce: TianJin:, 2012.
- Ni, N.; Wang, Z. Y.; Han, Z. H.; He, F.; Pan, H.; Mu, G. F.; Zhang, D. Q. Effect of pH on Heat-Induced Gel of Myofibrillar Protein from Lamb M. Longissimus Dorsi Muscle. Sci. Agricultura Sin. 2013, 46(17), 3680–3687.
- Fei, Y.; Han, M. Y.; Yang, L. H.; Zhou, G. H.; Xu, X. L. Studies on the Secondary Structure and Heat-Induced Gelation of Pork Myofibrillar Proteins as Affected by pH. Sci. Agricultura Sin. 2010, 43(1), 164–170.
- Wu, Y.; Xu, K.; Xu, X. L.; Niu, L. Effect of pH on Gelation Properties of Rabbit Myosin. Food Sci. 2010, 31(09), 6–11.
- Dong, Q. Y.; Yang, Y. L.; Xu, T. Study on Molecular Forces in the Formation in Myofibrillar Protein Gelation from Textural Perspective. Food Ferment. Ind. 2009, 35(5), 45–49.
- Yang, S. P.;. Studies on Gelation Properties of Salt-Soluble Protein of Beef and the Factors Affecting Functional Characteristics of Gelation; Hebei Agricultural University: Baoding:, 2003.
- Ryan, M.; Young-Hee, C.; Brian, F.; Jones, O. G. Control of Thermal Fabrication and Size of B-Lactoglobulin-Based Microgels and Their Potential Applications. J. Colloid Interface Sci. 2015, 447, 182. DOI: 10.1016/j.jcis.2014.09.067.
- Ferry, J. D.;. Protein Gels. Protein Chem. 1948, 4(1), 1–2.