?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Lupin is a major rotational crop produced abundantly in Australia. Three varieties of sweet lupins are grown in Australia depending on soil types and climatic conditions; these are white lupin (Lupinus albus), blue lupin (Lupinus angustifolius), and yellow lupin (Lupinus luteus). In this study, polysaccharides from the three lupin species have been extracted with hot water and a range of their biological activities investigated. The antioxidant activities of lupin polysaccharides have been measured in terms of ABTS•+ radical and hydroxyl radical scavenging activities, and their affinity to chelate with iron. Immune-enhancing power of the lupin polysaccharides was examined by measuring their capacity to stimulate the macrophages (RAW 264.7) to release TNF-α and nitric oxide. The prebiotic activities were determined against the beneficial probiotics such as Lactobacillus rhamnosus DR20, Lactobacillus acidophilus LAFTI L10, Bifidobacterium lactis DR10, and Bifidobacterium animalis BB-12. Polysaccharides from the three lupin species have exhibited significant antioxidant, immunostimulatory, and prebiotic activities. The results demonstrated that blue lupins displayed the best activities, and therefore are expected to possess high potential to be used as nutraceutical and functional ingredients with significant therapeutic value. FT-IR spectroscopic technique was employed for a preliminary structural characterization. Studies involving surface morphology and thermal behavior have indicated that the lupin polysaccharides are suitable for end product development.
Introduction
Lupin is a major crop cultivated by Australian farmers to fix soil nitrogen in wheat and other cereal producing fields.[Citation1] Consequently, Australia is the largest producer and exporter of sweet lupin seeds.[Citation2] The three species of sweet lupins produced in Australia are: white lupin (Lupinus albus L.), blue lupin (Lupinus angustifolius L.) and yellow lupin (Lupinus luteus L.). Some of the beneficial health outcomes of the foods based on sweet lupins are improved bowel function and lowering the risk of colon cancer[Citation3], blood pressure lowering[Citation4,Citation5], blood glucose control, improved cardiovascular health[Citation5] and promoting the growth of colonic microbiota.[Citation6]
Lupin offers a suitable option for sustainable use of land and hence is of immense value to Australian farmers. However, the enormous health beneficial effects of this product are not fully exploited for human consumption5. Further research to improve commercial value of the grain will be beneficial to farmers and food/nutraceutical industry as well as the consumers. Investigations of bioactivities of the major constituents from the three sweet lupins are likely to reveal the nutraceutical/pharmaceutical benefits and hence will improve the value of lupin seeds. Literature demonstrates[Citation5,Citation7,Citation8] that lupin has large amounts of beneficial polysaccharides and protein. Therefore, these two nutrients can be isolated from lupin and can be utilized for nutraceutical/pharmaceutical purposes.
The literature on the biological and structural analysis of the polysaccharides from the three Australian sweet lupins is limited.[Citation9,Citation10] Research investigating bioactive polysaccharides from the three Australian sweet lupin species is therefore important to evaluate their relative nutraceutical potential. This research aims to extract polysaccharides from the whole seeds of the three lupin species and study their biological activities. The results on scavenging abilities of lupin polysaccharides against ABTS•+ and hydroxyl radicals, their affinity to chelate with iron, their immunomodulatory and prebiotic activities are presented in this paper. Surface studies and thermal behavior of lupin polysaccharides were investigated with a view to assess their suitability in food industry. FT-IR based structural characterization and their relationship with the activities are also presented in this paper.
Experimental methods
Materials and chemicals
Coorow seeds, Western Australia have donated the seeds of Lupinus angustifolius, Lupinus luteus and Lupinus albus. Penicillin, streptomycin, Dulbecco’s modified eagle’s medium (DMEM), Lipopolysaccharide (LPS), Fetal bovine serum (FBS) and glutaMAX™ were obtained from Life Technologies (Australia). ELISA kits and TNF-α were procured from BD biosciences (USA). Mouse macrophages (RAW 264.7) were purchased from American type culture collection. Rest of the chemicals and reagents used were of analytical grade.
Extraction of lupin polysaccharides and preliminary analysis
A kitchen blender (Sunbeam PB7600) was used to grind the seeds of L.angustifolius, L.luteus, and L.albus to powder form. The extraction of water-soluble polysaccharides9 was carried out by autoclave method for 2 h at 121°C. The extract was allowed to cool at room temperature and then filtered. The supernatant was mixed with 1:4 vol/vol ratio of 95% ethanol for overnight at 4.1°C to obtain the precipitate of polysaccharides. The polysaccharides were then separated by centrifuging the ethanol-treated solution (12,000 g for 30 min) and then redissolved in deionized water. The samples were subsequently lyophilized to dryness and kept at approximately −20°C until further investigations.
Phenol–sulfuric acid method was used to measure the total sugar contents of the polysaccharides from the three lupin species.[Citation11] Bradford’s assay[Citation12] was employed to determine their total protein contents. In order to determine the monosaccharide contents of these polysaccharides, gas chromatography[Citation13] with FID detection technique was used (Hewlett Packard 7890B, capillary column model HP-5 from Agilent Technologies, USA). Polysaccharide samples were first hydrolyzed with trichloroacetic acid and the resulting samples were acetylated before performing GC analysis. Acetylation of all monosaccharide standards (mannose, galactose, ribose, glucose, xylose, fucose, rhamnose, and arabinose) was also performed.
Scanning electron microscopy
The morphological features of the powdered samples of the three lupin polysaccharides were studied by recording scanning electron micrograms (SEM) using a JEOL (6510 LV) instrument. Samples were mounted on aluminum stub with carbon film and the micrograms recorded.
Thermal analysis
Thermogravimetric analysis (TGA) is a useful technique, which helps to identify the thermal behavior and decomposition pattern of a sample, which will be helpful in determining the end product development and commercialization. TGA of blue, white, and yellow lupin polysaccharides was carried out on the simultaneous thermal analyzer Netzsch STA449C Jupiter (Germany). The sample was placed in the sample pan and heated from 0°C to 600°C at 10°C/min rate under the air and argon atmosphere.
Fourier transform infrared (FT-IR) spectroscopy
Fourier transform infrared Spectrometer (Perkin Elmer Spectrum 100) equipped with universal ATR accessory was used to record FT-IR spectral data. The spectra were recorded in the range of 4000 to 400 cm−1, resolution of 1 cm−1and 32 scans.
Antioxidant activities of polysaccharides
ABTS•+radical scavenging activity
The antioxidant properties of lupin polysaccharides have been estimated using ABTS•+ radical scavenging assay.[Citation14] The diammonium salt of ABTS•+ (0.35 mL at 7.4 mmol/L) was first mixed with K2S2O8 (0.35 mL at 2.6 mmol/L) to obtain ABTS•+ free radicals. The tube containing the mixture was wrapped with aluminum foil and kept overnight in the darkroom to facilitate total radical formation. Ninety-five percent ethanol was used to dilute ABTS•+ radical solution to set its absorbance reading to 0.74 ± 0.02 at 734 nm. The ABTS•+ scavenging abilities of polysaccharide samples were determined by the addition of 2 mL of ABTS•+ radical solution to 0.2 mL of sample. The absorbance of the samples was measured (at 734 nm) after incubating them at 25°C for 20 min. The negative control was 95% ethanol. ABTS•+ scavenging activity was determined using the equation below.
Percentage of ABTS•+ scavenging Activity (%) =(1 – A/A0) ×100
where A0 represents the absorbance value of negative control, and A represents the absorbance of the sample. All the measurements were done in triplicate.
Hydroxyl radical scavenging assay
Polysaccharide sample preparation and the incubation procedures used for this assay are similar to that described in previous publications.[Citation9,Citation15] The absorbance of the samples was measured at 536 nm. The OH• scavenging activity was determined using the equation below.
% OH• radical scavenging activity = [(Asample – Acontrol)/(Ablank – Acontrol)] X 100
where Asample represents the absorbance of the sample and Acontrol represents the absorbance of control solution containing 1,10-phenanthroline, FeSO4, and H2O2; Ablank represents the absorbance of blank solution containing 1,10-phenanthroline and FeSO4. All the measurements were done in triplicate.
Ferrous chelating activity
In order to measure the chelating activity of the samples, their capacity to coordinate with Fe2+ was observed in the presence of ferrozine. Polysaccharide sample with five concentrations (1–5mg/mL) was prepared in order to measure the dose-dependant chelating abilities. 1 millliter of each sample was mixed with 0.1 mL iron(II) chloride (2 mM), 0.15 mL ferrozine (5 mM), and 0.55 mL MeOH. Mixing of samples was performed by vortexing and incubated at room temperature for 15 min (allowing complexation reaction to complete). The absorbance of the incubated samples was then measured at 562 nm. The positive control was EDTA.[Citation14] Percentage chelating activity was determined using the equation below.
where A0 represents the absorbance of positive control, and A represents the absorbance of polysaccharide samples. All the measurements were done in triplicate.
Immunostimulatory activities
Griess assay
For the measurement of immunomodulatory effects, the solutions of polysaccharides of varying concentrations were made (0.05–5 mg/mL). The procedure employed for this assay was similar to that presented in previous publications.[Citation9,Citation16] Sodium nitrite was used as a standard in this method with LPS as positive control.
Determination of TNF-α production
Sandwich ELISA (TNF-α, BD Biosciences, San Jose, CA, USA) was employed and the procedure described in the manual was followed to measure the concentration of TNF-α produced. Procedure for the establishment of calibration curve for TNF-α standard and the determination of TNF-α released from the samples was similar to that presented in previous publications.[Citation10,Citation17]
Cell viability assay
Viability of RAW 264.7 macrophages was determined using MTT assay[Citation18] at different concentrations of polysaccharide samples. The procedure employed in this assay was similar to that presented in previous publications.[Citation9,Citation10] After a 30-min incubation of samples, the absorbance values were measured at 595nm. LPS was used as positive control. The cell viability was determined using the equation below:
where A represents the absorbance of positive control and A0 represents the absorbance with polysaccharide samples.
Prebiotic assessment of lupin polysaccharide fractions
The prebiotic activities of lupin polysaccharides were determined against four beneficial probiotic strains, namely, L. rhamnosus DR20, L. acidophilus (LAFTI L10), B. lactis DR10 and B. animalis BB-12 that were obtained from Microbiology Culture Collection, Western Sydney University (Hawkesbury, Australia). The procedure employed in this assay was similar to that presented in a previous publication.[Citation10] Decrease in pH of the media and increase in its turbidity were measured after 0, 8, 24, and 48 h which indicated the growth of the tested bacteria.[Citation19]
Data analysis
SPSS (Version 20) and Microsoft Excel were used for statistical analysis. Triplicate data was collected in all biological assays. One-way analysis of variance and Duncan’s multiple range tests were employed for data analysis. Statistical significance of the data was established based on p-value (p < 0.05). Smaller p-value indicates greater significance of the observed value.
Results and discussion
Chemical analysis of lupin polysaccharides
Water-soluble polysaccharides have been isolated from the seeds of white, blue and yellow lupins and their compositions were analyzed. The results () indicate that blue lupin seeds have produced maximum polysaccharide yield which is followed by white lupin and yellow lupin. also presents the sugar and protein contents of the polysaccharides extracted from the three lupin species. The polysaccharides extracted from blue, white and yellow lupins are designated as BLP, WLP, and YLP, respectively.
Table 1. Extraction Yield, carbohydrate, and protein contents of sweet lupin polysaccharides
Monosaccharide analysis
Monosaccharide compositions of blue, white and yellow lupin polysaccharides have been determined using gas chromatographic method, and the results are shown in . Galactose and mannose are the two major monosaccharides present in the polysaccharides extracted from all the three lupin species. Xylose, glucose, and fucose are the other monosaccharides present in these polysaccharides. Small quantity of rhamnose is present in blue lupin polysaccharides which is absent in the other two species. It should be noted that a total of nine monosaccharide standards were used in this analysis. However, three of the monosaccharides (fucose, ribose, and arabinose) were absent in all the three lupin species. The observations presented in and the literature reports[Citation20,Citation21] substantiate the occurrence of galactans and galactomannans as major polysaccharides in all the three lupin species. The results presented in also indicate that the other possible biopolymers comprising lupin polysaccharides include Xyloglucans.[Citation20,Citation21]
Table 2. Monosaccharide composition of different lupin polysaccharides
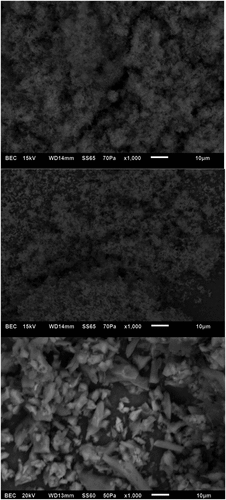
Scanning electron microscopy (SEM)
SEM is a powerful microscopic method for investigating the surface morphology of biomolecules, which helps in understanding their physical properties that may be relevant to food processing. Scanning electron micrograms of blue, white and yellow lupin polysaccharides are presented in . White and yellow lupin polysaccharides show porous and sponge-like surface. The surface morphology of white and yellow lupin polysaccharides has indicated amorphous material. The surface morphology of blue lupin polysaccharides showed quite different structure exhibiting granular structure consisting of irregular granules with varying sizes. These porous and granular structures are likely to be useful for improving organoleptic and sensory quality of food/nutraceutical products from sweet lupin polysaccharides.
Thermogravimetric analysis (TGA)
TGA is a useful technique to identify the thermal behavior and decomposition pattern of a food/nutraceutical ingredient which will be useful for end product development. Thermo-gravimetric analysis of blue, white and yellow lupin polysaccharides was carried out in the temperature range from room temperature to 590°C, and the results are presented in . These diagrams show mass changes as temperature is increased. All the three species of lupin polysaccharides showed three decomposition stages (). For white lupin polysaccharide: stage 1 (60°C–161°C) shows loss of absorbed and bound water; stage 2 (161°C–359°C) represents decomposition of polysaccharides in which CO, CO2, and H2O are lost; Stage 3 (359°C–587°C) represents complete decomposition of white lupin polysaccharides. Similar behavior was observed for blue and yellow lupin polysaccharides (). The observed decomposition patterns indicate that the lupin polysaccharides are suitable for food product development.
Table 3. Decomposition patterns of lupin polysaccharides
FT-IR characterization
Preliminary structural features of lupin polysaccharides were analyzed by FT-IR spectroscopy. FT-IR spectra of these polysaccharides consist of peaks that are characteristic of polysaccharides[Citation22] (). Characteristic absorption bands for white lupin polysaccharides are shown in . Four bands detected in the range of 797 to 919 cm−1 indicate the presence of glycosidic linkages (Bands labeled 1–4 in ). The band at 881 cm−1 and 919 cm−1 relate to β-glycosidic bond.[Citation22] The peaks at 797 and 837 cm−1 relate to α-glycosidic linkage22. The bands at 1021, 1048, and 1078 cm−1 correspond to the C-O stretching modes in C-O-H bonds in pyranose ring structures. The bands around 2928 cm−1 correspond to the stretching modes of C-H. Finally, the broad peak at 3274 cm−1 relates to – OH stretching vibrations.
Figure 2. FT-IR spectrum of white lupin polysaccharides. Relevant vibrational peaks in the spectrum are represented with numbers 1 to 11 (Peak 1: 797 cm−1; Peak 2: 837 cm−1; Peak 3: 881 cm−1; Peak 4: 919 cm−1; Peak 5: 1021 cm−1; Peak 6: 1048 cm−1; Peak 7: 1078 cm−1 Peak 8: 1533 cm−1; Peak 9: 1626 cm−1; Peak 10: 2928 cm−1 and Peak 11: 3274 cm−1)
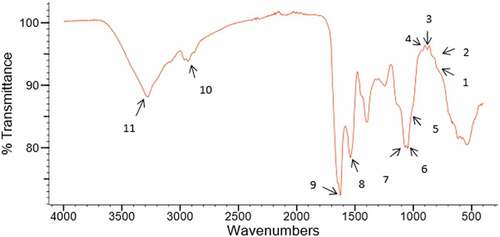
Similar FT-IR spectra were detected for yellow and blue lupin polysaccharides (Results not presented). Monosaccharide compositions of these polysaccharides together with FT-IR findings indicate the likely presence of galactans and galactomannans, which are known to contain pyranose sugars with α- and β-glycosidic bonds. These observations are in agreement with literature findings[Citation10]
Radical scavenging and iron chelating abilities of blue, white and yellow lupin polysaccharides
Prolonged existence of reactive free radicals in the body is likely to cause oxidative stress that can lead to serious diseases. Regular consumption of antioxidants alleviates oxidative stress. Several food-derived polysaccharides were found in the literature to be important sources of natural antioxidants.[Citation22–Citation24] Therefore, the polysaccharides extracted from the three lupin species were tested for their antioxidant potential. These results are presented below in .
Table 4. Antioxidant activities of polysaccharides from white, yellow, and blue lupins
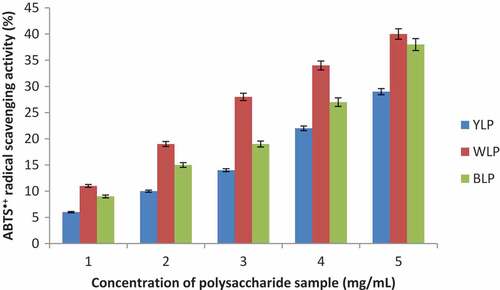
ABTS•+radical scavenging activity
ABTS•+ radical scavenging activities of blue, white and yellow lupin polysaccharides are shown in , and the corresponding IC50 values are given in . These polysaccharides (BLP, WLP, and YLP) exhibited significant scavenging activity against ABTS•+ radical in a dose-dependent manner. The polysaccharides extracted from all the lupin species displayed good IC50 values (). WLP showed the best ABTS•+ radical scavenging ability amongst the three lupin species and YLP displayed the least activity (WLP > BLP > YLP).
Hydroxyl radical scavenging activity
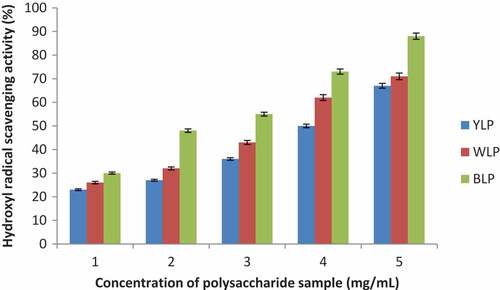
Hydroxyl radical scavenging abilities of the lupin polysaccharides from the three lupin species are presented in , and the corresponding IC50 values are given in . A closer look at , indicates that these polysaccharides (BLP, WLP, and YLP) exhibit significant scavenging activity against Hydroxyl radicals in a concentration-dependent manner. Low IC50 values were obtained for BLP (1.56 ± 0.32 mg/mL), WLP (2.26 ± 0.48 mg/mL) and YLP (2.82 ± 0.26 mg/mL). BLP exhibited the best activity amongst the three lupin species studied, and YLP displayed relatively lower activity (BLP > WLP > YLP). Extremely small IC50 values displayed by the polysaccharides from all the three lupin species indicate their high potential to scavenge biologically significant hydroxyl radical.
Figure 4. Dose-dependent activities of the yellow, white, and blue lupin polysaccharides (Hydroxyl radical scavenging activities are expressed in percentage with respect to the scavenging ability of Vitamin C at a concentration of 0.532 mg/mL taken as 100%) (n = 3, p < 0.03)
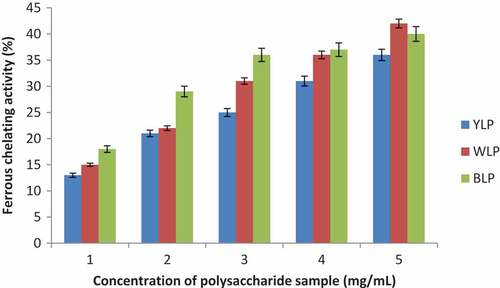
Ferrous chelating activity The chelating abilities of blue, white and yellow lupin polysaccharides are presented in , and the corresponding IC50 values are given in . BLP, WLP, and YLP have all showed good activities as a function of concentration. WLP showed highest chelating activity (IC50 = 3.48 ± 0.37 mg/mL) amongst all the species and YLP displayed relatively lower chelating ability (WLP > BLP > YLP).
Figure 5. Dose-dependent activities of the yellow, white, and blue lupin polysaccharides (Ferrous chelating activities are expressed in percentage with respect to the chelating ability of EDTA at a concentration of 0.562 mg/mL taken as 100%) (n = 3, p < 0.04)
It is of interest to note from that BLP and WLP have larger quantities of galactose and mannose than YLP. The observed antioxidant activities of BLP and WLP are also larger than YLP. These observations indicate that galactose and mannose may be the major contributors to the antioxidant potential of lupin polysaccharides, and this observation is in agreement with the literature[Citation9]
The findings presented above reveal that the polysaccharides from the three lupin species display high antioxidant activities (radical scavenging as well as metal chelating abilities) and they are highly potential candidates to be used as natural antioxidants. Results indicate that these activities are likely to be connected to their galactose and mannose contents, and these findings are in agreement with the literature.[Citation9,Citation10,Citation25,Citation26] The radical scavenging activities of mung bean polysaccharides have been shown to correlate well with the galactose and mannose contents.[Citation26] BLP showed the best antioxidant potential which is followed by WLP while YLP displayed relatively lower activity ( and –).
Immunostimulatory activities
Immunostimulatory activities of polysaccharides isolated from the three lupin species were measured by the treatment of macrophages (RAW264.7) with lupin polysaccharides. The results demonstrated that the production of TNF-α and NO were increased as the concentration of polysaccharide samples were increased ( and ). These results are presented and discussed below.
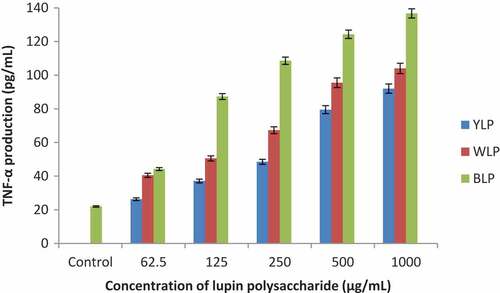
Tnf-α production
The results presented in show that the polysaccharides from all the three lupin species have significantly activated macrophages and produced TNF-α. When treated with BLP, a sevenfold enhancement in the release of TNF-α (at 1000 µg/mL) was observed in relation to control. Whereas WLPs have produced about fourfold increase and YLPs have increased threefold. These results indicate that the BLPs are the most active amongst the three lupin species with respect to TNF-α production, and the yellow lupin polysaccharides (YLPs) displayed lowest activity.
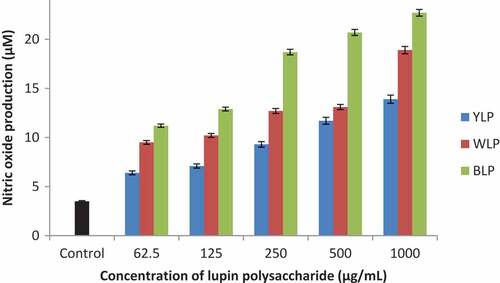
NO production
The polysaccharides from all the three lupin species have activated the macrophages to produce NO as a function of concentration (). BLPs have displayed highest activity. This is evident from a 6-fold increase (for BLP), about 4.5 fold increase (for WLP) and about 4-fold increase (for YLP) in the production of NO at 1000 µg/mL.
These results indicate that the polysaccharides from the three lupin species studied in this research are suitable candidates to enhance the immune function. These immunomodulatory activities of lupin polysaccharides together with their antioxidant activities are expected to make them extremely suitable candidates for nutraceutical and functional applications. Particularly, the blue lupin polysaccharides with high activities hold great importance for such applications. These results are very significant considering the fact that they are food polysaccharides and can be consumed in large quantities.
As can be seen from the literature[Citation23], the immunomodulatory function plays a key role in anti-cancer activity. It is therefore concluded that the sweet lupin polysaccharides are potential candidates for further exploitation as anti-cancer agents.
Cell viability
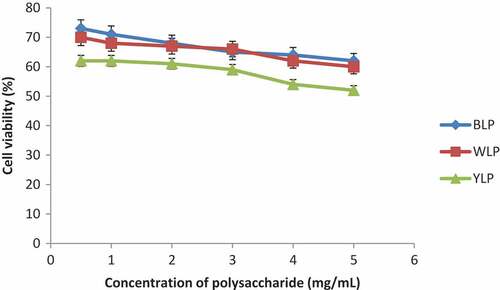
The viabilities of macrophage cells upon treatment with lupin polysaccharides was tested by MTT assay, and the results are shown in . Polysaccharides from the three lupin species have displayed least toxicities (good viability) even at large concentrations indicating that they are non-toxic. These results are consistent with the literature[Citation9,Citation17] findings that botanical polysaccharides display least toxicity.
Because of the fact that most of the modern therapies to treat lifestyle diseases involve serious side effects, there is a growing interest in the use of natural therapeutics for prevention and treatment of many human diseases caused by oxidative stress that include immune disorders and cancer. In this context, Australian sweet lupin seeds possess huge potential as they are nutritionally rich and contain non-starch polysaccharides that display a range of important biological activities[Citation9,Citation25–Citation29] with least side effects.
Prebiotic assessment of lupin polysaccharides
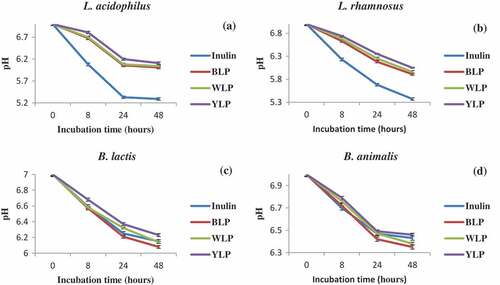
Prebiotic potential of the BLP, WLP, and YLP have been assessed against four probiotic species. Each of the tested bacteria fermented the polysaccharide samples as demonstrated by a decrease in pH of the medium as a function of incubation time (). Prebiotic activities of BLP, WLP and YLP were also evidenced by a clear increase of turbidity as a function of incubation time reflecting bacterial growth. These results indicate that BLP, WLP, and YLP exhibit good prebiotic activities.
BLP was the best amongst the three lupin species and was superior to inulin (a known standard). These findings demonstrate that BLP, WLP, and YLP are excellent natural polysaccharides with prebiotic potential. The results are consistent with the literature findings on the health benefits of lupin-based foods that have been shown to promote the growth of colonic microbiota, decrease the risk of colon cancer and many other positive effects on health.[Citation3,Citation5,Citation6]
Overall, the results presented in this paper demonstrate that sweet lupin polysaccharides possess high potential for antioxidant, immunostimulatory and prebiotic applications. They are expected to offer tremendous nutraceutical and functional benefits. BLP has displayed extremely high activities followed by WLP and YLP. The activities reported in this paper for BLP are comparable to the activities of some of the herbal polysaccharides[Citation24] and this is very important finding considering the fact that these are food polysaccharides and can be consumed in large quantities.
Tremendous nutraceutical, functional and therapeutic potential of sweet lupin polysaccharides revealed by this research is expected to open many avenues to utilize lupins to further develop the technology to produce novel lupin-based foods. In recent years, there has been significant progress in the development of lupin-based foods.[Citation30] Further developments in this direction are expected to advance the commercial value of sweet lupins and their use in developing a variety of functionally rich food products.
Conclusion
The polysaccharides extracted from the three lupin species displayed significant scavenging activities against two of the free radicals studied and displayed excellent iron chelating abilities indicating their potential to be used as nutraceuticals with antioxidant capacity. Sweet lupin polysaccharides have also exhibited significant immunostimulatory and prebiotic activities with BLP showing highest activity, followed by WLP and YLP. Polysaccharides from all the lupin species have been found to be non-toxic with excellent cell viabilities even at high concentrations. In addition to displaying superior biological activities, the blue lupin has yielded largest quantity of polysaccharides per gram of seeds, followed by white lupin and the yellow lupin yielded least quantity of polysaccharides. These findings suggest that the blue lupins have the best antioxidant, immunostimulatory, and prebiotic potential per gram of seeds, and the yellow lupins have the least. It is therefore expected that the blue and white lupin polysaccharides possess high potential to be used as nutraceutical and functional ingredients with significant therapeutic value. A closer look at the observed activities presented in this paper and the mono-sugar contents indicate that galactose and mannose are the major contributors to the biological activities of lupin polysaccharides. FT-IR findings indicate the presence of galactans and galactomannans.
Acknowledgments
The authors would like to thank School of Science and Health, Western Sydney University for their financial support for this research. ST would like to thank the university for the WSU Postgraduate Research Award. We thank Coorow seeds, Western Australia for providing blue, white and yellow lupin seeds.
References
- Doyle, A.; Moore, K.; Herridge, D. The Narrow-Leafed Lupin (Lupinus Angustifolius L.) As a Nitrogen-Fixing Rotation Crop for Cereal Production. III. Residual Effects of Lupins on Subsequent Cereal Crops. Aust. J. Agric. Res. 1988, 39, 1029–1037. DOI: 10.1071/AR9881029.
- Sedláková, K.; Straková, E.; Suchý, P.; Krejcarová, J.; Herzig, I. Lupin as a Perspective Protein Plant for Animal and Human Nutrition–A Review. Acta Vet. Brno. 2016, 85, 165–175. DOI: 10.2754/avb201685020165.
- Johnson, S. K.; Chua, V.; Hall, R. S.; Baxter, A. L. Lupin Kernel Fibre Foods Improve Bowel Function and Beneficially Modify Some Putative Faecal Risk Factors for Colon Cancer in Men. Br. J. Nutr. 2006, 95, 372–378.
- Lee, Y. P.; Mori, T. A.; Puddey, I. B.; Sipsas, S.; Ackland, T. R.; Beilin, L. J.; Hodgson, J. M. Effects of Lupin Kernel Flour–Enriched Bread on Blood Pressure: A Controlled Intervention Study. Am. J. Clin. Nutr. 2009, 89, 766–772. DOI: 10.3945/ajcn.2008.26708.
- Belski, R.;. Chapter 4 - Fiber, Protein, and Lupin-Enriched Foods: Role for Improving Cardiovascular Health. Adv. Food Nut. Res. 2012, 66,147–215.
- Smith, S. C.; Choy, R.; Johnson, S. K.; Hall, R. S.; Wildeboer-Veloo, A. C.; Welling, G. W. Lupin Kernel Fiber Consumption Modifies Fecal Microbiota in Healthy Men as Determined by rRNA Gene Fluorescent in Situ Hybridization. Eur. J. Nutr. 2006, 45, 335–341. DOI: 10.1007/s00394-006-0603-1.
- Wrigley, C.;. The Lupin–The Grain with No Starch. Cereal Foods World. 2003, 48, 30.
- Thumad Al-Kaisey, M.; Wilkie, K. C. B. The Polysaccharides of Agricultural Lupin Seeds. Carbohydr. Res. 1992, 227, 147–161.
- Thambiraj, S. R.; Phillips, M.; Koyyalamudi, S. R.; Reddy, N. Antioxidant Activities and Characterisation of Polysaccharides Isolated from the Seeds of Lupinus Angustifolius. Ind. Crops Prod. 2015, 74, 950–956. DOI: 10.1016/j.indcrop.2015.06.028.
- Thambiraj, S. R.; Phillips, M.; Koyyalamudi, S. R.; Reddy, N. Yellow Lupin (Lupinus Luteus L.) Polysaccharides: Antioxidant, Immunomodulatory and Prebiotic Activities and Their Structural Characterisation. Food Chem. 2018, 267, 319–328. DOI: 10.1016/j.foodchem.2018.02.111.
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S. I.; Lee, Y. C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. DOI: 10.1016/j.ab.2004.12.001.
- Bradford, M. M.;. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254.
- Lee, E. J.; Kim, J. S.; Kim, H. P.; Lee, J. H.; Kang, S. S. Phenolic Constituents from the Flower Buds of Lonicera Japonica and Their 5-Lipoxygenase Inhibitory Activities. Food Chem. 2010, 120, 134–139. DOI: 10.1016/j.foodchem.2009.09.088.
- Kao, T. H.; Chen, B. H. Functional Components in Soybean Cake and Their Effects on Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 7544–7555. DOI: 10.1021/jf061586x.
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate. Food Chem. 2008, 106, 444–450. DOI: 10.1016/j.foodchem.2007.04.067.
- Cui, S.; Reichner, J. S.; Mateo, R. B.; Albina, J. E. Activated Murine Macrophages Induce Apoptosis in Tumor Cells through Nitric Oxide-Dependent Or-Independent Mechanisms. Cancer Res. 1994, 54, 2462–2467.
- Jeong, S. C.; Koyyalamudi, S. R.; Jeong, Y. T.; Song, C. H.; Pang, G. Macrophage Immunomodulating and Antitumor Activities of Polysaccharides Isolated from Agaricus Bisporus White Button Mushrooms. J. med. food. 2012, 15, 58–65. DOI: 10.1089/jmf.2011.1704.
- Mosmann, T.;. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods. 1983, 65, 55–63.
- Azmi, A. F. M. N.; Mustafa, S.; Hashim, D. M.; Manap, Y. A. Prebiotic Activity of Polysaccharides Extracted from Gigantochloa Levis (Buluh Beting) Shoots. Molecules. 2012, 17, 1635–1651. DOI: 10.3390/molecules17021635.
- Buckeridge, M. S.;. Seed Cell Wall Storage Polysaccharides: Models to Understand Cell Wall Biosynthesis and Degradation. Plant Physiol. 2010, 154, 1017–1023. DOI: 10.1104/pp.110.158642.
- Jarvis, M. C.; Apperley, D. C. Direct Observation of Cell Wall Structure in Living Plant Tissues by Solid-State 13C NMR Spectroscopy. Plant Physiol. 1990, 92, 61–65.
- Yang, L.; Zhang, L. M. Chemical Structural and Chain Conformational Characterization of Some Bioactive Polysaccharides Isolated from Natural Sources. Carbohydr. Polym. 2009, 76, 349–361. DOI: 10.1016/j.carbpol.2008.12.015.
- Zheng, Y.; Wang, Q.; Zhuang, W.; Lu, X.; Miron, A.; Chai, -T.-T.; Zheng, B.; Cytotoxic, X. J. Antitumor and Immunomodulatory Effects of the Water-Soluble Polysaccharides from Lotus (Nelumbo Nucifera Gaertn.) Seeds. Molecules. 2016, 21, 1465. DOI: 10.3390/molecules21111465.
- Zhang, L.; Koyyalamudi, S. R.; Jeong, S. C.; Reddy, N.; Bailey, T.; Longvah, T. Immunomodulatory Activities of Polysaccharides Isolated from Taxillus Chinensis and Uncaria Rhyncophylla. Carbohydr. Polym. 2013, 98, 1458–1465. DOI: 10.1016/j.carbpol.2013.07.060.
- Yao, Y.; Xue, P.; Zhu, Y.; Gao, Y.; Ren, G. Antioxidant and Immunoregulatory Activity of Polysaccharides from Adzuki Beans (Vigna Angularis). Food Res. Int. 2015, 77, 251–256. DOI: 10.1016/j.foodres.2015.05.029.
- Yao, Y.; Zhu, Y.; Ren, G. Antioxidant and Immunoregulatory Activity of Alkali-Extractable Polysaccharides from Mung Bean. Int. J. Biol. Macromol. 2016, 84, 289–294. DOI: 10.1016/j.ijbiomac.2015.12.045.
- Yang, L.; Sun., Y.; Gangliang, H. Preparation and Antioxidant Activities of Important Traditional Plant Polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. DOI: 10.1016/j.ijbiomac.2018.01.086.
- Gangliang, H.; Xinya, M.; Jinchuan, H. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets. 2017, 18, 1296–1300. DOI: 10.2174/1389450118666170123145357.
- Qilin, T.; Gangliang, H. Progress in Polysaccharide Derivatization and Properties. Mini-Rev. Med. Chem. 2016, 16, 1244–1257.
- Jayasena, V.; Leung, P. P. Y.; Nasar-Abbas, S. M. Effect of Lupin Flour Substitution on the Quality and Sensory Acceptability of Instant Noodles. J. Food Qual. 2010, 33, 709–727. DOI: 10.1111/j.1745-4557.2010.00353.x.