ABSTRACT
The objective of this study was to investigate the effect of cooking and in vitro digestion on antioxidant activity of peptides in duck meat after 7 days postmortem aging. The 1, 1-diphenyl-2- picrylhydrazyl (DPPH) free radical scavenging activity and reducing power of the obtained peptides were evaluated. Results showed that the cooked sample possessed higher antioxidant activity than the raw sample and upon cooking at different temperatures, sample aged for 7 days had significantly higher antioxidant activity than that of unaged (day 0). The samples which showed higher antioxidant activity after cooking (65°C–30 min, 100°C–20 min, 121°C–10 min) were selected for in vitro digestion, and it is found that their DPPH radical scavenging activity decreased significantly but the reducing power increased significantly after gastrointestinal digestion. These results show the potential of aged duck meat as a good source of antioxidant peptides with high bioactivity after cooking and gastrointestinal digestion.
Introduction
Traditionally, food proteins have been recognized for their nutritional and functional properties. In recent years, consumer attention has been increasingly oriented toward the potential of food to provide physiological effect in the body, beyond that of nutrition.[Citation1] Peptides derived from food have been shown to display a wide range of physiological functions, such as antioxidative, antithrombotic, antihypertensive, antimicrobial, prebiotic, hypocholesterolemic, and immunomodulatory activities.[Citation2,Citation3] These peptides are characterized with short sequences of approximately 2–30 amino acids residues in length and a low molecular weight, and, once released, they can be absorbed through the intestine to enter the blood circulation or can produce a local effect within the gastrointestinal tract.[Citation2]
Oxidative stress in the body is closely related to human degenerative diseases including Parkinson, atherosclerosis, emphysema, diabetes, and aging[Citation4]; it is the consequence of an imbalance between free radicals and biological antioxidants in the body.[Citation5,Citation6] Thus, interest has been emerging to investigate the use of bioactive peptides as antioxidative agents not only as natural alternatives to synthetic antioxidants, but for their beneficial effects in terms of stable structures with higher activity and absorptivity and nonresidual side effects in food systems.[Citation7,Citation8] The integrity and activity of proteins experience changes during postmortem aging as a result of proteolytic degradation.[Citation2] A large number of peptides and free amino acid are released due to severe degradation of polypeptide fragments induced by peptidyl peptidases and aminopeptidases.[Citation9] Recent studies showed that postmortem aging not only contributes to meat tenderness but also generates peptides with biological function, such as antioxidant and antihypertensive activities.[Citation10–Citation12] Meat is always cooked before consumption, and the thermal treatments are critical in the development of oxidation and denaturation processes because they can affect the structural properties and physical–chemical state of proteins and peptides.[Citation10,Citation13] In addition, the bioactive activities of peptides, which are encrypted and inactive within the sequence of meat proteins, might have a positive impact on the body once released during in vitro digestion.[Citation3,Citation14]
Up to date, a large number of studies have been conducted to investigate the antioxidative properties of natural peptides from different food proteins[Citation5,Citation15,Citation16], but few studies focus on the peptides generated from meat products during postmortem aging. Our group has recently discovered that proteolytic degradation by endogenous enzymes can release antioxidant peptides in duck meat[Citation11], and there is still a lack of information related to the effect of different cooking treatments on the bioactivity of these antioxidant peptides. Therefore, the main objective of this study was to evaluate and compare the effect of different cooking treatments and simulated gastrointestinal digestion on the antioxidant activity of the peptides from duck meat after postmortem aging.
Materials and methods
Materials
Forty-day-old duck (Anas platyrhychos) were provided by Suqian Baishun Agricultural Science and Technology Co. Ltd. in Jiangsu, China. Pepsin (400 U/mg protein), trypsin (285 U/mg protein), 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, and 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), were obtained from Sigma-Aldrich (MO, USA). All other chemicals used were of analytical grade.
Sample preparation and heating procedure
Duck were slaughtered and breast muscles were rapidly removed. One part of the sample was frozen in liquid nitrogen and stored at −80°C (0-day samples), whereas the other part was kept for 7 days at 4°C before being frozen in liquid nitrogen and stored at −80°C until sample preparation and analysis (7-day samples). Three cooking conditions, 65°C, 100°C, and 121°C, were applied for cooking which were chosen to represent low, medium, and high sterilization temperature, respectively. The duck breast thawed at 4°C and incubated at various temperatures (65°C and 100°C) for different times (0,10,20, and 30 min) in thermostat bath. In addition, the remainder of samples were put into an autoclave (HVE-50 type, Hirayama Corporation) at 121°C for 0, 10,20, and 30 min. And then, all solutions were rapidly cooled to room temperature on ice and stored at −20°C prior to analysis.
Peptide extraction
The extraction was performed according to Bauchart et al.[Citation17] with slight modification. Frozen duck meat samples (20 g) were homogenized in 100 mL of 3% perchloric acid (PCA) using an Ultra-Turrax homogenized for three times (20 s each at 10,000 rpm with cooling in ice). The homogenate was centrifuged (Allegra 64R centrifuge, Beckman Coulter, USA) at 10,000 g for 20 min at 4°C, and the supernatant was collected and filtered using a cellulose acetate filter of 0.22 μm pore size. The extracts were neutralized to pH 7 using sodium hydroxide. The salt precipitate was eliminated by filtering twice using the cellulose acetate filter. The supernatant was submitted to an ultrafiltration with a 5 kDa cutoff (Vivaspin15, VIVASCIENCE, Hanover, Germany) at 4000 g for 1 h at 4°C. Subsequently, the sample was lyophilized and stored at −20°C prior to analysis.
Antioxidant activity measurements
DPPH radical scavenging activity
The DPPH radical scavenging activity was determined with a modified procedure from the research of Xing et al.[Citation18] The sample group was obtained by mixing 1 mL of sample with 1 mL of 0.2 mmol/L DPPH (dissolved in ethanol), the control group was 1 mL of ethanol mixed with 1 mL of 0.2 mmol/L DPPH, and the blank group consisted of 1 mL of sample and 1 mL of ethanol. The mixture was shaken and incubated for 30 min at room temperature protected from light. Then, the absorbance was measured at 517 nm using a multifunctional microplate reader (model Spectral Max M2e, Molecular Devices, USA). The following equation was used to calculate the scavenging activity:
where Asample, Ablank, and Acontrol represent the absorbance of the sample, the blank, and the control groups, respectively.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was conducted according to Benzie and Strain[Citation19] with some modifications. The FRAP solution was prepared by mixing 0.3 M acetate buffer (pH 3.6), 20 mM ferric trichloride hexahydrate (FeCl3 6H2O), and 10 mM TPTZ at a ratio of 10:1:1 (v/v/v). The resulting solution was mixed and incubated at 37°C for 60 min. Subsequently, test samples (20 μL) were mixed with 150 μL FRAP working solution and the mixture was shaken and incubated for 4 min. Then, the absorbance was measured at 593 nm. The change in absorbance was compared to that of a standard that was run simultaneously. Standard of known FeSO4 7H2O ran in triplicate at varying concentration in the range of 25–1000 μM. A standard curve was constructed, and the FRAP values for the samples were then determined using this standard curve and expressed as micromole Fe (II) equivalent per g sample.
In vitro simulated gastrointestinal digestion
Crude peptides were extracted from the heated (65°C for 30 min, 100°C for 20 min and 121°C for 10 min) meat samples after 7 days postmortem aging, which were then lyophilized and digested by pepsin–trypsin. In vitro gastrointestinal digestion of antioxidant peptides was performed by the method of You et al.[Citation20] with a slight modification. The pH of crude peptide solutions (1 mg/mL) was first adjusted to 2.0 with 4 M HCl. Then, pepsin was added to a level of 4% in comparison of the protein content and the mixture was incubated at 37°C for 2 h. After that, the pH was adjusted to 5.3 with 0.9 M NaHCO3 solution and subsequently to pH 7.0 with 4 M NaOH. To terminate the digestion, the tubes were placed in boiling water for 10 min. The solution was divided into two parts. One was lyophilized and the other was readded to trypsin at the same proportion. After 2 h incubation at 37°C, the mixture was kept in boiling water for 10 min. Then, the samples were cooled to room temperature and centrifuged at 10,000 g for 15 min. The obtained supernatant was submitted to an ultrafiltration with a 5 kDa cutoff (Vivaspin15, VIVASCIENCE, Hanover, Germany) at 4000 g for 1 h at 4°C. Finally, the filtrated sample was lyophilized and stored at −20°C prior to analysis.
Statistical analysis
All the experiments were repeated in triplicate. Data were evaluated using SAS 9.3 software. Duncan’s multiple range tests in one-way analysis of variance (ANOVA) were employed to determine any significant difference between treatments.
Results and discussion
Effect of cooking treatment on the antioxidant activities
Most natural antioxidant or newly formed antioxidants upon processing are multifunctional, and in complex heterogeneous foods such as meat and meat products, their activity cannot be evaluated by a single method.[Citation21] Two or more radical scavenging capacity assays are required to investigate samples since each assay involves different chemical mechanisms and may reflect a different aspect of their antioxidant properties.[Citation22] Here two methods were used to assess in vitro antioxidant activity of peptides from duck meat. DPPH is a stable free radical that shows maximum absorbance at 517 nm. When DPPH radicals encounter proton-donating substrates such as antioxidants to become a stable diamagnetic molecular, the radicals are scavenged and the absorbance is reduced.[Citation23] While the FRAP assay utilizes the reducing potential of antioxidant compounds to react with a ferric–tripyridyltriazine (Fe3+–TPTZ) complex, producing a blue color ferrous (Fe2+-TPTZ) that can be monitored by measuring the change in absorption at 593 nm.[Citation19]
The influence of thermal treatment on the antioxidant activity of peptides from duck meat was measured using the DPPH radical scavenging and FRAP represented by and , respectively. Similar changing trend was obtained for DPPH radical scavenging or FRAP studied. The changing trend of antioxidant activity at 0 days postmortem was similar to the samples at 7 days postmortem aging (,, ,).
Figure 1. The DPPH radical scavenging of the extracted peptides from duck breast. (a) Effect of cooking temperature and time on DPPH radical scavenging activity of peptides from duck meat at 0 days postmortem. (b) Effect of cooking temperature and time on DPPH radical scavenging activity of peptides from duck meat at 7 days postmortem. (c) Effect of postmortem aging on DPPH radical scavenging activity of peptides from duck meat at different cooking temperatures. The value of each datapoint is presented as mean ± SE (n = 3). The data with different highercase letters are significantly different at the same cooking temperature; data with different lowercase letters are significantly different at the same cooking time (P < .05)
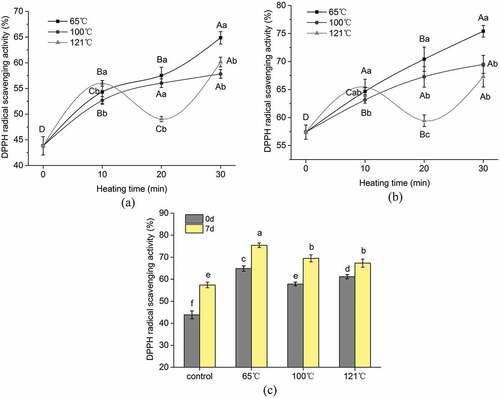
Figure 2. The ferric reducing antioxidant power (FRAP) of the extracted peptides from duck breast. (a) Effect of cooking temperature and time on FRAP of peptides from duck meat at 0 days postmortem. (b) Effect of cooking temperature and time on FRAP of peptides from duck meat at 7 days postmortem. (c) Effect of postmortem aging on FRAP of peptides from duck meat at different cooking temperatures. The value of each datapoint is presented as mean ± SE (n = 3). The data with different highercase letters are significantly different at the same cooking temperature; data with different lowercase letters are significantly different at the same cooking time (P < .05)
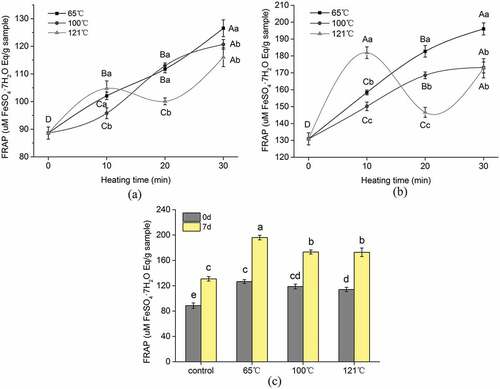
Within the tested temperature, the antioxidant activity of samples cooked at 65°C gradually increased and reached a maximum at 30 min (P < .05). Similarly, small peptides (<3 kDa) derived from longissimus thoracis (LT) and semitendinosus (ST) samples of beef at different aging days cooked at 65°C exhibited increasing antioxidant activity as cooking time prolonged.[Citation12] Although the caplpains of meat sample become rapidly inactivated at 55°C[Citation24], the author explained that the remaining endogenous peptidases such as cathepsins, collagenase, and metalloproteinase in cooked samples may still catalyze the proteolysis in beef during cooking, resulting in higher abundance of small peptides.[Citation15] To some extent, some endogenous peptidases in duck meat might induce the increased antioxidant activity of sample cooked at 65°C, as duck meat is quite different from beef, and further study is needed to confirm this and verify specific peptidase. Moreover, it is not guaranteed that higher concentration of the smaller peptides always induces impaired bioactivities as there may be certain antagonistic effects within a mixture of bioactive peptides.[Citation25,Citation26]
Similar to the sample cooked at 65°C, the antioxidant activity of the sample cooked at 100°C increased with the progress of cooking time. However, there was no pronounced change (P > .05) in DPPH radical scavenging activity for the sample cooked at 100°C during 20–30 min. Similarly, the peptides derived from ham by-products cooked at 100°C increased the antioxidant activity as cooking time prolonged.[Citation10] In the present study, samples subjected to cooking (100°C) showed higher antioxidant activity than uncooked samples according to the results of all two methods evaluated. This fact suggests that there has been no reduction, but rather an increase of potentially bioactive peptides after cooking; this is probably due to the generation of novel antioxidant peptides with satisfactory heat stability. On the other hand, small peptides (< 5000 Da) do not have the tertiary and quaternary structure, but they still can form secondary structures, which are the key factors affecting the antioxidant activity of peptides.[Citation27] Thermal treatments can alter the tertiary and quaternary structure of proteins, resulting in modifications of their physical properties.[Citation28,Citation29] Unfolding of the peptides can increase their antioxidant activity by increasing the solvent exposure of antioxidant amino acids that would normally be located in the core of the native peptide structure.[Citation30] Therefore, the heat treatments used here might increase the antioxidant activity of some peptides through changing their secondary structure.
Regarding the sample cooked at 121°C, the antioxidant activity increased at the beginning of the cooking (during 0–10 min). The antioxidant activity started to decrease during 10–20 min, but at the longest heating time, a further increase of antioxidant activity is seen. The trend of antioxidant activity of peptides in the early stage of heating can be explained by the reason that, in the initial period, the consumption of antioxidant substances is less markedly compared to the exposure of “hidden” antioxidant amino acids due to protein denaturation. Moreover, some antioxidant compounds such as glutathione(GSH) can be released as a consequence of cell membrane destruction, thus being more reactive toward the radical probes. Prolongation of the heating process reduced the antioxidant activity of small peptides from duck meat. This might be due to the accumulation of oxidized proteins and the loss in functionality of active meat peptides. Moreover, degradation of endogenous antioxidative factors such as vitamin E, vitamin C, ubiquinols, polyphenols, and cellular thiols could be promoted by heating.[Citation22] In the last stage of the heating process, the antioxidant activity of peptides from duck meat showed a marked increase in both assays. This may be related to Maillard reactions between reducing sugars and free amino acids or free amino groups in proteins.[Citation22,Citation31] Since the Maillard reaction is favored at low water activity, a significant increase in the Maillard reaction products(MRPs) concentration could be achieved at the end of the cooking.
Effect of aging time on the antioxidant activities
and shows that the effect of aging time on the antioxidant activity of small peptides from duck meat after different temperatures cooked for 30 min. Aging time and cooking can influence the generation of peptides and their related antioxidant activity. All the peptides derived from raw and cooked samples show that the sample at 7 days postmortem possessed higher antioxidant activity than sample of day 0. Similar results were also reported for beef meat during postmortem aging and cooked at 65°C.[Citation12].
The bioactivity of the extracted peptides derived from the raw and cooked samples was evaluated based on the normalized protein concentration (1 mg/mL). Therefore, changes in bioactivity with aging time and cooking are ascribed to certain active peptides generated in the different phases of aged or cooked duck meat. Postmortem proteolysis is a dynamic and variable procedure where endopeptidases exert their cleavages randomly to produce a complex mixture of peptides,[Citation32] which might be responsible for the aged samples having higher antioxidant activity than hot fresh meat (the sample of day 0).
Effect of simulated gastrointestinal digestion on the antioxidant activities
DPPH radical scavenging of simulated gastrointestinal digestion
The sample at 7 days postmortem showing higher antioxidant activity after cooking (65°C–30 min, 100°C–20 min, 121°C–10 min) were selected for in vitro digestion. As shown in a, the sample cooked at 65°C exhibited highest DPPH·scavenging activity, reaching 73.51 ± 1.57%. The DPPH·scavenging activity of different heat treatment was similar during in vitro digestion. After 2 h of digestion with pepsin, the scavenging activity of samples was significantly increased (except 121°C–10 min which was not significant). However, further digestion with trypsin resulted in a sharp decrease in DPPH·scavenging activity (P < .05). Among the heat treatment samples, the highest DPPH·scavenging activity after in vitro digestion was in sample cooked at 65°C for 30 min (43.20 ± 1.60%), whereas the lowest level (33.22 ± 1.69%) after in vitro digestion was obtained from that sample cooked at 121°C for 10 min.
Figure 3. Effect of pepsin–trypsin digestion on antioxidant activity DPPH radical scavenging of peptides from aged duck meat after cooking. (a) DPPH radical scavenging activity of peptides. (b) FRAP of peptides. The value of each datapoint is presented as mean ± SE (n = 3). The data with different highercase letters are significantly different during pepsin–trypsin digestion; data with different lowercase letters are significantly different between different cooking methods (P < .05)
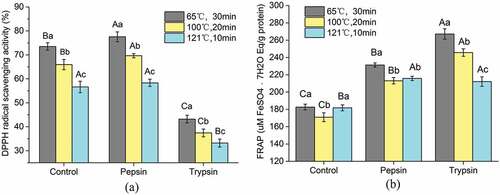
Oxidation and aggregation of proteins can lead to an increase of surface hydrophobicity that modified the rate of digestion by gastrointestinal enzymes depending on the nature of protease, temperature and time of cooking.[Citation10,Citation33] Recent studies showed that the temperature of cooking affects the digestion rate of proteins more than digestibility, and conformational changes due to protein denaturation favored the bioaccessibility of the digestive proteases to their cleavage sites.[Citation10,Citation34] In the present study, we found that the DPPH radical scavenging activity increased with pepsin treatment but then significantly decreased following trypsin digestion. During the pepsin digestion, more hydrophobic amino residue side chain groups were expected to be exposed because the peptides were completely hydrolyzed, which would make the peptides more accessible to the DPPH radicals and stabilized toward the DPPH radicals.[Citation35,Citation36] However, further digestion with trypsin resulted in a sharp decrease in DPPH radical scavenging activity. That is because, during the trypsin digestion, the peptides were intensively hydrolyzed, which could lead to high accumulation of shorter peptides and free amino acids, thus making the digestion sample to be more hydrophilic. It has been reported that the digestion with increased polarity can prevent the peptides from reacting with lipid-soluble DPPH radicals.[Citation20] Therefore, the DPPH radical scavenging activity was decreased during trpsin treatment.
FRAP of simulated gastrointestinal digestion
To further evaluate the effect of in vitro digestion on antioxidant activity of peptides, the digested samples were tested for reducing power. For the reducing power assay, the presence of antioxidants in the samples tested reduced the Fe3+ complex to the Fe2+ form.[Citation37] The FRAP value of different heat treatment was similar during in vitro digestion (b). After 2 h of digestion with pepsin, the FRAP value of samples was significantly increased, and the sample cooked at 65°C exhibited highest reducing power, from the initial 180.70 ± 3.42 to 231.29 ± 2.42. After further incubation with trypsin, the FRAP value of sample significantly increased compared to that of the peptides before digestion (except the samples cooked at 121°C, where this was not significant). Overall, the reducing power of cooked sample was significantly increased after simulated gastrointestinal digestion.
The increase in the reducing power of samples shows that antioxidant peptides can be more effective hydrogen or electron donors after in vitro digestion. With the increase in the degree of hydrolysis, polar or charged moieties were exposed heavily and the peptides could become effective hydrogen or electron donors to reduce Fe3+–TPTZ complex.[Citation35,Citation36] This result is in accordance with that of You et al.[Citation20], who found that the reducing power of loach protein hydrolysates significantly increased after simulated gastrointestinal digestion.
Overall, it is found that the DPPH radical scavenging activity was significantly decreased, but the reducing power significantly increased after gastrointestinal digestion.
The antioxidant activity of peptides is associated with the sequence, structure molecular weight, as well as the amino acid composition.[Citation2,Citation18,Citation38] Previous studies have shown that the presence of hydrophobic amino acids played an important role in scavenging free radicals including Leu、Ile、Val、Met、Ala、Phe and Trp.[Citation3,Citation11] Peptides consisted of Glu, Gln and Lys and were identified to have stronger ability to chelate metal ions as well as scavenge radicals.[Citation39,Citation40]
Conclusion
In conclusion, the small peptides that are generated by endogenous enzymes in duck meat through postmortem aging after thermal treatment possessed varying degrees of in vitro antioxidant activities. Generally, cooking and postmortem aging increased the antioxidant activity, and within the tested temperature, the aged duck meat cooked at 65°C for 30 min exhibited highest antioxidant activity. In addition, the sample cooked at 100°C for 20 min and 121°C for 10 min possessed higher antioxidant activity compared to other samples at the same temperature. The samples displayed higher antioxidant activity after in vitro digestion, all of which showed pronounced decrease of DPPH radical scavenging activity with significant increase in reducing power. These results could provide a theoretical basis for improving the nutritional and functional value of duck meat when using it as a good source of antioxidant peptides. The next steps will be the characterization of small peptides in cooked duck meat after simulated gastrointestinal digestion.
Acknowledgments
We gratefully acknowledge Dr. Ron Tume from CSIRO, Animal, Food and Health Sciences for his helpful manuscript corrections. This work was financially supported by the National Natural Science Foundation of China (No. 31671872), Self-Initiated Research Project of State Key Laboratory of Food Science and Technology Jiangnan University (SKLF-ZZA-201906) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Additional information
Funding
References
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients. 2010, 2, 611–625. DOI: 10.3390/nu2060611.
- Lafarga, T.; Hayes, M. Bioactive Peptides from Meat Muscle and By-Products: Generation, Functionality and Application as Functional Ingredients. Meat Sci. 2014, 98, 227–239. DOI: 10.1016/j.meatsci.2014.05.036.
- Samaranayaka, A. G. P.; Li-Chan, E. C. Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods. 2011, 3, 229–254. DOI: 10.1016/j.jff.2011.05.006.
- Simonian, N.; Coyle, J. Oxidative Stress in Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. DOI: 10.1146/annurev.pa.36.040196.000503.
- Wang, L.-S.; Huang, J.-C.; Chen, Y.-L.; Huang, M.; Zhou, G.-H. Identification and Characterization of Antioxidant Peptides from Enzymatic Hydrolysates of Duck Meat. J. Agric. Food Chem. 2015, 63, 3437–3444. DOI: 10.1021/jf506120w.
- Al Ghouleh, I.; Khoo, N. K. H.; Knaus, U. G.; Griendling, K. K.; Touyz, R. M.; Thannickal, V. J.; Barchowsky, A.; Nauseef, W. M.; Kelley, E. E.; Bauer, P. M.; et al. Oxidases and Peroxidases in Cardiovascular and Lung Disease: New Concepts in Reactive Oxygen Species Signaling. Free. Radical. Biol. Med. 2011, 51, 1271–1288. DOI: 10.1016/j.freeradbiomed.2011.06.011.
- Liu, Q.; Kong, B. H.; Xiong, Y. L.; Xia, X. F. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate as Influenced by the Degree of Hydrolysis. Food Chem. 2010, 118, 403–410. DOI: 10.1016/j.foodchem.2009.05.013.
- Ryan, J. T.; Ross, R. P.; Bolton, D.; Fitzgerald, G. F.; Stanton, C.; Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Nutrients. 2012, 3, 60–65. DOI: 10.3390/nu3090765.
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of Processing on Bioactive Proteins and Peptides. Trends Food Sci. Technol. 1998, 9, 307–319. DOI: 10.1016/S0924-2244(98)00054-5.
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Effect of Cooking and in Vitro Digestion on the Antioxidant Activity of Dry-Cured Ham By-Products. Food Res. Int. 2017, 97, 296. DOI: 10.1016/j.foodres.2017.04.013.
- Liu, D.; Chen, X.; Huang, J.; Huang, M.; Zhou, G. Generation of Bioactive Peptides from Duck Meat during Post-Mortem Aging. Food Chem. 2017, 237, 408–415. DOI: 10.1016/j.foodchem.2017.05.094.
- Fu, Y.; Young, J. F.; Therkildsen, M. Bioactive Peptides in Beef: Endogenous Generation through Postmortem Aging. Meat Sci. 2017, 123, 134–142. DOI: 10.1016/j.meatsci.2016.09.015.
- Santélhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of Meat Cooking on Physicochemical State and in Vitro Digestibility of Myofibrillar Proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. DOI: 10.1021/jf072999g.
- Simonetti, A.; Gambacorta, E.; Perna, A. Antioxidative and Antihypertensive Activities of Pig Meat before and after Cooking and in Vitro Gastrointestinal Digestion: Comparison between Italian Autochthonous Pig Suino Nero Lucano and a Modern Crossbred Pig. Food Chem. 2016, 212, 590–595. DOI: 10.1016/j.foodchem.2016.06.029.
- Escudero, E.; Mora, L.; Fraser, P. D.; Aristoy, M. C.; Toldrá, F. Identification of Novel Antioxidant Peptides Generated in Spanish Dry-Cured Ham. Food Chem. 2013, 138, 1282. DOI: 10.1016/j.foodchem.2012.10.133.
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of Chicken Breast Protein Hydrolysate and Analysis of Its Antioxidant Activity. Food Chem. Toxicol. 2012, 50, 3397–3404. DOI: 10.1016/j.fct.2012.07.047.
- Bauchart, C.; Chambon, C.; Mirand, P. P.; Savary, A. I.; Rémond, D.; Morzel, M. Peptides in Rainbow Trout (Oncorhynchus Mykiss) Muscle Subjected to Ice Storage and Cooking. Food Chem. 2007, 100, 1566–1572. DOI: 10.1016/j.foodchem.2005.12.023.
- Xing, L. J.; Hu, Y. Y.; Hu, H. Y.; Ge, Q. F.; Zhou, G. H.; Zhang, W. G. Purification and Identification of Antioxidative Peptides from Dry-Cured Xuanwei Ham. Food Chem. 2016, 194, 951–958. DOI: 10.1016/j.foodchem.2015.08.101.
- Benzie, F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. BiochemI. 1996, 239, 70–76. DOI: 10.1006/abio.1996.0292.
- You, L.; Zhao, M.; Joem, R.; Ren, J. Changes in the Antioxidant Activity of Loach (Misgurnus Anguillicaudatus) Protein Hydrolysates during a Simulated Gastrointestinal Digestion. Food Chem. 2010, 120, 810–816. DOI: 10.1016/j.foodchem.2009.11.018.
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature Data May Underestimate the Actual Antioxidant Capacity of Cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. DOI: 10.1021/jf050049u.
- Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Meat Sci. 2012, 90, 60–65. DOI: 10.1016/j.meatsci.2011.05.027.
- Escudero, E.; Aristoy, M. C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive Effect and Antioxidant Activity of Peptide Fractions Extracted from Spanish Dry-Cured Ham. Meat Sci. 2012, 91, 306–311. DOI: 10.1016/j.meatsci.2012.02.008.
- Christensen, L.; Ertbjerg, P.; Løje, H.; Risbo, J.; Berg, F. W. J. V. D.; Christensen, M. Relationship between Meat Toughness and Properties of Connective Tissue from Cows and Young Bulls Heat Treated at Low Temperatures for Prolonged Times. Meat Sci. 2013, 93, 787–795. DOI: 10.1016/j.meatsci.2012.12.001.
- Hartmann, R.; Meisel, H. Food-Derived Peptides with Biological Activity: From Research to Food Applications. Curr. Opin. Biotech. 2007, 18, 163–169. DOI: 10.1016/j.copbio.2007.01.013.
- Hernándezledesma, B.; Recio, I.; Amigo, L. Beta-Lactoglobulin as Source of Bioactive Peptides. Amino Acids. 2008, 35, 257–265. DOI: 10.1007/s00726-007-0585-1.
- Zhu, C. Z.; Zhang, W. G.; Kang, Z. L.; Zhou, G. H.; Xu, X. L. Stability of an Antioxidant Peptide Extracted from Jinhua Ham. Meat Sci. 2013, 96, 783–789. DOI: 10.1016/j.meatsci.2013.09.004.
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of Oxidation on in Vitro Digestibility of Skeletal Muscle Myofibrillar Proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. DOI: 10.1021/jf070252k.
- Tironi, V. A.; Tomas, M. C.; Anon, M. C. Structural and Functional Changes in Myofibrillar Proteins of Sea Salmon (Pseudopercis Semifasciata) by Interaction with Malonaldehyde (RI). J.Food Sci. 2002, 67, 929–935. DOI: 10.1111/j.1365-2621.2002.tb09430.x.
- Elias, R. J.; Mcclements, D. J.; Decker, E. A. Impact of Thermal Processing on the Antioxidant Mechanisms of Continuous Phase β-lactoglobulin in Oil-In-Water Emulsions. Food Chem. 2007, 104, 1402–1409. DOI: 10.1016/j.foodchem.2007.01.072.
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A New Procedure to Measure the Antioxidant Activity of Insoluble Food Components. J. Agric. Food Chem. 2007, 55, 7676–7681. DOI: 10.1021/jf071291z.
- Gallego, M.; Mora, L.; Fraser, P. D.; Aristoy, M. C.; Toldrá, F. Degradation of LIM Domain-Binding Protein Three during Processing of Spanish Dry-Cured Ham. Food Chem. 2014, 149, 121–128. DOI: 10.1016/j.foodchem.2013.10.076.
- Bax, M. L.; Aubry, L.; Ferreira, C.; Daudin, J. D.; Gatellier, P.; Rémond, D.; Santé-Lhoutellier, V. Cooking Temperature Is a Key Determinant of in Vitro Meat Protein Digestion Rate: Investigation of Underlying Mechanisms. J. Agric. Food Chem. 2012, 60, 2569–2576. DOI: 10.1021/jf205280y.
- Sayd, T.; Chambon, C.; Santé-Lhoutellier, V. Quantification of Peptides Released during in Vitro Digestion of Cooked Meat. Food Chem. 2016, 197, 1311–1323. DOI: 10.1016/j.foodchem.2015.11.020.
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y. Reducing, Radical Scavenging, and Chelation Properties of in Vitro Digests of Alcalase-Treated Zein Hydrolysates. J. Agric. Food Chem. 2008, 56, 2714–2721. DOI: 10.1021/jf703697e.
- Liu, D.; Chen, X.; Huang, J.; Zhou, X.; Huang, M.; Zhou, G. Stability of Antioxidant Peptides from Duck Meat after Post‐Mortem Ageing. Int. J. Food Sci. Tech. 2017, 52, 2513–2521. DOI: 10.1111/ijfs.13536.
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. DOI: 10.1006/abio.1996.0292.
- Mendis, E.; Niranjan Rajapakse, N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. DOI: 10.1021/jf048877v.
- Zhu, C.-Z.; Zhang, W.-G.; Zhou, G.-H.; Xu, X.-L.; Kang, Z.-L.; Yin, Y. Isolation and Identification of Antioxidant Peptides from Jinhua Ham. J. Agric. Food Chem. 2013, 61, 1265–1271. DOI: 10.1021/jf3044764.
- Majumder, K.; Wu, J. A New Approach for Identification of Novel Antihypertensive Peptides from Egg Proteins by QSAR and Bioinformatics. Food. Res. Int. 2010, 43, 1371–1378. DOI: 10.1016/j.foodres.2010.04.027.
