ABSTRACT
Postmortem characteristics of Patinopecten yessoensis adductor muscle (PYAM) were evaluated by biochemical, chemical and textural changes during iced storage for 14 days. Triphosphate (ATP) and its breakdown products, K-value, total volatile basic nitrogen (TVB-N), pH, water-holding capacity (WHC), color, texture, protein degradation and cathepsin activities were monitored. K-value increased linearly from 5.9 ± 0.9% at day 0 to 28.1 ± 2.4% at day 2 and 70.2 ± 1.8% at day 12. Spoilage indicator TVB-N (mg/100 g) increased from 10.0 ± 0.6 to 34.6 ± 3.1 at day 12. Textural parameters (e.g., hardness, chewiness, springiness, adhesiveness, and shear force) followed a declining trend over the storage. The WHC decreased from 85.1 ± 3.1% at day 0 to 70.5 ± 1.8% at day 12. SDS-PAGE result indicated that proteolysis occurred in actin and myosin heavy chain (MHC) at day 14. Both cathepsin B and L increased throughout the iced storage, peaking at 1.47-fold and 1.08-fold, respectively, suggesting that cathepsin B and L played important roles in the deterioration of PYAM quality. The overall results indicated that PYAM was suitable to be consumed raw within the first 2 days, and to be processed in no more than 11 days.
Introduction
Scallop Patinopecten yessoensis (P. yessoensis) is a bivalve mollusk widely found in Hokkaido, Japan, Kuril(e) Islands in Russia, and Bo Hai bay in China, where it is considered an important marine resource. P. yessoensis is a rich source for proteins, vitamins, and minerals, and popular among consumers for its unique taste and mouthfeel. The total output of P. yessoensis reached 2.0 million tons in China in 2017.[Citation1] It is an important aquaculture species due to its high economic value and fast-growing nature. The adductor muscle is the main edible part of P. yessoensis (referred to as PYAM in this study) and an important commercial food product. PYAM can be consumed raw, cooked, canned, or as processed, ready-to-eat food.
Postmortem changes in P. yessoensis usually go through several stages: rigor mortis, dissolution of rigor mortis and autolysis.[Citation2] Postmortem changes affect the eating quality and shelf-life of P. yessoensis. In muscular tissues of seafood, adenosine triphosphate (ATP) degrades to adenosine diphosphate (ADP), adenosine monophosphate (AMP) and inosine monophosphate (IMP) promptly postmortem due to endogenous enzymatic reactions.[Citation3] IMP then decompose into inosine (HxR) and hypoxanthine (Hx). IMP is a main umami substance in meat, which is hydrolyzed by AMP deaminase (ADA) and acid phosphatase (ACP). It has been shown that activities of endogenous enzymes may cause muscle tissues to autolyze. In addition, subsequent bacterial growth may lead to further muscle corruption in seafood.[Citation4]
Many evaluation indices for the freshness of aquatic products have been utilized over the years, in which various aspects of sensory properties, physical and chemical changes, and microbiological growth are assessed.[Citation5] Among them, studies have shown that K-value, which evaluates levels of nucleotide degradation and chemical spoilage[Citation6], and total volatile basic nitrogen (TVB-N) are useful indicators for evaluating freshness of fish quality.[Citation7,Citation8] Texture properties are also important in affecting consumer acceptance of muscle seafood like shellfish. Postmortem textural changes in shellfish are usually induced by endo-enzyme like lysosomal (cathepsins) and cytosolic enzymes (calpains).[Citation9]
PYAM is often stored on ice for extended time to be consumed as sashimi in restaurants. Changes in physicochemical properties of PYAM during iced storage remain largely unknown. In the present study, postmortem changes in PYAM during iced storage were characterized. Our findings could provide guidelines for the handling and processing of PYAM to generate better returns for producers and satisfying experiences for consumers.
Materials and methods
Sample preparation and treatments
Live P. yessoensis (50 ± 5 g) samples were bought from a seafood market located in Dalian, China. All of the P. yessoensis were caught from Yellow sea around Dalian city in the early morning of the same day. After removing shell, gonad, and viscera, washing, chilling, the adductor muscle were prepared. The PYAM was then packed in heat-sealed bags weighing about 5 g each (day 0 sample), then placed in heat-insulated bubble foam container embedded in crushed ice, and stored in a cool room at 4°C for 14 days. During storage, water from molten ice was removed through weep holes at the bottom of each container, and fresh ice was added when necessary. Iced stored samples and fresh PYAM (as control) were analyzed at designated time points (i.e., day 0, 2, 12 and 14, respectively).
Assessment of ATP-related compounds and K-value
ATP-related compounds were extracted according to Li et al.[Citation10] Briefly, 5 g of PYAM was homogenized with 5 mL of cold water for 30 s, then 5 mL of cold perchloric acid (20%, v/v) were added, and the PYAM sample was centrifuged at 7,800 g for 10 min at 4°C. After collecting the supernatant, the pellet was wash with 10 mL of cold perchloric acid (5%, v/v) and centrifuged again following the same procedure. This process was repeated twice. The combined supernatant was adjusted to pH 6.35–6.45 with 1mol/L KOH solution and centrifuged at 7,800 g for 3 min at 4°C. Finally, the supernatant, which contains ATP-related compounds, was collected and diluted to 25 mL at pH 6.4 for analysis.
ATP-related compounds (ATP, ADP, AMP, IMP, Hxr, and Hx) were analyzed by high-performance liquid chromatography (HPLC) (Shimadzu, LC-20AT series, Kyoto, Japan).[Citation11] K-value was calculated by the following equation:
Determination of total volatile basic nitrogen (TVB-N)
TVB-N value was determined using the semi-micro steam distillation method[Citation12] with some modifications. Ten grams of the PYAM sample were homogenized and dispersed in 50 mL of distilled water and stirred for 30 min. The mixture was then centrifuged at 3,000 g for 15 min at 25°C. Five millilitre of filtrate was made alkaline by adding 5 mL of 10 g/L MgO. Steam distillation was performed using Kjeldahl distillation unit (KjeldahlApparatus (UDK+129, Italy)) for 5 min. The distillate was collected in a flask containing a 10 mL aqueous solution of boric acid (20 g/L), and a mixed indicator produced from dissolution of 0.1 g of methyl red and 0.1 g of methylene blue to 100 mL of ethanol. Afterward, the boric acid solution was titrated with a 0.01 mol/L hydrochloric acid solution. The TVB-N value was determined according to the consumption of hydrochloric acid.
Measurement of pH
PYAM (5 g) was homogenized in 50 mL distilled water and stirred for 30 min. Then the mixture was centrifuged at 7,800 g for 10 min at room temperature to collect the supernatant. The pH of the supernatant was measured with a digital pH meter (Mettler Toledo PHS-3, Shanghai, China).
Determination of Water-holding capacity (WHC)
WHC was measured according to Suarez et al.[Citation13] with some modifications. PYAM samples (5 g) were placed in a 50 mL centrifuge tube, which contained tissue paper to absorb water. The tube was then centrifuged at 4,500 g for 10 min. Samples were then removed from the tube and weighed. The initial water content was determined by measuring the loss value of 5 g PYAM before and after drying at 105°C for 16 h. The WHC was calculated as follows:
Analysis of textural properties
PYAM sample was cut into 10 × 10 × 10 mm cubes. Textural parameters including hardness and springiness were measured by TA-XT2i texture analyzer (Stable Micro Systems, Surrey, UK) with a TPAP/50 square probe. The probe was pressed against the cube at the speed of 1 mm/s. Each cube was compressed to 30% total deformation with 5 s interval between two consecutive test cycles.
Color evaluation
Color changes of the PYAM were determined with a tristimulus colorimeter (Model UltraScan-PRO, HunterLab, US). The L*,a*, and b* values were measured at the surface of the PYAM samples, each of which was 12 to 20 mm high and 12 to 13.5 mm in diameter. Six random samples of each group were taken for analysis at each sampling time (0, 1/3, 1, 2, 4, 6, 8, 10, 12, and 14 d)
Protein analysis with SDS-PAGE
Protein degradation was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 5% stacking gel and 8% running gel. One gram sample was homogenized with 9 mL 5% (w/v) SDS solution, then the mixture was incubated at 85°C in water bath for 1 h. The supernatant was collected after centrifugation at 5,000 g for 15 min. The supernatant was mixed with SDS-PAGE sample buffer (0.25 M Tris-HCl (pH 7.5), 8 M Urea, 5% SDS, 5% mercaptoethanol) at a ratio of 4:1 and boiled for 5 min. The boiled sample (20 μg) and markers were loaded into the sample well in the stacking gel. After separation and staining with 0.05% Coomassie Brilliant Blue R-250, the gel was destained using methanol and acetic acid solution.
Measurement of Cathepsin activity
Samples were homogenized with protein extraction solution (50 mM phosphate buffer (pH 7.0), 0.1% Triton X-100, 1 mM EDTA) at 4°C by 1:3 (w/v). Then, the homogenate was centrifuged at 12,000 g for 10 min at 4°C, supernatant was then collected for analysis of cathepsin activity. Cathepsin activity was determined using substrates Z-Arg-Arg-MCA for cathepsin B activity, and the Z-Phe-Arg-Mec for cathepsin L activity. The activity of cathepsin was measured according to Ge et al.[Citation14], with some modifications. The cathepsin activity was expressed as U/mg of protein.
Statistical analysis
All experiments were run in triplicate. Data were analyzed by the SPSS 16.0 (SPSS Inc, 2001, Chicago, IL, USA) and one-factor analysis of variance (ANOVA) was conducted. Means ± SE was calculated. Differences between means were considered to be significant at p< .05.
Resuls and discussion
Atp-related compounds and K-value
For the majority of aquatic products, nucleotide degradation in them follows a well-defined process: ATP → ADP → AMP → IMP →Hxr → Hx.[Citation15] Transformation of ATP, and the contents of relevant compounds in PYAM during the 14-day iced storage are illustrated in . The total amount of ATP-related compounds was 5.6 ± 0.3 μmol/g. This value is similar to the 6.6 μmol/g reported in adductor muscle of Catarina scallop[Citation16], but was much lower than the 10.2 μmol/g in the adductor muscle of Japanese baking scallop (Pecten albicans).[Citation17] The metabolism of ATP in scallop was affected by many factors, including the species, the season, physiology, fishing technique, and killing method employed.[Citation16]
Figure 1. ATP and related endogenous degradation products of PYAM during iced storage
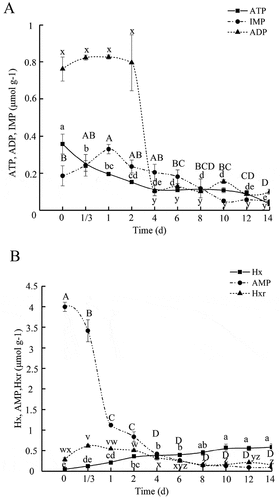
In this study, the ATP content of PYAM in the initial stage (8 h after treatment in laboratory) was 0.36 ± 0.05 μmol/g of muscle, and it fell gradually throughout the duration of the iced storage. Similar results were reported for Catarina scallop, where the ATP concentration was reduced rapidly during storage.[Citation16] It has been reported that ATP could be converted into ADP, AMP and IMP within a short period of time postmortem[Citation18], consistent with our observation: the initial IMP concentration was 0.19 ± 0.05 μmol/g, then increased until the highest content (0.33 ± 0.03 μmol/g) was reached at day 1. From that point on the IMP content gradually declined from 0.33 ± 0.03 μmol/g to 0.050 ± 0.004 μmol/g at day 14. Evidently, a rapid degradation of ATP to IMP occurred in PYAM, similar to the result reported in silver carp fish muscle by Shi et al.[Citation19] The IMP could enhance the flavor of fish muscle, its disappearance has been correlated with the loss of flavor and freshness in some fish species.[Citation20] Therefore, the accumulation of IMP in PYAM shortly postmortem could contribute to an improved flavor of freshness. The ADP content declined during the iced storage after a small increase at first, while the AMP content dropped sharply within the first day. The similar results have also been observed by Ramon et al. in ADP and ADP content of Pacific lions-paw scallop.[Citation21] The Hxr content increased within the first 2 days, and then began to decrease. The Hx content gradually increased till the end of the iced storage from 0.048 ± 0.001 μmol/g to 0.59 ± 0.07 μmol/g, due to both autolytic and enzymatic activities that caused spoilage. Hx tastes bitter, and it is regarded as a contributor to off-flavors. After 1 day, IMP was slowly degraded to Hxr and then to Hx, contributing to the progressive loss of desirable flavor in PYAM.
Decomposition of ATP to ADP, AMP, IMP, Hxr, and Hx was widely reported in fish. It has been used widely as the basis of calculating the K-value, or freshness index for aquatic species.[Citation22] Changes in K-values of PYAM during iced storage were shown in . It is generally believed that seafood with K-value less than 20% could be consumed raw as sashimi.[Citation23] While seafood with K-value less than 50% is considered as moderately fresh, and could not be directly eaten as sashimi, it could be consumed after thermal processing, or cooking. Seafood with K-value over 70% is considered not fresh, and not suitable for human consumption.[Citation24]
Figure 2. Changes of K value in PYAM during iced storage
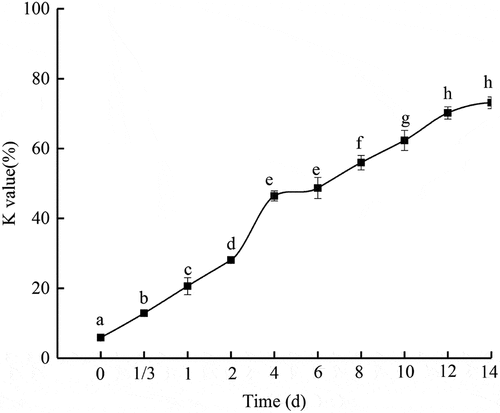
In the present study, K-value of the PYAM increased from 5.9 ± 0.9% to 28.1 ± 0.8% within the first 2 days. The K-value was 44.63 ± 0.08% on the 4th day and 70.2 ± 1.7% on the 12th day. If K-value of 70% is defined as the threshold for acceptable freshness, the shelf life of the stored PYAM in ice could be set to be 11 days. The increase in K-value might be correlated to a rapid decrease in ATP and ADP, and a sharp increase in Hx.
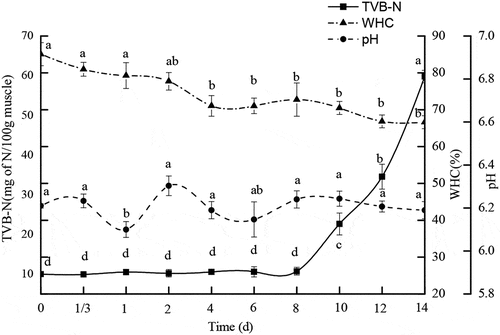
Changes in ph
Changes in pH of the PYAM during 14 days of storage were shown in . Apparently, pH remained at 6.21 ± 0.01 with no significant change observed during the 14-day iced storage. It is consistent with the value (pH 6.3) reported by Pacheco–Aguilar.[Citation21]The stable pH may be correlated to the insignificant microbial growth during the iced storage.
Total volatile basic nitrogen (TVB-N)
TVB-N analysis is commonly used as an evaluation method for monitoring quality in aquatic products during iced storage.[Citation25] TVB-N measures nitrogen in ammonia as well as primary, secondary, and tertiary amines. The increase of TVB-N is related to the activities of spoilage bacteria and endogenous enzymes.[Citation18]
In the present study, the initial TVB-N content on day 0 was 10.0 ± 0.6 mg/100 g. It is lower than the previously reported TVB-N values of untreated ice-stored scallops (13.5 mg/100 g).[Citation26] . showed that the amount of TVB-N in PYAM remained almost constant in 8 days, below 11mg/100 g, suggesting that to this point bacterial growth was under effective control due to iced storage. However, after day 8, a rapid increase in TVB-N was observed, reaching a value of 34.6 ± 3.1 mg/100 g on day 12, and 59.8 ± 1.6 mg/100 g on day 14. It suggested that microbial growth could no longer be held check. As the growth of microbes accelerated, so did protein decomposition. According to Riaz & Qadri’s reports[Citation27], fish flesh with a level of 35–40 mg/100 g of TVB-N can be regarded as spoiled. Taking the 35 mg/100g as the threshold for acceptable quality, the shelf life of stored PYAM in ice was about 11 days. This result was consistent with the shelf-life determined through K-value, as discussed earlier.
Changes of textural properties and WHC
Texture properties of PYAM such as hardness and adhesiveness can be impacted by several factors including the change of pH, the degradation of myofibrils[Citation24,Citation28], and the effects of endo-enzymes.[Citation29] As shown in , adhesiveness and shear force of PYAM increased postmortem by day 1, hardness increased postmortem by day 2, then all indicators started to decline till the end of the iced storage. The change in hardness was in accordance with that of Songpu mirror carp.[Citation13] Chewiness decreased to a certain extent at the entire storage.
Table 1. Changes of the hardness, chewiness, springiness, adhensiveness, shear force in PYAM during storage in ice
Springiness measures elasticity and describes the ability of the PYAM to recover from deformation. Springiness of the PYAM reached their maximum in day 1, and later declined. The decline might be caused by cathepsins, as Bahuaud[Citation30] reported negative correlations between cathepsin activity and texture in Atlantic salmon (Salmo salar L.).
WHC is a primary qualitative parameter that is widely used in food industry. It is correlated to sensory characteristics, shelf life, and the functionality of food product. In the present study, PYAM had an initial WHC of 85.09 ± 0.03% (), which decreased in all the samples over time during the iced storage. Generally, adductor muscle of shellfish tends to become tougher, experiences a progressive loss of fluid and a reduction of WHC.[Citation24] The gradual decline of WHC in the PYAM could be attributed to the denaturation of myofibrillar proteins during the storage, consistent with what has been reported in chilled pork muscle.[Citation9]
Changes in color
Color is a significance index to evaluate the quality of seafood products. shows the change of surface color in PYAM during iced storage for 14 days. Over time, the yellow-blue value of the PYAM (b*) did not change (p > .05), while the luminance value (L*), and red and green values (a*) of PYAM showed a slight decreasing trend, it might be due to a decrease in water retention. These results are similar to previous report by Pacheco–Aguilar[Citation24], that no significant difference (p > .05) in color was observed for the adductor muscle of Pacific lions-paw scallop (Nodipecten subnodosus) during iced storage for 15 days.
Table 2. Changes in color parameters in PYAM during storage in ice
Changes in PYAM proteins
In order to identify the changes of the proteins in PYAM, the proteins were extracted and analyzed by SDS-PAGE. Myosin heavy chain (MHC) (200 kDa) and actin (45 kDa) bands were observed in PYAM (). The MHC began to degrade on day 4 and was completely degraded by day 14. The actin obviously degraded on day 14 too. Both MHC and actin gradually became lighter, suggesting the degradation of myofibrillar protein. The similar results were found in stored Indian squid mantle protein in ice.[Citation31] These results indicated that structural proteins degraded over time during iced storage. It has been suggested that the high activities of fish muscle protease might affect the protein structure.[Citation32] In the present study, we also observed that cathepsin B and L () were released from the lysosome in cells, and then went through activation/deactivation cycles during the iced storage. The increased cathepsin B and L activities might modulate changes in protein content composition.
Figure 4. SDS-PAGE photographs of protein degradation in PYAM during iced storage
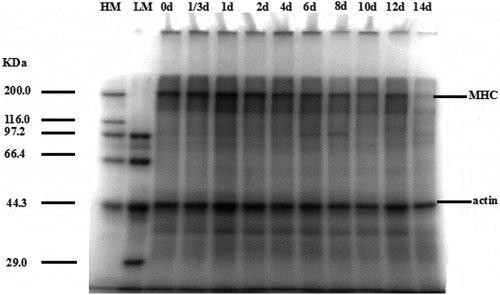
Table 3. Cathepsin activities changes of PYAM during storage in ice
Cathepsin B and L activities
The activities of cathepsin B and L are involved in proteolysis of muscle proteins.[Citation11] These enzymes are involved in mitochondria-mediated apoptosis, which in turn affects muscle protein degradation.[Citation33] Thus meta-analysis of cathepsin activity in subcellular fractions may reveal their relocation in myocyte postmortem, thereby show the role of cathepsin in affecting the eating quality of the PYAM. Cathepsin B and L are responsible for the degradation of MHC and a-actin, and may contribute to textural changes.[Citation34] Changes of cathepsin B and L activities in lysosome of PYAM during 14 days are depicted in .
A similar trend was observed for cathepsin B and L in PYAM. The activities of cathepsin B and L increased at first, then obviously declined. The same trend was also reported by Ge et al.[Citation4] in grass carp (Ctenopharyngodon idella) fillets in iced storage. Cathepsin B and L activities in PYAM increased up until day 4 of storage, peaking at 1.47-fold and 1.08-fold, respectively. Cathepsin B had the highest activity, while change for cathepsin L was minor at day 4. Similar trend was also reported by Ge et al.[Citation14], super chilled grass carp cathepsin B had the highest activity. Cathepsin B activity in PYAM stated to decline on day 12, and cathepsin L declined on the last day. Overall, cathepsin B activity was higher than that of cathepsin L. The elevated cathepsin B and L activities might explain the reduction in PYAM springiness as well as hardness at the later stage of iced storage. Li et al.[Citation11] found that the decrease in hardness of carp muscle was correlated with the activities of cathepsins.
Conclusion
The present study shows that iced storage could be an effective way for PYAM preservation as long as the timing is well controlled to meet specific goals. Biochemical, chemical and physical evaluations in this study indicated that iced PYAM would remain suitable for raw consumption until day 2, and suitable for processing until day 11. K-value, as well as TVB-N, could be used as good indicators for monitoring loss of freshness during the iced storage of PYAM. These results provided important timelines and control points guidelines for seafood manufacturers and processers to maintain high quality in their PYAM products. Further study will focus on understanding the mechanism of PYAM quality changes during iced storage.
Additional information
Funding
References
- Bureau of Fisheries in Ministry of Agriculture. China Fishery Statistical Yearbook; China Agriculture press: Beijing, 2017; pp pp29.
- Wongso, S.; Ushio, H.; Ohshima, T.; Yamanaka, H. Changes in Content of Octopine, Acidic Opines, Related Amino Acids and Phosphoarginine in the Adductor Muscle of Three Species of Scallop during Storage. J. Food Biochem. 2007, 22(1), 65–81. DOI: 10.1111/j.1745-4514.1998.tb00231.x.
- Howgate, P.;. Kinetics of Degradation of Adenosine Triphosphate in Chillstored Rainbow Trout (Oncorhynchus Mykiss). Int. J. Food Sci. Technol. 2005, 40(6), 579–588. DOI: 10.1111/ifs.2005.40.issue-6.
- Ge, L.; Xu, Y.; Jiang, X.; Xia, W.; Jiang, Q. Broad‐Spectrum Inhibition of Proteolytic Enzymes by Allicin and Application in Mitigating Textural Deterioration of Icestored Grass Carp (Ctenopharyngodon Idella) Fillets. Int. J. Food Sci. Technol. 2016, 51(4), 902–910. DOI: 10.1111/ijfs.13047.
- Ohashi, E.; Okamoto, M.; Ozawa, A.; Fujita, T. Characterization of Common Squid Using Several Freshness Indicators. J. Food Sci. 2010, 56(1), 161–163. DOI: 10.1111/j.1365-2621.1991.tb08001.x.
- Cheng, J. H.; Sun, D. W.; Pu, H.; Zhu, Z. Development of Hyperspectral Imaging Coupled with Chemometric Analysis to Monitor K Value for Evaluation of Chemical Spoilage in Fish Fillets. Food Chem. 2015, 185(1), 245–253. DOI: 10.1016/j.foodchem.2015.03.111.
- Özyurt, G.; Kuley, E.; Özkütük, S.; Özogul, F. Sensory, Microbiological and Chemical Assessment of the Freshness of Red Mullet (Mullus Barbatus) and Goldband Goatfish (Upeneus Moluccensis) during Storage in Ice. Food Chem. 2014, 114(4), 505–510. DOI: 10.1016/j.foodchem.2008.09.078.
- Andrade, S.; Mársico, E.; Godoy, R.; Franco, R. M.; Conte Junior, C. A. Chemical Quality Indices for Freshness Evaluation of Fish. J. Food Stud. 2014, 3(1), 71–87. DOI: 10.5296/jfs.v3i1.
- Zhang, Y.; Ertbjerg, P. Effects of Frozen-Then-Chilled Storage on Proteolytic Enzyme Activity and Water-Holding Capacity of Pork Loin. Meat Sci. 2018, 145(5), 375. DOI: 10.1016/j.meatsci.2018.07.017.
- Li, D.; Jia, S.; Zhang, L.; Wang, Z.; Pan, J.; Zhu, B. Effect of Using a High Voltage Electrostatic Field on Microbial Communities, Degradation of Adenosine Triphosphate, and Water Loss When Thawing Lightly-Salted, Frozen Common Carp (Cyprinus Carpio). J. Food Eng. 2017, 212, 226–233. DOI: 10.1016/j.jfoodeng.2017.06.003.
- Li, Q.; Zhang, L.; Lu, H.; Song, S.; Luo, Y. Comparison of Postmortem Changes in ATP-related Compounds, Protein Degradation and Endogenous Enzyme Activity of White Muscle and Dark Muscle from Common Carp (Cyprinus Carpio) Stored at 4°C. LWT - Food Sci. Technol. 2017, 78, 317–324. DOI: 10.1016/j.lwt.2016.12.035.
- Özogul, F.; Özo Gul, Y. Comparison of Methods Used for Determination of Total Volatile Basic Nitrogen (TVB-N) in Rainbow Trout (Oncorhynchus Mykiss). Turk. J. Zool. 2000, 24, 113–120.
- Shi, C.; Cui, J.; Luo, Y.; Zhou, Z. Effect of Lightly Salt and Sucrose on Rigor Mortis Changes in Silver Carp (Hypophthalmichthys Molitrix) Stored at 4°C. Int. J. Food Sci. Technol. 2014, 49, 160–167. DOI: 10.1111/ijfs.12291.
- Ge, L.; Xu, Y.; Xia, W. The Function of Endogenous Cathepsin in Quality Deterioration of Grass Carp (Ctenopharyngodon Idella) Fillets Stored in Chilling Conditions. Int. J. Food Sci. Technol. 2015, 50(3), 797–803. DOI: 10.1111/ijfs.12713.
- Lu, F.; Liu, D. H.; Ye, X. Q.; Wei, Y. X.; Liu, F. Alginate-Calcium Coating Incorporating Nisin and Edta Maintains the Quality of Fresh Northern Snakehead (Channa Argus) Fillets Stored at 4°C. J. Sci. Food Agric. 2010, 89(5), 848–854. DOI: 10.1002/jsfa.3523.
- Ocaño-Higuera, V. M.; Maeda-Martínez, A. N.; Lugo-Sánchez, M. E.; Pacheco-Aguilar, R. Postmortem Biochemical and Textural Changes in the Adductor Muscle of Catarina Scallop Stored at 0°C. J. Food Biochem. 2006, 30(4), 373–389. DOI: 10.1111/j.1745-4514.2006.00071.x.
- Wongso, S.; Yamanaka, H. Extractive Components of the Adductor Muscle of Japanese Baking Scallop and Changes during Refrigerated Storage. J. Food Sci. 2010, 63(5), 772–776. DOI: 10.1111/j.1365-2621.1998.tb17897.x.
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and Preservation Performance of Active Polyethylene Films Containing Rosemary and Cinnamon Essential Oils for Pacific White Shrimp Packaging. Food Control. 2018, 92, 37–46. DOI: 10.1016/j.foodcont.2018.04.052.
- Shi, C.; Cui, J.; Luo, Y.; Zhou, Z. Effect of Lightly Salt and Sucrose on Rigor Mortis Changes in Silver Carp (Hypophthalmichthys Molitrix) Stored at 4℃. Int. J. Food Sci. Technol. 2014, 49, 160–167. DOI: 10.1111/ijfs.12291.
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements between Various Amino Acids and Imp. Chem. Senses. 2002, 27(8), 739–745. DOI: 10.1093/chemse/27.8.739.
- Pacheco-Aguilar, R.; Marquez-Ríos, E.; Lugo-Sánchez, M. E.; García-Sanchez, G.; Maeda-Martínez, A. N.; Ocaño-Higuera, V. M. Postmortem Changes in the Adductor Muscle of Pacific Lions-Paw Scallop (Nodipecten Subnodosus) during Ice Storage. Food Chem. 2008, 106(1), 253–259. DOI: 10.1016/j.foodchem.2007.05.079.
- Alasalvar, C.; Taylor, K. D. A.; Öksüz, A.; Garthwaite, T.; Alexis, M. N.; Grigorakis, K. Freshness Assessment of Cultured Sea Bream (Sparus Aurata) by Chemical, Physical and Sensory Methods. Food Chem. 2001, 72(1), 33–40. DOI: 10.1016/S0308-8146(00)00196-5.
- Fatih., Ö.; Yesim., Ö.; Esmeray, K. Nucleotide Degradation and Biogenic Amine Formation of Wild White Grouper (Epinephelus Aeneus) Stored in Ice and at Chill Temperature (4°C). Food Chem. 2008, 108(3), 933–941. DOI: 10.1016/j.foodchem.2007.11.070.
- Jht, L.; Male, K. B.; Huynh, M. D. Applications of Polargraphy for Assessment of Fish Freshness. J. Food Sci. 2010, 56(2), 335–337.
- Han, L.; Luo, Y.; Zhou, Z. The Quality Changes of Songpu Mirror Carp (Cyprinus Carpio) during Partial Freezing and Chilled Storage. J. Food Process. Preserv. 2014, 38(3), 948–954. DOI: 10.1111/jfpp.12049.
- Okpala, C. O. R.; Choo, W. S.; Dykes, G. A. Quality and Shelf Life Assessment of Pacific White Shrimp (Litopenaeus Vannamei) Freshly Harvested and Stored on Ice. LWT - Food Sci. Technol. 2014, 55(1), 110–116. DOI: 10.1016/j.lwt.2013.07.020.
- Riaz, M.; Qadri, R. B. Time-Temperature Tolerance of Frozen Shrimps. 1. High Quality Life and Acceptability Time of Frozen Glazed Shrimps. Trop. Sci. 1987, 27(3), 167–173.
- Kaale, D. L.; Eikevik, M. T.; Rustad, T Ice Crystal Development in Pre-Rigor Atlantic Salmon Fillets during Super Chilling Process and following Storage. Food Control. 2013, 31(2), 491–498. DOI: 10.1016/j.foodcont.2012.11.047.
- Ahmed, S.; Akand, N. R.; Islam, M. T.; Arafat-Al -Mamun,; Bari, M. L.; -Mamun, -A.-A. Effectiveness of Scallop Powder Ice in Reducing Bacterial Load on Fresh Whole Fish and in the Melted Ice Water. LWT - Food Sci. Technol. 2015, 64(1), 270–274. DOI: 10.1016/j.lwt.2015.06.002.
- Bahuaud, D.; Mørkøre, T.; Østbye, T. K.; Veiseth-Kent, E.; Thomassen, M. S.; Ofstad, R. Muscle Structure Responses and Lysosomal Cathepsins B and L in Farmed Atlantic Salmon (Salmo Salar, L.) Pre-, and Post-Rigor, Fillets Exposed to Short and Long-Term Crowding Stress. Food Chem. 2010, 118(3), 602–615. DOI: 10.1016/j.foodchem.2009.05.028.
- Mehta, N. K.; Chouksey, M. K.; Balange, A. K.; Tripathi, G.; Nayak, B. B. Physicochemical and Gel Properties of Myofibrillar Protein from Sin Croaker (Johnius Dussumieri) Fish during Ice Storage. J. Aquat. Food Prod. Technol. 2016, 26(1), 71–85. DOI: 10.1080/10498850.2015.1092485.
- Hu, Y.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Effect of Iced-Storage on the Activity of Cathepsin L and Trypsin-Like Protease in Carp Dorsal Muscle. LWT - Food Sci. Technol. 2015, 60(2), 1249–1253. DOI: 10.1016/j.lwt.2014.09.004.
- Duun, A. S.; Hemmingsen, A. K. T.; Haugland, A.; Rustad, T. Quality Changes during Superchilled Storage of Pork Roast. LWT - Food Sci. Technol. 2008, 41(10), 2136–2143. DOI: 10.1016/j.lwt.2008.02.001.
- Xu, Y.; Ge, L.; Jiang, X.; Xia, W.; Jiang, Q. Inhibitory Effect of Aqueous Extract of Allium, Species on Endogenous Cathepsin Activities and Textural Deterioration of Ice-Stored Grass Carp Fillets. Food Bioprocess Technol. 2015, 8(10), 2171–2175. DOI: 10.1007/s11947-015-1564-2.