?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The production of chitooligosaccharide (COS) from chitosan (molecular weight [MW] at 362 kDa and degree of deacetylation [%DD] at 100) using commercial enzymatic hydrolysis (commercial pectinase, Pectinex® Ultra SP-L) was investigated. The commercial pectinase hydrolyzed (COS) chitosan was produced by optimal condition (i.e., pH of sodium acetate at 4.0, enzyme:substrate ratio at 1:4, and incubation time at 180 min). This condition yielded COS with highest yield (98.0%). COS possessed %DD at 100 and MW at 99.77 kDa. The result in this study implied that COS can be easily dissolved in dilute organic acid and water. COS was applied to improve the quality of reduced fat-low sugar Chinese pork sausage. The shelf life of sausage with 0.5% and 1.0% w/w COS was extended from 7 days (control) to 14 days, when packed in plastic bags (Poly Ethylene, PE) at room temperature. Moreover, the inside color, skin color, gumminess, chewiness and proximate compositions of the sausage did not affect by COS (p > .05). The produced sausage was accepted by consumers.
Introduction
Chitosan is the deacetylated form of chitin, the second most abundant biopolymer after cellulose.[Citation1] It can isolate from shells of crustacean such as crab, shrimp, and crawfish. In Thailand, there are abundant of shrimp shells as the waste from frozen shrimp industry. Hence, shrimp shells can be used as raw material for chitosan production. The value of shrimp shell is also increased.[Citation2] The report in 1995 described that chitosan was a linear copolymer composed of approximately 5–25% N-acetyl-d-glucosamine and 75–95% d-glucosamine units which connected through (1,4)-linked β-glycosidic linkages.[Citation2] The molecular weight (MW) of chitosan varied widely and could be as high as 106 Da. Low MW chitosan was received by some degraded treatment using enzyme, alkali, or acid and was called chitooligosaccharide or COS. Chitosan had the reactive hydroxyl and high primary amine groups in its structure and usually had less crystalline than chitin. This made chitosan more accessible to reagent. Chitosan was insoluble in neutral or alkaline aqueous solutions while it was dissolved in some proper acidic/water solutions such as formic acid, acetic acid, and lactic acid.[Citation2] Moreover, chitosan is now widely produced commercially from crab and shrimp shell wastes with different deacetylation grades and MWs and hence, different functional properties.[Citation3] Chitosan has various prominent functional properties and has been interested for its applications in the biomedical, food, and chemical industries.[Citation4] COS can corporate into food product as functional ingredients, antioxidant and antimicrobial agent in meat,[Citation5] edible film,[Citation6] antioxidant in meat,[Citation7] antimicrobial agent in sausage,[Citation8] and Chinese-style sausages,[Citation9] stabilizers in meat product,[Citation10] and preservative in pork model burgers.[Citation11] COS from cicada slough was also reported and used as antibacterial agent against Bacillus subtilis, Staphylococcus aureus, and Escherichia coli at 100 mg/ml.[Citation12] Moreover, chitosan can absorb in the human intestine and provide the health benefits.[Citation13]
Chitosan has the limiting application in food and biomedicine since it is water insoluble. Unlike chitosan, its hydrolyzed products (low MW chitosan or chitosan oligosaccharides or COS) are readily dissolved in water due to their short chain lengths and free amino groups in d-glucosamine units.[Citation13]
COS can produce with either chemical or enzymatic hydrolysis. However, chemical hydrolysis has some drawbacks, due to the development of toxic compounds and environmental pollution, and low production yields.[Citation14] For enzymatic hydrolysis, the low MW chitosan was effectively obtained from complex enzyme.[Citation15] The incubation time, temperature, pH, and enzyme ratio affected MW of the produced chitosan. Due to the expensive cost of the purified enzyme, this research was aimed to produce COS from commercial pectinase enzyme (Pectinex® Ultra SP-L). The quality characteristics of the obtained COS, including MW, yield (%), moisture content, water activity, degree of deacetylation (%DD), and antimicrobial activity using disc diffusion test, were determined. COS with highest yield and prominent internal properties was added in reduced fat-low sugar Chinese pork sausage. The changes of physical, chemical, microbial, and sensory properties of the produced Chinese pork sausage were investigated.
Materials and methods
Materials
Shrimp shells were obtained from Ta Ming Enterprise Co. Ltd., (Samut Sakhon, Thailand). Commercial pectinase or polygalacturonase named Pectinex® Ultra SP-L (9500 PGU/ml) (Novozyme) was purchased from Brenntag Ingredients Thailand Co. Ltd. (Bangkok, Thailand). All chemical reagents were analytical reagent grade, except sodium hydroxide pallet (99.5%) for chitosan preparation (commercial grade).
Production of chitosan from shrimp shells and preparation of COS
Chitosan was prepared from dried and cleaned shrimp shells by the method described by Refs. [Citation16,Citation17]. The method consisted of four steps including deproteinization (soaking in 1.5 M sodium hydroxide solution for 12 h two times), demineralizaton (soaking in 1.5 M hydrochloric solution for 12 h two times), decoloration (soaking in 90% ethanol at 50°C for 1 h), and deacetylation (soaking in 50% w/w sodium hydroxide solution at room temperature for 72 h and 90°C for 48 h). Various properties of chitosan were determined. Water activity was assessed using AquaLab CX3 TE (Decagon Co., USA). The measurement was performed for five replicates. The MW was measured using gel permeation chromatography (Waters 600E, USA) and the method was modified from Ref. [Citation18]. Degree of deacetylation (%DD) was evaluated using nuclear magnetic resonance (Bruker Biospin DPX-300, USA).[Citation19] The electron micrograph was collected using scanning electron microscope (JEOL JSM-6400, Japan). The functional groups of chitin and chitosan were detected using Fourier-transformed infrared (FT-IR) spectrometer (Perkin Elmer Spectrum 400, USA). The method used in this measurement was modified from Ref. [Citation20]. The production yield of chitosan was also calculated using the following equation:
COS was produced from enzymatic hydrolysis of chitosan under various conditions.[Citation21] The commercial enzyme (Pectinex® Ultra SP-L) was selected to be used in this study. The hydrolysis conditions were varied as follows: pH of sodium acetate buffer at 4.0, 4.5, and 5.0 and enzyme to substrate ratio at 1:1, 1:2, and 1:4. One gram of chitosan flake was dissolved in 100 ml of buffer at the specified pH. The solution was incubated in thermostatic shaking water bath until the temperature reached to 50°C. The enzyme was added into the flask at the abovementioned ratio.
Aliquots of reaction mixture were periodically withdrawn at 60, 120, and 180 min of incubation, heated at 95°C, 15 min to terminate the enzyme reaction. Hence, the solution of hydrolyzed chitosan was achieved. In this experiment, a 3 × 3 factorial design in Completely Randomized Design (CRD) was used. The experiment was carried out in duplicate. Statistical analysis was performed by using ANOVA and general linear model in SPSS version 12.0 (SPSS, 2002). Duncan’s new multiple range test (p < .05) was used to detect the differences among treatment means.
Recovery of COS from reaction mixture
Aliquots of hydrolyzate were neutralized with 10 M NaOH, filtered through Whatman filter paper NO.93 and rinsed with deionized water until the pH of hydrolyzate reach to 7. The precipitate was then dried at 55°C for 3 h and crushed with Waring blender (Waring, USA) at 11,000 rpm. The obtained COS powder was analyzed for water activity, MW, %DD, electron micrograph and functional groups using the method described. The antimicrobial activity of the produced COS was determined using disc diffusion test[Citation22] (Kirby–Bauer method) and expressed in term of clear zone or zone of inhibition (mm). The detection was performed in five replicates for each sample. The production yield of chitosan was calculated using the following equation:
Application of COS into reduced fat-low sugar Chinese pork sausage
COS with the highest yield and prominent internal properties was selected to use as natural antimicrobial agent in reduced fat-low sugar Chinese pork sausage. Sausage was produced using the method described by Ref. [Citation23]. From preliminary study, the basic formulation and appropriate production method of Chinese pork sausage was obtained. The fat was reduced from 29.65% to 20.765% and sugar was reduced from 15.10% to 9.06% by adding 0.01% sucralose (). COS was mixed homogeneously with pork batter at 0.0%, 0.5%, and 1.0% w/w. The batter was stuffed into 2.5 cm diameter collagen casing and dried at 60°C for 24 h. The physical, chemical, biological, and sensory characteristics of the Chinese pork sausage were analyzed in order to clarify the benefit of COS. Water activity was determined with AquaLab CX3 TE (Decagon Co., USA.) for five replicates. The color was measured using the method of Ref. [Citation24] (five replicates per sample) and expressed in term of CIE L*a*b* (Spectrophotometer, CM3500d, Japan). The texture profile analysis (TPA) was assessed with Lloyd Instrument, TA500, (England). The sample was cut into 2.0 cm height and measured by the following conditions: determined test speed 10 mm/s by diameter of cylinder is 30 mm, pressed into samples at 75% of its height for two times.[Citation25] The TPA measurement was performed in two times and five replicates. The proximate compositions of all samples were performed according to the method of AOAC.[Citation26] The microbial load of each sample was evaluated in term of total plate count and total yeast and mold, using the method of BAM.[Citation27] The sensory evaluation was performed using 9-point hedonic scoring test.[Citation28] The experimental design in this experiment was CRD with three treatments. The experiment was carried out in duplicate. Statistical analysis was performed with ANOVA in SPSS version 12.0 (SPSS, 2002). Duncan’s new multiple range test (p < .05) was used to detect the differences among treatment means.
Table 1. Ingredients of reduced fat-low sugar Chinese pork sausage with COS compared with control
Results and discussions
Properties of the produced chitosan
Internal properties of the produced chitosan
The obtained chitosan possessed MW at 362 kDa and DD at 100% (). The production yield, moisture content, and water activity of chitosan were 76.5%, 13.07% and 0.521%, respectively (). The process with deproteinization before demineralization yielded chitosan with desirable MW and %DD. According to previous work of Ref. [Citation29], they reported that the native chitosan should have the MW in between 1000–1500 kDa, which depended on preparation method. Hence, chitosan in this study was proposed as low MW chitosan. The obtained chitosan possessed 100%DD, which suggested that the acetyl group of chitin was totally eliminated. The acetyl group of chitin were completely substituted with amino group (–NH2 group).[Citation17,Citation30] Hence, the obtained chitosan was easily dissolved in dilute organic acid[Citation4] and used for producing COS in the next experiment.
Table 2. Properties of the obtained chitosan
Scanning electron micrograph of the produced chitosan
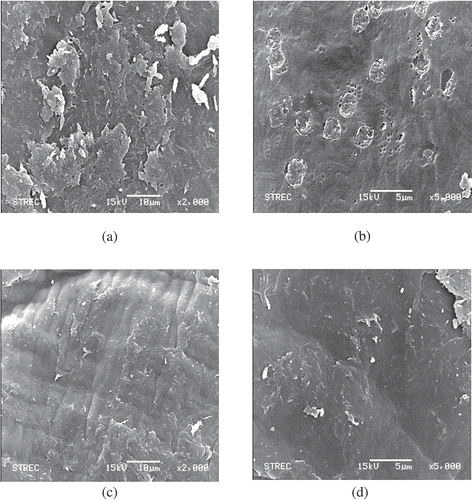
According to scanning electron micrograph shown in , the surface of chitosan was modified from that of the chitin. At 2000 times of magnification, the surface of chitin was rough, while the surface of chitosan was smooth ( and ). When the image was magnified to 5000 times, the chitin surface exhibited the rough surface with small pores ( and ). In contrast, the surface of chitosan was smooth with small and uniform pores ( and ). This electron micrograph exhibited the normal surface of chitosan from crustaceans.[Citation31] According to the previous work of Ref. [Citation32], chitosan structure was modified from that of the chitin. It had nonhomogenous and smooth surface with steeps and shrinkage. These results indicated that there was the modification of chitosan structure from chitin after deacetylation with 50% w/w NaOH solution.
FI-IR spectra of the produced chitosan
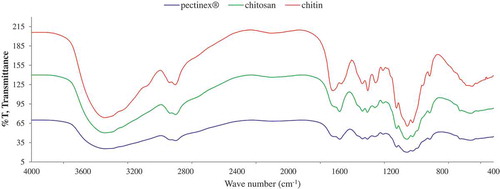
The FT-IR spectrum of chitosan was slightly different from that of the chitin (). The difference of wavenumbers was occurred at 3200–400 cm−1 that indicated the changes of chitosan structure and functional group from chitin. The peak at 3434.35 cm−1, which exhibited –NH2 and stretching aliphatic –OH group, was occurred. This result agreed with the research of Ref. [Citation15]. Chitosan had specific absorption peaks, such as O–H stretching vibration at 3480 cm−1. From deacetylation process, the acetyl group was eliminated from chitin. Moreover, %T of chitosan at 2877.88 cm−1 was lower than that of the chitin, indicating the reduction of acetyl group in chitosan chain.
Properties of the produced COS
Internal properties of the produced COS
COS was successfully produced with commercial Pectinex® hydrolysis. The factors studied in this experiment (pH and enzyme to chitosan ratio) directly affected properties of COS ( and ). The water activity of all treatments was varied in the range of 0.565–0.572, which was not significantly different from each other (p > .05) (). COS had water activity below 0.65, which was lower than the water activity required for microbial growth. Hence, the produced COS can be kept in dried-sealed plastic package at room temperature prior to use in the next experiment.
Table 3. The MW of the obtained COS at 60, 120, and 180 incubation time
Table 4. The water activity and yield (%) of the obtained COS
Table 5. The clear zone diameter (mm) of all COS samples after incubation for 180 min, obtained from disc diffusion test
The MW of the produced COS gradually reduced when increasing the incubation time (). The 50% MW reduction was found after 180 min of incubation. This result indicated that Pectinex® Ultra SP-L hydrolysis was the time-dependent reaction. The MW of COS gradually decreased along the incubation time. The rate of MW reduction was lowest in treatment with enzyme:substrate ratio at 1:4. These results suggested that the increase of substrate at the constant concentration of enzyme reduced the hydrolysis rate. The report in 2011[Citation15] reported that chitosan degradation could be appropriately divided into two phases. First, interior contact played the cardinal role during the early period of hydrolysis, enzymes hydrolyzed the β-(1,4) glycosidic bonds within chitosan molecular, chitosan solution viscosity decreased rapidly, MW lowered from 1000,000 to100,000, and the reaction did not conform to Michaelis–Menten kinetic equations. Due to the specificity of enzymes, the degradation of β-(1,4) glycosidic bonds within chitosan molecular began to decrease after some periods of reaction. Second, pectinase undertook selective cross-degradation on chitosan fragments obtained from degradation by different enzymes, and the MW of chitosan lowered from 100,000 to 10,000. Reaction rate during this process was quite slow compared to the former phase. When oligomeric chitosan concentration reached a certain level, they began to inhibit enzyme activities and limit degradation reaction, and at this time, enzymatic degradation terminated.[Citation15] According to the result in , the optimal enzyme:substrate ratio at pH 4 was 1:1 with COS with lowest MW (72.46 kDa) was obtained after 180 min incubation () as shown in COS with high yield (92.95a). The lowest production yield (68.00b) was found when hydrolyzed at pH 4.5 at the ratio 1:1. The similar result was found in treatments with pH 4.5 and 5. The lowest MW COS was found in treatments with enzyme:substrate ratio at 1:1 (39.86 and 58.02 kDa for pH 4.5 and 5, respectively). The highest hydrolysis rate was shown after 60 min of incubation with enzyme:substrate ratio at 1:1. The MW sharply reduced from 362 to 94.04 kDa in treatments with pH 4.5 and 117.19 kDa in treatments with pH 5.
Furthermore, the MW was continually declined from 60 to 180 min of incubation time. The lowest MW of COS was found at 39.86 and 58.02 kDa for pH 4.5 and 5, respectively. Hence, the change of enzyme to substrate ratio from 1:1 to 1:2 and 1:4 resulted in lower hydrolysis reaction and the COS with higher MW was obtained. When considering all factors studied (pH, enzyme:substrate ratio, and incubation time), it can be suggested that the pH and enzyme:substrate ratio were the prominent parameters that control hydrolysis reaction of Pectinex®. Pectinex® could hydrolyze actively at pH 4.5 with the incubation time at 180 min. The COS with lowest MW (39.86 kDa) was received. When pH value was lower than 4.5, enzyme activity was reduced. When pH value was higher than 5.5, enzyme activity was also decreased. This phenomenon could be mainly described by enzyme property. Enzyme was an amphoteric electrolyte protein. The pH value could affect not only the stability of enzyme molecular structure but also the relevant group dissociation, chitosan dissolution, and ionization in its activity center.[Citation15]
Antimicrobial activity of the produced COS
The diameter of clear zone was resulted from disc diffusion test. The results revealed that antimicrobial activity of chitosan and COS directly depended on their MW (). Both chitosan and COS possessed the antimicrobial activity against Gram positive (S. aureus and Steptococcus faecalis) and Gram negative (E. coli and Salmonella typhimurium). The COS with MW at 191.05 kDa (pH 4.5 and enzyme:substrate ratio at 1:4) effectively inhibited E. coli (p < .05) (). The highest diameter of zone of inhibition (10.5a mm) was occurred (p < .05) (). COS with MW at 99.77 and 95.37 could inhibit Gram positive effectively. COS with 87.53 kDa (treatment with pH 4.5 and enzyme:substrate ratio at 1:2) inhibited Gram negative bacteria as shown in the wide clear zone diameter (). At the MW of COS equal to 58.02 kDa (treatment with pH 5.0 and enzyme:substrate ratio at 1:1), the highest inhibition of Gram positive bacteria (S. aureus and S. faecalis) was achieved. The largest clear zone was resulted with diameter at 10.75a mm for S. aureus and 9.30a mm for S. faecalis (p < .05), respectively. Moreover, the COS with lowest MW (39.86 kDa) exhibited the highest inhibition against S. typhimurium (P < .05) which resulted in the largest clear zone with diameter of 10.50a.
Among various COS samples produced in this study, the treatment with highest yield (98.00%) was selected due to the feasibility to transfer the technology to pilot plant and industrial scale. This sample had MW at 99.77 kDa and could produce successfully with Pextinex® hydrolysis in buffer pH 4.0, enzyme:substrate ratio at 1:4 and incubation time at 180 min. The remained study, including the study of SEM and FT-IR and the application into reduced fat-low sugar Chinese pork sausage, was performed using the selected COS sample.
Scanning electron micrograph of the selected COS
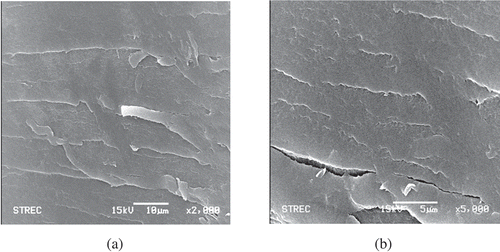
The structure of the selected COS ( and ) was totally changed from that of the chitin and chitosan (). At 2000 times of magnification, the surface structure of COS was not smooth as chitosan. The stretching mark and crack were found (). The porous texture of chitosan was disappeared, indicating the structure modification of Pectinex® hydrolysis. This phenomenon might occur during the preparation of COS. Chitosan was dissolved in 1% acetic acid solution prior to hydrolyze with Pectinex®, and then precipitated using 10 N NaOH before rinsing and drying. This process changed the porous chitosan structure into loose and crack structure of COS ( and ). As a result, the COS was easily dissolved in diluted organic acid and also dispersed homogeneously in water.
FT-IR spectra of the produced COS
The FT-IR spectra of the selected COS were not different from the spectra of chitosan (). The wavenumber at 3432–3427 cm−1 (N–H stretching), 2877 cm−1 (C–H stretching), 2119–2123 cm−1 (CO stretching), 1598–1600 cm−1 (C = O conjugated with phenyl group), 1383 cm−1 (N–O stretching), 1115 cm−1 (C–O stretching), 1030–1031 cm−1 (C–O stretching with R–OH), 898–897 cm−1 (C–H bend disubstituted 1,1), and 577–581 cm−1 (amide VI group) were not different from that of the chitosan, while the slight different at wavenumber 2919 cm−1 (CH3–CH2 group) was resulted. This result suggested that Pectinex® hydrolysis did not affect functional group and internal structure of chitosan. The hydrolysis reaction only reduced the chain length of chitosan. This result exhibited the effective hydrolysis of commercial pectinase, which can be utilized for producing COS with high yield.
Effect of COS on quality characteristics of reduced fat-low sugar Chinese pork sausage
Physical properties of reduced fat-low sugar Chinese pork sausage
The addition of COS in Chinese pork sausage affected increased water activity from 0.846b to 0.893a and springiness from 6.70b to 7.62a and 7.40a (P < .05), while the hardness and cohesiveness were decreased (p < .05) (). The gumminess, chewiness, and color (inside and peel color) of the produced Chinese pork sausage with COS were not significantly different from that of the control (). These results indicated that COS could modify texture of Chinese pork sausage from hard and cohesive texture to soft and springy texture. This phenomenon might occur because of water binding property of COS.[Citation33] After the hydrolysis reaction, the increase of hydroxyl group along the COS chain was occurred and the increase of reactive group that able to bind with water inside the product was resulted. Hence, the water activity () and moisture content () of Chinese pork sausage were increased (p < .05). Moreover, the soft texture of Chinese pork sausage with COS was resulted.
Table 6. The water activity, texture profiles, and color of reduced fat-low sugar Chinese pork sausage with COS and without COS (control)
Table 7. Proximate compositions of reduced fat-low sugar Chinese pork sausage with COS and without COS (control)
Proximate compositions of the reduced fat-low sugar Chinese pork sausage
Protein, ash, and moisture contents of the developed Chinese pork sausage were not significantly different from that of control (p > .05) (). However, the significant effect on fat reduction was found (p < .05) in treatment with COS, which indicated the fat binding property of COS. According to the work of Ref. [Citation34] in 1983, chitosan also demonstrated lipid-binding and water-binding capacities. The energy from fat of treatment with 0.5% COS (255.69 kcal) and 1.0% COS (252.45 kcal) was 25% lower than the energy from fat of basic formulation. The developed Chinese pork sausage had lower energy from normal Chinese pork sausage.
Microbial property of the reduced fat-low sugar Chinese pork sausage
When adding COS into reduced fat-low sugar Chinese pork sausage, the water activity and moisture content of the product were increased. This phenomenon encouraged the microbial growth after storage in PE bag at room temperature for 14 days. The concentration of COS did not affect bacterial count of reduced fat-low sugar Chinese pork sausage (p > .05) (). After storage at room temperature for 14 days, the control sausage had total yeast and mold at 1.25 × 102 CFU/g (), which indicated the spoilage of product. The total yeast and mold count was over the standard of Chinese pork sausage,[Citation35] which controlled at less than 100 CFU/g. In the other hand, the total yeast and mold count was not detected in treatment with COS during storage for 14 days (). The standard was also specified that the total bacterial count of Chinese pork sausage must lower than 1 × 105 CFU /g. At 21 days of storage, the total bacterial counts of Chinese pork sausage without COS (control) was 6.95 × 104, while the sausage, with 0.5% COS and 1.0% COS possessed the table at 5.34 × 104 and 5.00 × 104, respectively. Hence, Chinese pork sausage with and without COS had total bacterial count less than the standard. This result implied that COS had the ability to retard the yeast/mold growth better than bacterial growth and can be used to extend the shelf life of product packed in PE bags from 7 to 14 days at room temperature ().
Table 8. Microbial population changes (CFU/g) (total plate count and total yeast and molds counts) of reduced fat-low sugar Chinese pork sausage applied COS in packaging during storage at room temperature for 0–28 days
Conclusion
COS was effectively produced with commercial enzymatic hydrolysis with Pectinex® Ultra SP-L. The obtained COS possessed desirable properties and production yield. The optimal conditions for producing COS with highest yield (98.0%) and low MW (99.77 kDa) was pH of sodium acetate at 4.0, enzyme:substrate ratio at 1:4 and incubation time at 180 min. The obtained COS possessed the similar functional group to chitosan. When COS was homogeneously mixed into reduced fat-low sugar Chinese pork sausage, the inside color, skin color, gumminess, chewiness, and proximate compositions (percentage of protein, fat, ash, and moisture content) were not significantly different from that of the control (p > .05). COS had the ability to retard the yeast/mold growth better than bacterial growth and can be used to extend the shelf life of product packed in PE bags from 7 to 14 days at room temperature.
Additional information
Funding
References
- Amaral, I. F.; Granfa, P. L.; Barbosa, M. A. Chemical Modification of Chitosan by Phosphorylation an XPS, FT-IR and SEM Study. J. Bio. Sci. Polym. 2005, 16(12), 1575–1593. DOI: 10.1163/156856205774576736.
- Winterowd, J. G.; Stanford, P. A. Chitin and Chitosan. In Food Polysaccharides, first ed.; Stephen, A. M., Ed.; Marcek Dekker: New York, 1995; pp 441–462.
- No, H. K.; Meyers, S. P. Preparation and Characterization of Chitin and Chitosan-a Review. J. Aquat. Food Prod. Technol. 1995, 4(2), 27–52. DOI: 10.1300/J030v04n02_03.
- No, H. K.; Mayers, S. P.; Prinyawiwatkul, W.; Xu, Z. Applications of Chitosan for Improvement of Quality and Shelf Life of Foods: A Review. J. Food Sci. 2007, 72, 87–100. DOI: 10.1111/j.1750-3841.2007.00383.x.
- Georgantelis, D. I.; Ambrosiadis, P.; Katikou, G.; Blekas,; Georgakis, S. A. Effect of Rosemary Extract, Chitosan and α-tocopherol on Microbiological Parameters and Lipid Oxidation of Fresh Pork Sausages Stored at 4 ºC. Meat Sci. 2007, 76, 172–181. DOI: 10.1016/j.meatsci.2006.10.026.
- Beverly, R. L.; Janes, M. E.; Prinyawiwatkula, W.; No, H. K. Edible Chitosan Films on Ready-to-eat Roast Beef for the Control of Listeria Monocytogenes. Food Microbiol. 2008, 25, 534–537. DOI: 10.1016/j.fm.2007.11.002.
- Rao, M. S.; Chander, R.; Sharma, A. Synergistic Effect of Chitooligosaccharides and Lysozyme for Meat Preservation. LWT Food Sci. Technol. 2008, 41, 1995–2991. DOI: 10.1016/j.lwt.2008.01.013.
- Jo, C.; Lee, J. W.; Lee, K. H.; Byun, M. W. Quality Properties of Pork Sausage Prepared with Water-soluble Chitosan Oligomer. Meat Sci. 2001, 59, 369–375. DOI: 10.1016/S0309-1740(01)00089-4.
- Lin, K. W.; Chao, J. Y. Quality Characteristics of Reduced-fat Chinese-style Sausage as Related to Chitosan’s Molecular Weight. Meat Sci. 2001, 59, 343–351. DOI: 10.1016/S0309-1740(01)00084-5.
- Benjakul, S.; Visessanguan, W.; Tanaka, M.; Ishizaki, S.; Suthidham, R.; Sungpech, O. Effect of Chitin and Chitosan on Gelling of Surimi from Barred Gafish (Hemiramphus Far). J. Sci. Food Agric.. 2000, 81, 102–108. Doi:10.1002/1097-0010(20010101)81:1<102::AID-JSFA792>3.0.CO;2-O.
- Sayas-Barbera, E.; Quesada, J.; Sancez-Zapata,; Viuda-Matos, M.; Fernandez-Lopez, F. Effect of the Molecular Weight and Concentration of Chitosan in Pork Model Burgers. Meat Sci. 2011, 88, 740–749. DOI: 10.1016/j.meatsci.2011.03.007.
- Wu, S. J.; Pan, S. K.; Wang, H. B.; Wu, J. H. Preparation of Chitooligosaccharides from Cicada Slough and Their Antibacterial Activity. Int. J. Biol. Macromol. 2013, 62, 348–351. DOI: 10.1016/j.ijbiomac.2013.09.042.
- Jeon, Y. J.; Shahidi, F.; Kim, S. K. Preparation of Chitin and Chitosan Oligomers and Their Applications in Physiological Functional Foods. Food Rev. Int. 2000, 61, 159–176. DOI: 10.1081/FRI-100100286.
- Kim, S. K.; Rajapakse, N. Enzyme Product and Boligical Activities of Chitosan Oligosaccharides (COS): A Review. Carbohydr. Polym. 2005, 65, 357–368. DOI: 10.1016/j.carbpol.2005.08.012.
- Jia, X. H.; Huang, Z. J.; Zhang, C. Preparation of Low Molecular Weight Chitosan by Complex Enzymes Hydrolysis. Int. J. Chem. 2011, 3(2), 180–186.
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the Preparation of Chitins and Chitosan with Controlled Physic-chemical Properties. Polymer. 2003, 44(26), 7939–7952. DOI: 10.1016/j.polymer.2003.10.025.
- Kachanechai, T.; Jantawat, P.; Pichyangkura, R. The Influence of Chitosan on Physico-chemical Properties of Chicken Salt-soluble Protein Gel. Food hydrocolloids. 2008, 22, 74–83. Doi:10.1016/j.foodhyd.2007.04.010.
- Terbojevich, M.; Cosani, A. Molecular Weight Determination of Chitin and Chitosan. In Chitin Handbook; Riccardo A. A. Muzzarelli and Martin G. Peter, Ed.; Atec Edizioni: Grottammare AP, Italy, 1997; pp 87–101.
- Muzzarelli, R. A. A.; Rocchett, I. R.; Stanic, V.; Weckx, M. Methods for the Determination of the Degree of Acetylation of Chitin and Chitosan. In Chitin Handbook; Riccardo A. A. Muzzarelli and Martin G. Peter, Ed.; Atec Edizioni: Grottammare AP, Italy, 1997; pp 109–119.
- Pawlak, A.; Mucha, M. Thermogravimetic and FTIR Studies of Chitosan Blends. Thermochim. Acta. 2003, 396, 153–166. DOI: 10.1016/S0040-6031(02)00523-3.
- Trongchittum, I. Glucosamine Extraction from Silk Worm Molt. M.S. Thesis, Department of Chemistry, Faculty of Science, King Mongkut’s University of Technology North Bangkok, Thailand, 2005
- No, H. K.;. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. DOI: 10.1016/S0168-1605(01)00717-6.
- Phunchaisri, C.; Siripin, U.; Daendprok, W. Production of Low Fat Chinese Sausage from Konjac. Research Report. Department of food science, Faculty of engineering and agro-industry, Mae-Jo University, Chiangmai, Thailand, 2004.
- Songsiri, P. Use of Kappa Carrageenan, Soduim Alginate and Xanthan Gum in Low- Fat Chinese Sausage. Thesis, Department of Food Science and Technology. Faculty of Agro-Industry, Kasetsart University, Thailand, 2000.
- Warodomwichit, D. Reduction of Fat in Frankfurter Sausage Using Carbohydrated- Based Fat Replacers. Thesis, Department of product development, Faculty of Agro- Industry, Kasetsart University, Thailand, 2001
- AOAC. Official Methods of Analysis, 17 th ed.; The Association of Official Analytical Chemists: Maryland, U.S.A, 2000.
- Bacteriological Analytical Manual (BAM). Food and Drug Administration Bacteriological Analytical Manual, 8th; AOAC International, USA, 2002. https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam
- Chompreeda, P.;. Sensory Evaluation and Consumer Acceptance; Department of Product Development, Faculty of Agro-Industry, Kasetsart University: Bangkok, Thailand, 2007.
- Rhoadas, J.; Roller, R. Antimicrobial Action of Degraded and Native Chitosan against Spoilage Organisms in Laboratiory Media and Food. Appl. Environ. Microbiol. 2000, 66, 80–86. DOI: 10.1128/AEM.66.1.80-86.2000.
- Aye, K. N.; Karuppuswamy, R.; Ahamed, T.; Stevens, W. F. Peripheral Enzymatic Deacetylation of Chitin and Reprecipitated Chitin Particles. Bioresour. Technol. 2006, 97, 577–582. DOI: 10.1016/j.biortech.2005.03.030.
- Paulino, A. T.; Simionato, J. I.; Garcia, J. C.; Nozaki, J. Characterization of Chitosan and Chitin Produced from Silkworm Chrysalides. Carbohydr. Polym. 2005, 64, 98–103. DOI: 10.1016/j.carbpol.2005.10.032.
- Islam, M. M.; Masum, S. M.; Rahman, M. M.; Molla, M. A.; Shaikh, A. A.; Roy, S. K. Preparation of Chitosan from Shrimp Shell and Investigation of Its Properties. Int. Res. J. Appl. Basic Sci. 2011, 11(1), 116–130.
- Knorr, D.;. Functional Properties of Chitin and Chitosan. J. Food Sci. 1982, 47, 593–595. DOI: 10.1111/jfds.1982.47.issue-2.
- Knorr, D.;. Dye Binding Properties of Chitin and Chitosan. J. Food Sci. 1983, 48, 36–41. DOI: 10.1111/jfds.1983.48.issue-1.
- Thai Industrial Standard for Chinese Pork Sausage. TIS. 2003, 103–2546