?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The aim of this study was to investigate the effect of black plum peel puree (40-60%) and pectin (0%, 0.25%, and 0.5%) concentrations on the chemical compositions, sensory attributes, rheological properties, antioxidant activity, and total phenolic compounds of black plum peel sharbat. Increasing the pectin concentration decreased the acidity. The results of sensory properties showed that increasing the pectin concentration reduced color, consistency, water-solubility, and sweetness. The increase of black plum puree led to a rise in aroma score and decreased water-solubility. The sample with 50% black plum peel puree without pectin had the highest acceptability. Black plum peel sharbat behaved as a pseudo-plastic fluid. The power-law model was found to be the most appropriate to fit the flow curves of black plum peel sharbat. Increasing the pectin level increased the consistency coefficient. Flow behavior index, consistency coefficient, and apparent viscosity of black plum peel sharbat were in the range of 0.41–0.72, 1.16–41.78 (Pa.sn) and 11.09–72.78 (Pa.s), respectively. Antioxidant activity and phenolic compounds of black plum peel sharbat increased with increasing the puree concentration. PLS analysis showed that the results of sensory and rheological properties were in agreement with each other.
Introduction
Plum (Prunus subg. Prunus) belongs to the family Rosaceae and is one of the important fruits of temperate regions. There are more than 2000 varieties of plums, among which relatively few are of commercial importance.[Citation1]It contains a variety of minerals including iron, calcium, phosphorus, magnesium, manganese, fluorine, sulfur, potassium, and sugar as well as vitamins A, B1, B2, C, PP.[Citation2] Also because of phenolic acids, anthocyanins, carotenoids, and pectin can be used as a useful material in the human diet. The results of studies have shown that the presence of polyphenols and antioxidant compounds in plums and stone fruits can prevent diabetes, hypertension, and cardiovascular diseases.[Citation3,Citation4] According to literature data, depending on the variety, environmental conditions and applied analytical methods, contents of phenolic acids in plums fall within a wide range of values[Citation5-.Citation8] According to the FAO report, in 2017 the amount of plum production in the world exceeded 18.5 million tons. China, Romania, and the United States are the largest producers of this product in the world. Iran is the world’s fifth largest producer of plums with 298,893 tons .[Citation9] Neyshabur city is the largest producer of plum in Iran with around 50,000 tons in 2019. About 20% of this product being marketed fresh and 80% are used to dry. Generally, out of every 5 kg of plums, 1 kg of plum peel is produced. Therefore, about 10,000 tons of plum peel are produced annually in Neyshabur.[Citation10] During the plum drying process, a considerable amount of plum peel remains that accumulate in the gardens and villages. Moisture content, bulk of this material, and its spoilage can pollute the environment and impose environmental cleanup costs. In cases where they are used as animal feed, they cause diseases, especially fungal contamination in the environment. There is no indication of any use for this valuable material, so far, although it contains valuable compounds including vitamins, minerals, anthocyanins, and phenolic compounds (similar to the black plum) and it can be used to produce high-value products.
Sharbat (syrup) is a popular drink in Iran and prepared from mixing fruits puree, sweeteners (usually sugar), acids (mostly citric acid), and other additives like gums (usually pectin) and aromatic substance to achieve the desired concentration and usually served chilled. In addition to fruits and juices (such as orange, cherry, pineapple, apple, mango, lemon, etc.), plants and flowers are also converted to sharbat by boiling with a sweetener. Occasionally, the plant seeds are added to the sharbat, such as flixweed and balangu, which is served at religious ceremonies.[Citation11] Some of these sharbats also have medicinal properties, such as pussy willow, menthe, echium, and ipomoea sharbat.[Citation12] Nowadays in industrial factories, sharbats are made from fruit concentrates, and after pasteurization, they are filled in glass and plastic containers. Sharbat can be served in concentrated form and eaten with a spoon or diluted with water to create the drink. Usually, grade 2 or 3 fruits are used to produce sharbat. Although plum is one of the most important agricultural products in Iran and is widely used by people, but plum sharbat produced in limited quantities. In addition, since plum peel has nutritional properties of plum and it has more attractive than color, it can also be used for sharbat production. Black plum peel sharbat is a new product. Innovative aspects and unique features of this product include the use of plum by-products and reduced environmental pollution, low cost and high profitability, to produce natural products without any artificial additives and production a special product as Iran souvenirs.
There are a limited number of researches about the different properties of syrups[Citation13–Citation22] and some of them are about date syrups that are different from other syrups, due to no use of sweeteners and gums in the formulation. So far, no studies have been published on the production of sharbat from by-products of black plum processing. Therefore, the purpose of this study was to use black plum peel for sharbat production and to study its physico-chemical and sensory properties. For this purpose, the effect of sharbat formulation with regard to the pectin and puree of black plum peel concentrations on chemical properties (acidity, pH, brix, and moisture content), sensory attributes (color, flavor, consisnency, aroma, water-solubility, sweetness, and total acceptance), rheological properties (consistency coefficient, flow behavior index, and apparent viscosity based on appropriate rheological models), antioxidant activity, and phenolic compounds of black plum peel sharbat would be studied to obtain complete characterizations of black plum peel sharbat properties.
Materials and methods
Raw materials
Raw materials included frozen black plum peel, pectin, glucose syrup, sugar, and citric acid. Plum peel was obtained from Kharve village, Neyshabur, Iran, pectin (Green Ribbon, Citrus, 57-62% degree esterification, NATUREX, Switzerland), citric acid (Jovein, Sabzevar, Iran) glucose syrup, and sugar from Neyshabour supermarkets, Khorasan Razavi, Iran.
Sample preparation
After washing and removing impurities, the plum peel was crushed in an industrial crusher and was kept in the freezer until experiments. To produce sharbat, the crushed peel mixed with water and passed through the filter to obtain a smooth and uniform composition. The obtained solution was named plum puree. Brix and pH of black plum puree were 24 and 3.79, respectively. Egbekun et al. (1996)[Citation23] reported the brix and pH of black plum (Vitex doniana) pulp 5.2 and 4.38, respectively. For the production of sharbat, water was added to the black plum peel puree at a ratio of three times the weight and was heated with glucose syrup and sugar to reach brix 60. Subsequently, pectin and citric acid were added and the heating process was carried out until reaching 70% brix and pH <3.2. After cooking, the samples were cold and filled in glass containers and kept at room temperature (22°C ± 2) for 24 h to complete the gelation process. The obtained samples were used for tests. shows images of black plum, dried plum, and black plum peel sharbat. The constituent components of the black plum peel sharbat formulas were plum puree-sugar (40–60, 50–50 and 60-40%), 20% glucose syrup, 0.7% citric acid, and pectin at three levels (0%, 0.25%, and 0.5%). According to the Iranian standard, chemical parameters for various sharbats are different. Since there is no standard for plum sharbat and black plum peel sharbat is similar to sour cherry, the parameters considered for black plum peel sharbat were based on the standard of sour cherry sharbat. According to Iranian standard (number 1812, sour cherry sharbat[Citation24]), brix, pH, and acidity should be at least 63%, 3.6%, and 0.6% respectively.
Chemical analysis
Chemical compositions of the black plum peel sharbat samples were analyzed as hydrogen potential (pH), total soluble solids (brix), total acidity (TA), and moisture content (by the oven method at 70°C). To determine the acidity, diluted samples were titrated with 0.1 N sodium hydroxide solution and the results were given as a percentage of citric acid. Soluble solid (brix) was measured using a refractometer (RHBO_80, Link, Fuzhou, China). The hydrogen potential was controlled by a pH meter (Sartorius PB_11, Göttingen, Germany) and moisture content by an oven (Fan-Azma-Gostar, Iran). Measurements were performed at 20°C. Chemical experiments were performed at 2 replications.
Sensory properties
Black plum peel sharbat was examined organoleptically by 13 trained panelists (6 men and 7 women, 20–40 years old) from students and staff of Neyshabur University of Medical Sciences. Initially, the sensory characteristics of plum peel sharbat were explained to the panelists. As regards, the panelists were familiar with plum sharbat, so, they knew the sensory properties of the black plum peel sharbat, very well. Triangle test was used to identify sensory differences between the samples. Evaluations were performed at room temperature (22 ± 2°C) and under white fluorescent light. The 9-point hedonic score (1 = Dislike extremely, 9 = like extremely) was used to assess the sensory attributes of black plum peel sharbat. Throughout panel sessions, panelists were instructed to rinse their mouths with water before testing each sample. Therefore, 7 black plum peel sharbat factors including color, flavor, consistency, aroma, water-solubility, sweetness, and total acceptance were evaluated.
Rheological measurements
Rheological properties of black plum peel sharbat were measured by a Bohlin Visco 88 viscometer (Bohlin Instrument, Cirencester, UK.), equipped with a bob and cup geometry (bob length: 60 mm; bob diameter: 14 mm; gap width: 1 mm) and a heating circulator (model F12-MC, Julabo Labortechnik, Seelbach, Germany). The software Bohlin v06.32 was used to generate shear stress–shear rate data. The flow curves of black plum peel sharbat were measured at 25 ± 0.5°C by enhancing the shear rate from 20 to 300/s over a time lapse of 400 s. The apparent viscosity of black plum peel sharbat was determined at a shear rate of 40/s.
Modeling time-independent behavior: In the flow curve measurements, the shear stress (σ) as a function of shear rate (γ) was measured for time-independent behaviors. Then, the apparent viscosity (η) at a given shear rate could be calculated using the following equation[Citation25,Citation26]:
The time-independent flow curves of black plum peel sharbat samples were described by power-law (or Ostwald-Waele), Herschel–Bulkley, Casson, Bingham, and Sisko models.[Citation25]
Antioxidant activity and phenolic compounds
Extraction of antioxidant compounds was done according to the method of Moo-Huchin et al. (2015)[Citation27] .Five grams of the sharbat samples were weighed and stirred with 20 ml methanol/water solvent mixture (50:50) for 1 h at room temperature. Samples were centrifuged at 3500 rpm for 15 min and the supernatant was collected. Next, 20 ml of acetone/water solvent mixture (70:30) was added to the remaining sediments and extracted for 60 min by the above method and finally centrifuged. The methanol and acetone extracts were filtered with Whitman filter paper; the remaining solutions were mixed. The obtained extract was used to determine total phenolic compounds and antioxidant activity. Total phenolic compounds were determined using the Folin–Ciocalteu method reported by Spanos and Wrolstad (1990).[Citation28] One hundred microliters of each extract was mixed with 5 ml of distilled water. Five hundred microliters of Folic Acid Reagent and 1.5 ml of 20% sodium carbonate were added to it and finally made up to 10 ml with distilled water. The mixture was incubated for 2 h at room temperature and their absorbance was measured at 765 nm using UV/Vis spectrophotometer (Jenway 7315 model, UK.). The results were expressed based on different concentrations (10–100 ppm) of gallic acid using the calibration curve. Antioxidant activity was determined using 2,2-diPhenyl-1-Picrylhydrazyl (DPPH) method. Six different concentrations of each sharbat extract were made. One ml of DPPH ethanol solution (0.3 mmol/L) was added to 2.5 ml of sample solutions of varying concentrations and stirred at room temperature for 30 min. Then, the absorbance of the samples was read against appropriate blanks containing 1 ml of DPPH reagent and 2.5 ml of methanol at 517 nm at UV/Vis spectrophotometer. Antioxidant activity and phenolic compounds were measured at 2 replications.
Statistical analysis
A completely randomized factorial design was used to evaluate the results where analysis of variance (ANOVA) was carried out to compare the mean values. All significant differences were reported at P ≤ 0.05 level. Tukey test was employed to determine the difference among the formulations. Partial Least Square (PLS) method was applied to explore relationships between sensory properties, rheological characteristics, and antioxidant activity of black plum peel sharbat. Minitab statistical software (Version 16, USA, 2010) was used for all statistical analyses in the present research. GraphPad Prism (Version 6.07, USA, 2015) was also utilized to plot the curves.
Results and discussion
Chemical properties
Brix and pH: Because the brix of the samples was controlled by the device within a certain range, no statistical analysis was performed on them. Statistical analysis showed that increasing the percentage of pectin and puree concentrations had no significant effect on the pH of samples (P > .05) (). The results of this study showed that the brix and pH of samples were in the range of 72.75–74.50 and 2.86–3.02, respectively. Egbekun et al. (1996)[Citation23] reported the pH and brix of black plum (Vitex doniana) syrup to be 2.9 and 67% respectively, which are consistent with the results of this study and the Iranian standard (number 1812, sour cherry sharbat[Citation24]).
Figure 2. Effect of pectin (a) and puree (b) concentrations on chemical properties of black plum peel sharbat.
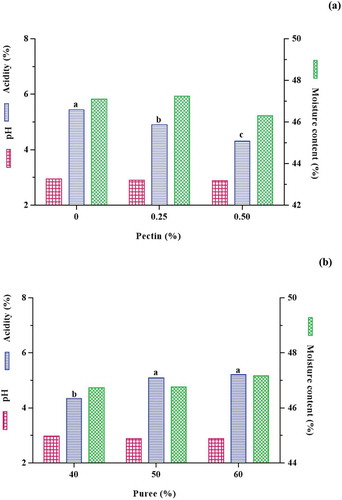
Acidity: Statistical analysis showed that increasing the percentage of pectin and black plum peel puree had a significant effect on black plum peel sharbat acidity (P < .05). The acidity decreased with increasing pectin content (). As the percentage of pectin increases, a larger and tighter gel network is formed and holds the acid inside. Therefore, the amount of acid in the aqueous medium decreased. So, the amount of NaOH needed to neutralize the acid reduced and acidity was decreased. As shown in , acidity increased with increasing of plum peel puree. This can be due to acidic property and low pH of black plum peel puree (pH = 3.79). The results showed that the acidity of the sharbat samples was in the range of 4.05–6.11%. Egbekun et al. (1996)[Citation23] reported the acidity of black plum syrup (Vitex doniana) 1.2%. This value is different from the results of this study. They produced black plum syrup as an alternative to sweeteners, while the aim of this study was to produce a new drink with a sour taste.
Moisture content: Increasing the percentage of pectin and black plum peel puree had no significant effect on the moisture content of the samples (P > .05) (). The moisture content of the samples ranged from 45.84% to 48.94%. Egbekun et al. (1996)[Citation23] reported moisture content of black plum syrup 20% that is different from the result of this study. This may be due to the use of plum syrup as a sugar substitute, which may require less moisture. Kunyanga et al. (2009)[Citation29] reported chemical properties of cactus fruit syrup including pH, acidity, and total solids 3.2, 1% and 7.5%, respectively.
Sensory properties
Color: Analysis of variance showed that the increase in pectin percentage had a significant effect on black plum peel sharbat color (P < .05), while the effect of black plum peel puree on the color score of samples was not significant (P > .05) (). As can be seen in , increasing the amount of pectin decreased the color score of the samples. It seems to be due to a decrease in the transparency and darkening of the sharbat color due to the increased pectin and the formation of the jelly network. According to the panelists, the color score of the samples ranged from 4.57 to 7.93. The results showed that the sample containing 0.25% pectin and 50% black plum peel puree had the lowest score and the sample containing 50% black plum peel puree and without pectin had the highest score.
Figure 3. Effect of pectin (a) and puree (b) concentrations on sensory properties of black plum peel sharbat.
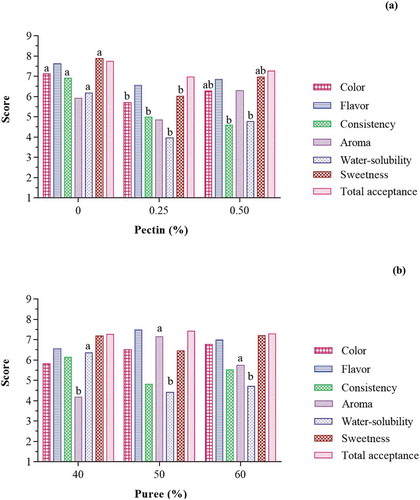
Flavor: The results showed that increasing the percentage of pectin and puree did not have a significant effect on the flavor score of black plum peel sharbat (P > .05) (). The sample containing 0.5% pectin and 40% black plum peel puree had the lowest score (6.06) and the sample containing 50% black plum peel puree without pectin had the highest score (8.50).
Consistency: Increasing percentage of black plum peel puree did not show a significant effect on black plum peel sharbat consistency (P > .05), although, the effect of pectin concentration was significant on consistency score (P < .05) (). As shown in , increasing the concentration of pectin decreased the consistency score. It seems that panelists liked diluted samples. The consistency score was in the range of 3.92 to 7.92. The results showed that a sample containing 50% black plum peel puree and 0.5% pectin had the lowest consistency score and sample with 50% black plum peel puree without pectin had the highest consistency score.
Aroma: Analysis of variance showed that increasing pectin percentage had no significant effect on aroma score (P > .05) (), though, an increasing percentage of puree showed a significant effect on aroma score of black plum peel sharbat (P < .05). shows that as the percentage of black plum peel puree increases, the aroma of the sharbat significantly increases. Black plum peel puree had a very strong aroma that was well breathable in the samples. As the amount of black plum peel puree increased, the aroma significantly changed. The results showed that the sample containing 40% black plum peel puree and 0.25% pectin had the lowest aroma score (3.33) and the sample with 50% black plum peel puree without pectin had the highest aroma score (7.67). Generally, panelists were less interested in samples with 40% and 60% black plum puree. According to panelist ideas, the aroma of samples with 40% black plum puree was very low and it was very much for samples with 60% puree.
Water-solubility: Increasing the percentage of black plum peel puree and pectin showed a significant effect on water-solubility of black plum peel sharbat (P < 0/05). As shown in , water-solubility decreased with increasing percentages of black plum peel puree and pectin. Increasing the amount of pectin and black plum peel puree (due to its fibrous structure) causes the samples to more viscose and reduce their water-solubility. The solubility score of the samples ranged from 2.79 to 7.42. The results showed that samples containing 50% black plum peel puree and 0.25% pectin had the least solubility and samples with 50% black plum peel puree without pectin had the highest water-solubility. Kuyanga et al. (2009)[Citation29] reported that the presence of 0.05% guar can improve the physical stability of cactus fruit syrup.
Sweetness: Analysis of variance showed that increasing the percentage of pectin had a significant effect on the sweetness of black plum peel sharbat (P < .05), whereas the effect of black plum peel puree on the quality and sweetness of samples was not significant (P > .05) (). As shown in , the sweetness of the sample decreased with increasing pectin content. It can be said that with increasing pectin content more sugar is trapped in the jelly network and its sweetness in the mouth decreases. The sweetness score of the samples ranged from 3.79 to 8.08. According to the results, the sample containing 50% black plum peel puree and 0.25% pectin had the least sweetness and the sample with 50% black plum peel puree without pectin had the highest sweetness.
Total acceptance: Analysis of variance showed that increasing the percentage of pectin and black plum peel did not have a significant effect on the total acceptance of black plum peel sharbat (P > .05). From the panelist’s point of view, all samples of black plum peel sharbat were acceptable. The total acceptance score ranged from 6.71 to 8.17. The results showed that the sample containing 50% black plum peel puree and 0.25% pectin had the lowest acceptance and the sample with 50% black plum peel puree without pectin had the highest acceptance. Egbekun et al. (1996)[Citation23] investigated the sensory properties of black plum syrup and compared it with honey. There was no significant difference in flavor score and total acceptance of the samples. According to the results of their study, black plum syrup was acceptable and could substitute for other syrups as a nutritive sweetener.
Rheological properties
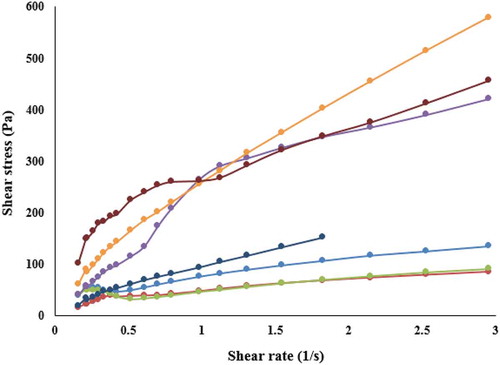
illustrates the flow curves of black plum peel sharbat at different levels of pectin and puree. According to the results, the shear stress – shear rate relation was nonlinear, indicating that all sharbat samples behaved as non-Newtonian fluid, pseudoplastic type. shows the results of time-independent rheological behavior modeling based on Power Law, Herschel‒Bulkley, Casson, and Bingham models. As can be seen, Herschel–Bulkley and Power-Law models had the ability to fit the rheological parameters of black plum peel sharbat. It is also observed that the Herschel-Bulkley model in some samples was not suitable because the yield stress was negative. Therefore, the Power-Law model was considered as an appropriate model to investigate the time-independent rheological properties of black plum peel sharbat. The R2 and RMSE values for the samples of sharbat were in the range of 0.9049–0.9999 and 0.94–37.32, respectively. Analysis of variance showed that increasing pectin and black plum puree concentrations and their interaction had a significant effect on rheological parameters (P < .05). However, the increase of the black plum puree level did not show a special trend on rheological parameters (). Increasing black plum puree from 40% to 50% decreased flow behavior index and apparent viscosity and increased consistency coefficient, while increasing from 50% to 60% increased flow behavior index and apparent viscosity and decreased consistency coefficient. Increasing the percentage of pectin increased the consistency coefficient. Flow behavior index, consistency coefficient, and apparent viscosity of black plum peel sharbat were in the range of 0.41–0.72, 1.16–41.78 (Pa.sn) and 11.09–72.78 (Pa.s), respectively. Maceiras et al. (2007)[Citation16] investigated the rheological properties of cooked plum puree at different temperatures. According to the researchers, the Power-Law and Herschel–Bulkley models were able to describe the flow behavior of cooked plum puree. Flow behavior index and consistency coefficient of cooked plum puree based on Power-Law model were in the range of 0.26–0.30 and 5.08–9.33 Pa.sn, respectively, that are less than the results of this study. Egbekun et al. (1996)[Citation23] reported the viscosity of black plum (Vitex doniana) syrup at 30°C, 2.10 (Pa.s).
Table 1. Statistical parameters of the rheological models determined for flow curves of black plum peel sharbat.
Antioxidant activity and phenolic compounds
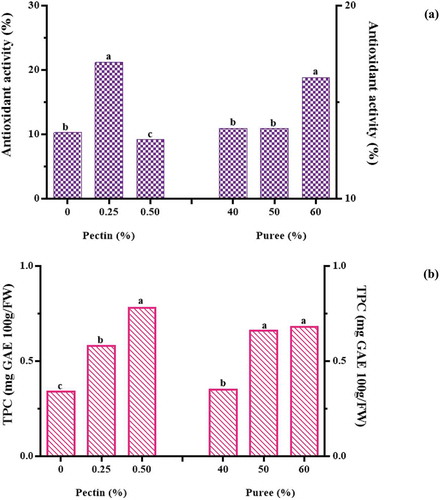
The results of ANOVA showed that increasing the percentage of pectin and black plum puree had a significant effect on the antioxidant activity and phenolic compounds of black plum peel sharbat (P < .05). shows the effect of pectin and puree concentrations on the antioxidant activity and phenolic compounds of black plum peel sharbat. As can be seen, the increase in the percentage of black plum peel puree increased antioxidant activity and phenolic compounds. It is also observed with increasing pectin percentage, total phenolic compounds increased, but the amount of antioxidant activity did not show any specific trend. Antioxidant activity and total phenolic compound of black plum peel sharbat ranged from 3.80% to 33.47% and 0.28–1.03 mg GAE 100/g FW, respectively. Belkheir et al., (2013)[Citation30] in the study of Tunisian Wild Crataegus azarolus (Yellow Azarole) and Crataegus monogyna (Red Azarole) syrup reported that low levels of phenolic compounds to antioxidant activity could be due to the presence of Hydroxymethylfurfural. This result may also be applied to black plum peel sharbat. Aamer (2016)[Citation13] reported antioxidant activity and phenolic compounds of doum fruit syrup in the range of 26.55–31.86% and 19.03–23.47 mg/100 g, respectively.
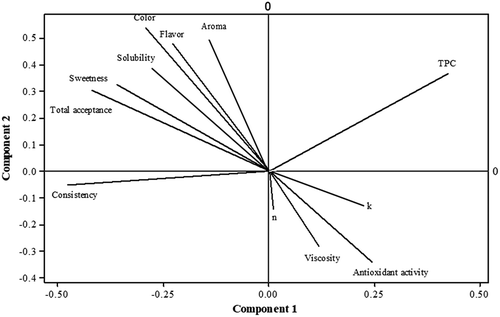
Correlation between sensory attributes, rheological parameters, and antioxidant activity of black plum peel sharbat
shows the correlation between sensory attributes, antioxidant activity, and rheological properties of black plum peel sharbat. It can be seen, aroma, flavor, color, sweetness, and water-solubility were positively related to total acceptance. Moreover, total acceptance showed a negative correlation with consistency, viscosity, consistency coefficient (K), flow behavior index (n), and antioxidant activity. These results are consistent with the results of sensory properties. Viscosity had a positive correlation with antioxidant activity, K, and flow behavior index.
Conclusion
The present study has shown that high potential exists for the use of black plum peel as a local source of raw materials for making sharbat than allowing it to waste as a nutritional material. The sensory properties indicated that the sample with 50% black plum peel puree and without pectin had the highest acceptability. It seems to be due to the attractive color, suitable water solubility, and flavor and moderate aroma at this formulation. Physicochemical and sensory evaluations suggest that the sharbat is of high value and is generally acceptable. Production of black plum peel sharbat in addition to producing a new product makes optimum use of plum processing by-products, which can play an important role in reducing environmental pollution.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgments
We thank the Neyshabur University of Medical Sciences for funding the project (Project number 76). The authors of this article declare that they have no conflict of interests.
References
- Somogai, L.;. Plums and Prunes Processing of Fruits Science and Technology. CRC Press. 2005, 21, 513–530.
- Bennett, L. E.; Singh, D. P.; Clingeleffer, P. R. Micronutrient Mineral and Folate Content of Australian and Imported Dried Fruit Products. Crit. Rev. Food Sci. Nutr. 2010, 51(1), 38–49. DOI: 10.1080/10408390903044552.
- Rupasinghe, H. V.; Jayasankar, S.; Lay, W. Variation in Total Phenolics and Antioxidant Capacity among European Plum Genotypes. Sci. Hortic. 2006, 108(3), 243–246. DOI: 10.1016/j.scienta.2006.01.020.
- Walkowiak-Tomczak, D.;. Characteristics of Plums as a Raw Material with Valuable Nutritive and Dietary Properties-a Review. Pol. J. Food Nutr. Sci. 2008, 58(4), 401–405.
- Donovan, J. L.; Meyer, A. S.; Waterhouse, A. L. Phenolic Composition and Antioxidant Activity of Prunes and Prune Juice (Prunus Domestica). J. Agric. Food Chem. 1998, 46(4), 1247–1252. DOI: 10.1021/jf970831x.
- S.-i., K.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant Activity of Prune (Prunus Domestica L.) Constituents and a New Synergist. J. Agric. Food Chem. 2002, 50(13), 3708–3712.
- Nakatani, N.; Kayano, S.-I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, Quantitative Determination, and Antioxidative Activities of Chlorogenic Acid Isomers in Prune (Prunus D Omestica L.). J. Agric. Food Chem. 2000, 48(11), 5512–5516. DOI: 10.1021/jf000422s.
- Tomás-Barberán, F. A.; Gil, M. I.; Cremin, P.; Waterhouse, A. L.; Hess-Pierce, B.; Kader, A. A. HPLC− DAD− ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49(10), 4748–4760. DOI: 10.1021/jf0104681.
- Faostat, F. Food and Agriculture Organization Statistical Database. accessed Feb 2019. http://www.fao.org/
- Agriculture-Jahad, M. O. 2019. https://www.maj.ir/.
- Montazemi, R. The Art of Cooking; Iran Book Publishing: Iran, Tehran, 1992.
- Dehkhoda, A. Dehkhoda Dictionary; Tehran University Publishing: Iran, 1931.
- Aamer, R. A.;. Characteristics of Aqueous Doum Fruit Extract and Its Utilization in Some Novel Products. Ann. Agric. Sci. 2016, 61(1), 25–33. DOI: 10.1016/j.aoas.2016.04.004.
- Abbès, F.; Kchaou, W.; Blecker, C.; Ongena, M.; Lognay, G.; Attia, H.; Besbes, S. Effect of Processing Conditions on Phenolic Compounds and Antioxidant Properties of Date Syrup. Ind. Crops Prod. 2013, 44, 634–642. DOI: 10.1016/j.indcrop.2012.09.008.
- García-Quiroga, M.; Nunes-Damaceno, M.; Gómez-López, M.; Arbones-Maciñeira, E.; Muñoz-Ferreiro, N.; Vázquez-Odériz, M.; Romero-Rodríguez, M. Kiwifruit in Syrup: Consumer Acceptance, Purchase Intention and Influence of Processing and Storage Time on Physicochemical and Sensory Characteristics. Food Bioprocess. Technol. 2015, 8(11), 2268–2278. DOI: 10.1007/s11947-015-1571-3.
- Maceiras, R.; Alvarez, E.; Cancela, M. Rheological Properties of Fruit Purees: Effect of Cooking. J. Food Eng. 2007, 80(3), 763–769. DOI: 10.1016/j.jfoodeng.2006.06.028.
- Masoumi, A.; Sadananda, G.; Krishna, H.; Prashanth, S.; Vasundhara, M.; Ravindra, U.; Rahimi, B. A. Effect of Preservative and Storage Condition on Biochemical and Sensory Quality of Pomegranate and Plum Blended Carbonated Drink. J. Pharmacogn. Phytochem. 2018, 7(4), 826–829.
- Oboh, F.; Imafidon, J. Antioxidant and Sensory Properties of New Beverage Formulations Composed of Palm Sugar, Aframomum Melegueta, and Citric Acid. Beverages. 2018, 4(3), 59. DOI: 10.3390/beverages4030059.
- Puoci, F.; Iemma, F.; Spizzirri, U. G.; Restuccia, D.; Pezzi, V.; Sirianni, R.; Manganaro, L.; Curcio, M.; Parisi, O. I.; Cirillo, G. Antioxidant Activity of a Mediterranean Food Product:“fig Syrup”. Nutrients. 2011, 3(3), 317–329. DOI: 10.3390/nu3030317.
- Yasni, S.; In Development Technology of Functional Drinks Made from Ginger Extracts as Products Model for Developing Small-medium Enterprises. IOP Conference Series: Earth and Environmental Science, Vietnam, IOP Publishing, 2018; p 012017.
- Al-Hooti, S. N.; Sidhu, J. S.; Al-Saqer, J. M.; Al-Othman, A. Chemical Composition and Quality of Date Syrup as Affected by Pectinase/cellulase Enzyme Treatment. Food Chem. 2002, 79(2), 215–220. DOI: 10.1016/S0308-8146(02)00134-6.
- Gabsi, K.; Trigui, M.; Barrington, S.; Helal, A. N.; Taherian, A. R. Evaluation of Rheological Properties of Date Syrup. J. Food Eng. 2013, 117(1), 165–172. DOI: 10.1016/j.jfoodeng.2013.02.017.
- Egbekun, M. K.; Akowe, J. I.; Ede, R. J. Physico-chemical and Sensory Properties of Formulated Syrup from Black Plum (Vitex Doniana) Fruit. Plant Foods Human Nutr. 1996, 49(4), 301–306. DOI: 10.1007/BF01091979.
- Iranian National Standards Organization, S. c. s.. http://www.isiri.gov.ir/. Vol. 1812, 2016.
- Steffe, J. F. Rheological Methods in Food Process Engineering; Freeman press, 1996.
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G. V. Rheology for the Food Industry. J. Food Eng. 2005, 67(1–2), 147–156. DOI: 10.1016/j.jfoodeng.2004.05.062.
- Moo-Huchin, V. M.; Moo-Huchin, M. I.; Estrada-León, R. J.; Cuevas-Glory, L.; Estrada-Mota, I. A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant Compounds, Antioxidant Activity and Phenolic Content in Peel from Three Tropical Fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. DOI: 10.1016/j.foodchem.2014.05.127.
- Spanos, G. A.; Wrolstad, R. E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric. Food Chem. 1990, 38(7), 1565–1571. DOI: 10.1021/jf00097a030.
- Kunyanga, C.; Strum, S.; Graham, S.; Sipitiek, J.; Imungi, J. In Physico-chemical Methods for Preservation of Opuntia Cactus Fruit Syrup, 9th African Crop Science. Conference Proceedings, African Crop Science Society, Cape Town, South Africa, 28 September-2 October, 2009, pp 333–337.
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Congiu, F.; Tuberoso, C. I. G.; Amri, M.; Fattouch, S. Comparative Analysis of Tunisian Wild Crataegus Azarolus (Yellow Azarole) and Crataegus Monogyna (Red Azarole) Leaf, Fruit, and Traditionally Derived Syrup: Phenolic Profiles and Antioxidant and Antimicrobial Activities of the Aqueous-acetone Extracts. J. Agric. Food Chem. 2013, 61(40), 9594–9601. DOI: 10.1021/jf405285m.