?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Detection of primary metabolites such as amino acids, carbohydrates along with the secondary metabolites is important for the quality control of herbal. Therefore, a simple amino acid analysis method was investigated for the quality control of Protaetia brevitarsis. Six batches of P. brevitarsis collected from different feeding batches were used to analyze the amino acid composition and content. The amino acid composition was consistent in the six batches of P. brevitarsis, and the amino acid content of six batches of P. brevitarsis was also relatively stable. Under these conditions, the six batches of P. brevitarsis also possessed stable antioxidant activity. The stable composition and content of the amino acids could guarantee the stable antioxidant in batch-to-batch of P. brevitarsis. So, the amino acid–activity relationship as an effective method can be used to assess the quality of P. brevitarsis.
Introduction
White-spotted flower chafer (Protaetia brevitarsis) is a species of the subfamily Cetoniinae that lives in East Asia and Europe.[Citation1–Citation3] They could feed on a large number of plants and fungi, decompose animal and plant debris, and eat other invertebrates.[Citation3] P. brevitarsis larvae possess therapeutic effects in the treatment of breast cancer,[Citation4,Citation5] antibacterial action,[Citation6] inflammatory disease, antioxidant[Citation3] and liver-related diseases.[Citation7] Furthermore, the majority of the previous studies on P. brevitarsis larvae were mainly focused on the biochemistry and the physiology of the insect itself.[Citation8–Citation10] Detection of primary metabolites such as amino acids, carbohydrates along with the secondary metabolites is important for the quality control of herbal.[Citation11] In addition, some amino acids have antioxidant effects including lysine, histidine, arginine, methionine, cysteine, tryptophan, tyrosine, and phenylalanine.[Citation12] However, the effect of the relationship between the amino acids and the antioxidant of P. brevitarsis on batch-to-batch quality control is not clear. Therefore, the aim of the present work was to develop amino acid–activity relationship of P. brevitarsis, which may be used as a marker for the quality evaluation of batch-to-batch of P. brevitarsis.
Materials and methods
Samples’ preparation
All of the samples of P. brevitarsis were collected from the Hebei langfang base of the Chinese academy of agricultural sciences (Beijing, P.R. China). Firstly, P. brevitarsis was cleaned with clean water. Secondly, P. brevitarsis were held in water at 100°C about 40 s, which were immediately removed from water and dried, and then dissected, intestinesremoved the intestines, and weighed. Afterward, they were soaked in anhydrous ether for 24 h, air-dried, and weighed.
Amino acid compositions and contents of P. brevitarsis
The amino acid compositions and contents of P. brevitarsis were detected by adopting the PRC standard detected by GB/T 1846–2000.[Citation13] Based on the amino acid testing standard analyzed the amino acid compositions and contents of P. brevitarsis. The formula of amino acid contents is as follows:
where is the content of an amino acid determined by an undegreased pattern (%).
is the content of colored amino acids from degreasing samples (%).
is the content of amino acids in hydrolyzate per milliliter, ng.
is the content of tryptophan per milliliter of computer solution, ng. m is sample quality, mg. D is trial dilution multiple. F is the fat content of the sample.
Antioxidant investigation
Ferric reducing/antioxidant power (FRAP) assay.[Citation14] Briefly, the reagent was prepared by mixing 10 mmol 2,4,6-Tris(2-pyridyl)-striazine (TPTZ)/L reagent with 20 mmol/L ferric chloride in acetate buffer (pH 3.6). Quantitative analyses were performed by the external standard method using ferrous sulfate (2 × 10−1 mmol/L) as the reference standard and correlating the absorbance (λ593 nm) with the concentration. Samples of mead (0.1 mL) were mixed in polystyrene cuvettes with 0.9 mL of distilled water and 3 mL of ferric complex. The results were calculated and expressed as micromoles of Trolox per milliliter of the mead. The absorbance was read in disposable polystyrene cuvettes using a spectrophotometer. All measurements were performed in triplicate. The correlation coefficient (r2) is ≥ 0.9979.
Data analysis
All data were shown as mean ± SD of three independent experiments. Data were statistically compared using one-way ANOVA with Tukey post hoc, and p < .05 was considered statistically significant.
Results and discussion
Amino acid compositions and contents analysis
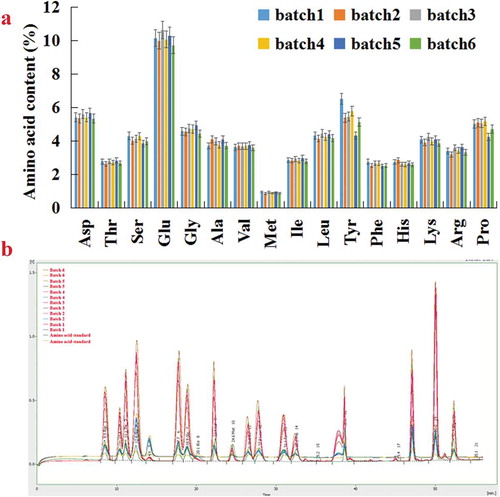
We analyzed the amino acid compositions and contents of six batches of P. brevitarsis, as shown in . Each of the six batches of P. brevitarsis contained the same amino acid compositions and the similar amino acid contents. The range of the content of Asp is 5.35 ~ 5.68. The range of the content of Thr is 2.665 ~ 2.855 and Ser is 3.875 ~ 4.315. The range of the content of Glu is 9.73 ~ 10.635. The range of the content of Gly is 4.45 ~ 4.96. The range of the content of Ala is 3.72 ~ 4.12. The range of the content of Val is 3.605 ~ 3.77. The range of the content of Met is 0.885 ~ 0.975. The range of the content of Ile is 2.805 ~ 2.945. The range of the content of Leu is 4.165 ~ 4.47. The range of the content of Tyr is 4.345 ~ 6.53. The range of the content of Phe is 2.515 ~ 2.78. The range of the content of His is 2.595 ~ 2.86. The range of the content of Lys is 3.895 ~ 4.245. The range of the content of Arg is 3.215 ~ 3.655. The range of the content of Pro is 4.245 ~ 5.19. These results demonstrated that favorable quality control could ensure the batch-to-batch stability of the amino acid compositions and contents of P. brevitarsis.
Relationship between amino acids and antioxidant activity
At the same time, the antioxidant ability of six different feeding batches of P. brevitarsis was stable and semblable (). The antioxidant capacity of six batches of P. brevitarsis ranged from 0.526 ± 0.043 to 0.548 ± 0.021 mM; therefore, the six batches of P. brevitarsis had stable antioxidant capacity. Previous research has shown that lysine, histidine, and arginine react with sugars and produce melanoidins to achieve the antioxidant effect.[Citation15] Methionine can be converted into reduced glutathione via redox cycle to antioxidant effect.[Citation16] Aromatic amino acids such as tyrosine and phenylalanine inhibit the chain reaction of free radicals to achieve antioxidant effect.[17] In our results, there were stable composition and content of Tyr, Met, Phe, His, Lys, and Arg in different batches of P. brevitarsis. Therefore, the results indicated that the stable amino acid composition and content in different batches of P. brevitarsis could guarantee the active stability of batch to batch of P. brevitarsis.
Table 1. Antioxidant activity of six different feeding batches of P. brevitarsis.
Conclusion
In this work, we found that there were 16 amino acids in P. brevitarsis, and the composition and content of the 16 amino acids were stable in different batches of P. brevitarsis. Therefore, controlling the stability of the amino acid composition and content could guarantee the active stability of batch-to-batch of P. brevitarsis. In addition, our data also suggest that P. brevitarsis could be used as a resource for the development of novel antioxidants against oxidative damage.
Additional information
Funding
References
- Noh, J. H. Jeong, J. S. Park, S. J.; Yun, E. Y. Hwang, J. S.; Kim, J. Y. Jung, K. J.; Park, H. J.; Son, H. Y.; Moon, K. S. Toxicological Safety Evaluation of Freeze-dried Protaetia Brevitarsis Larva Powder. Reprod. Toxicol. 2018, 5, 695–703. DOI: 10.1016/j.toxrep.2018.06.001.
- Yin, S.; Li, G.; Liu, M.; Wen, C.; Zhao, Y. Biochemical Responses of the Protaetia Brevitarsis Lewis Larvae to Subchronic Copper Exposure. Environ. Sci. Pollut. Res. 2018, 25, 18570–18578. DOI: 10.1007/s11356-018-2031-1.
- Suh, H. J.; Kang, S. C. Antioxidant Activity of Aqueous Methanol Extracts of Protaetia Brevitarsis Lewis (Coleoptera: Scarabaedia) at Different Growth Stages. Nat. Prod. Res. 2012, 26, 510–517. DOI: 10.1080/14786419.2010.530267.
- Yeo, H.; Youn, K.; Kim, M.; Yun, E. Y.; Hwang, J. S.; Jeong, W. S.; Jun, M. Fatty Acid Composition and Volatile Constituents of Protaetia Brevitarsis Larvae. Preventive Nutr. Food Sci. 2013, 18, 150–156. DOI: 10.3746/pnf.2013.18.2.150.
- Yoo, Y. C.; Shin, B. H.; Hong, J. H.; Lee, J.; Chee, H. Y.; Song, K. S.; Lee, K.-B. Isolation of Fatty Acids with Anticancer Activity fromProtaetia Brevitarsis Larva. Arch. Pharmacal Res. 2007, 30, 361–365. DOI: 10.1007/BF02977619.
- Yoon, H. S.; Lee, C. S.; Lee, S. Y.; Choi, C. S.; Lee, I. H.; Yeo, S. M.; Kim, H. R. Purification and cDNA Cloning of Inducible Antibacterial Peptides fromProtaetia Brevitarsis (Coleoptera). Arch. Insect Biochem. Physiol. 2003, 52, 92–103. DOI: 10.1002/arch.10072.
- Kang, M.; Kang, C.; Lee, H.; Kim, E.; Kim, J.; Kwon, O.; LEE, H.; KANG, H.; KIM, C.; JANG, H.;, et al. Effects of Fermented Aloe Vera Mixed Diet on Larval Growth of Protaetia Brevitarsis Seulensis (Kolbe) (Coleopteran:cetoniidae) and Protective Effects of Its Extract against CCl4-induced Hepatotoxicity in Sprague-Dawley Rats. Entomol .Res. 2012,42, 111–121. DOI: 10.1111/j.1748-5967.2012.00444.x.
- Wang, K.; Li, P. P.; Gao, Y. Y.; Liu, C. Q.; Wang, Q. L.; Yin, J.; Zhang, J.; Geng, L.; Shu, C. De Novo Genome Assembly Of The White-spotted Flower Chafer (Protaetia Brevitarsis). Gigascience. 2019, 8(4), giz019.
- Heo, J.; Kim, S. J.; Kim, J. S.; Hong, S. B.; Kwon, S. W. Paenibacillus Protaetiae Sp. Nov., Isolated from Gut of Larva of Protaetia Brevitarsis Seulensis. Int. J. Syst. Evol. Microbiol. 2020, 70, 989–994. DOI: 10.1099/ijsem.0.003860.
- Ahn, E. M.; Myung, N. Y.; Jung, H. A.; Kim, S. J. The Ameliorative Effect of Protaetia Brevitarsis Larvae in HFD-induced Obese Mice. Food Sci. Biotechnol. 2019, 28, 1177–1186. DOI: 10.1007/s10068-018-00553-w.
- Qureshi, M. N.; Stecher, G.; Bonn, G. K. Quality Control of Herbs: Determination of Amino Acids in Althaea Officinalis, Matricaria Chamomilla and Taraxacum Officinale. Pak. J. Pharm. Sci. 2014, 27, 459–462.
- Zhang, B.; Xia, T.; Duan, W. H.; Zhang, Z. J.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of Organic Acids, Amino Acids and Phenolic Compounds on Antioxidant Characteristic of Zhenjiang Aromatic Vinegar. Molecules. 2019, 24(20), 3799.
- PRC standard. Determination of Amino Acids in Feeds. GB/T 18246-2000.
- Kawa-Rygielska, J.; Adamenkoa, K.; Kucharskab, Z.; Szatkowska, A. Fruit and Herbal Meads-chemical Composition and Antioxidant Properties. Food Chem. 2019, 283, 19–27. DOI: 10.1016/j.foodchem.2019.01.040.
- Chen, T.; Gui, Q.; Shi, J. J.; Zhang, X. Y.; Chen, F. S. Analysis of Variation of Main Components during Aging Process of Shanxi Aged Vinegar. Acetic Acid Bact. 2013, 2, 6. DOI: 10.4081/aab.2013.s1.e6.
- Brosnan, J. T.; Brosnan, M. E. The Sulfur-containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. DOI: 10.1093/jn/136.6.1636S.
- Morozova, O. B.; Yurkovskaya, A. V. Modulation of the Rate of Reversible Electron Transfer in Oxidized Tryptophan and Tyrosine Containing Peptides in Acidic Aqueous Solution. J. Phys. Chem. B. 2015, 119, 140–149. DOI: 10.1021/jp511068n.