ABSTRACT
This study aimed to analyze the most important apple vitamin, L-ascorbic acid (AsA), and the most abundant fat-soluble one, α-tocopherol, in the pulp of 24 cultivars from harvest season 2010 and in six cultivars collected over three consecutive years (2008–2010). All cultivars were grown and stored under identical conditions and identified as ‘true to type’ by molecular genetic tools. The two vitamins were analyzed using high performance liquid chromatography with diode array detection (HPLC-DAD), involving two rapid methods with excellent recovery, linearity, precision, and sensitivity. Results showed an intriguing diversity of AsA contents and, to a lesser extent, of α-tocopherol in biological replicates, between cultivars and harvest years. Nevertheless, cultivar-specific relative vitamin contents can be deduced from this study. Among the 24 cultivars, a minimum AsA content of 0.43 ± 0.13 mg/100 g fresh weight (FW) and a maximum of 6.22 ± 1.06 mg/100 g FW have been found in ‘Gala’ and ‘Freiherr von Berlepsch,’ respectively. α-tocopherol ranged from 0.13 ± 0.02 mg/100 g FW to 0.33 ± 0.04 mg/100 g FW for ‘Freiherr von Berlepsch’ and ‘Brixner Plattling,’ respectively. The results of the present study suggest a potential of some old cultivars as a component of a healthy, vitamin-rich diet.
KEYWORDS:
Introduction
Apples (Malus x domestica Borkh.) are among the most consumed fruits worldwide and appreciated for their content of beneficial compounds.[Citation1–Citation6] The global annual production exceeds 70 Mio t, with the European Union being the second largest producer after China.[Citation7] South Tyrol, Italy, with ca. 18,500 hectares of apple orchards, accounts for more than 50% of Italian production and is reportedly the largest contiguous apple growing area in Europe.[Citation8] Apples have a balanced composition of sugars and acids, as well as a low content of proteins and lipids. The fruit is a rich source of minerals, fibers, and antioxidant compounds such as polyphenols and vitamins.[Citation1–Citation6,Citation9] Apples’ phytochemicals have been consistently associated with health benefits like reducing the risk of coronary heart disease, asthma, and diabetes.[,Citation3,Citation5] Moreover, apples have positive effects on the human lipid metabolism, resulting in reduction of total cholesterol[Citation10] and promoting weight loss.[Citation11]
Fruits are a relevant source of L-ascorbic acid (AsA), which is an antioxidant, required for collagen synthesis and important for the prevention of oxidative stress-related diseases, such as cancer.[Citation12] Apple pulp contains a reported average value of 4.0 mg/100 g fresh weight (FW) of AsA,[Citation13] which apparently cannot rival other fruits like kiwis, oranges, or strawberries. However, considering the large serving size of apples and apple-based products like apple juice, they substantially contribute to the daily assumption of AsA. Among the four vitamin E isomers (α, β, γ, δ), α-tocopherol is the biologically most active one. It is a fat-soluble compound with a defense role against endogenous and exogenous oxidants[Citation14] and relevance for neurological health.[Citation15] α-tocopherol is the most abundant fat-soluble vitamin present in apples.[Citation13]
The chemical composition of apples, including the vitamin content, depends on the cultivar, fruit tissue, environmental and agricultural factors, the growing site, ripeness, and storage.[Citation1,Citation16–Citation25] There are several reports about the content of antioxidant compound including AsA in different apple cultivars.[Citation26–Citation31] but studies that focus on well-characterized genotypes while holding the other parameters constant are rare.[Citation2] Traditionally, apple cultivars are characterized using morphological and agronomic traits, which are prone to misinterpretation. Therefore, an accurate identification of apple cultivars involving molecular genetic tools is considered an important prerequisite for studies on nutrient content.[Citation32]
In addition, few studies aimed at describing the content of vitamins in apples collected over several years are available. Previous studies report the changes in the concentrations of vitamin C in the peel of numerous apple varieties harvested over two consecutive years[Citation33] and in apple peel and pulp of two apple cultivars collected over two years.[Citation34] However, only one recent study was found about the amount of ascorbic acid in apple skin of the apple cultivar ‘Jonathan’ over two growing seasons.[Citation35]
So far, several analytical techniques have been reported for the determination of AsA in food, including titration, spectrophotometry, and HPLC.[Citation36–Citation41] The latter is preferred for the analysis of complex food matrixes because of its efficiency, separating power, accuracy, sensitivity, and specificity.[Citation42–44] Similarly, numerous analytical methods for tocopherols are reported,[Citation9,Citation45–Citation51] involving organic solvents, alkaline solutions, liquid-liquid extraction and others for extracting the vitamin from food, fruit, or vegetables. Most of them require numerous steps or use harsh conditions that may lead to a loss of tocopherols.[Citation51] A typical procedure for the extraction of vitamin E involves saponification or extraction into organic solvents and separation by normal or reversed-phase HPLC.[Citation52] Feliciano et al.[Citation9] reported a simple two steps extraction with n-hexane from fresh apple pulp, while Chun et al.[Citation45] used a method based on saponification of fresh material. Knecht et al.[Citation49] proposed a direct extraction of freeze-dried vegetable powders with acetone, demonstrating a higher stability of tocopherols in freeze-dried samples compared to fresh homogenates. The same study noted that a comparable stabilization of tocopherols was obtained by adding AsA to the fresh material.
For the present work, HPLC was chosen as a technique for the analysis of vitamins in apple pulp. Abe-Matsumoto et al.[Citation40] stated that HPLC is preferable to iodometric titration for the analysis of complex matrices containing other water-soluble vitamins than ascorbic acid. Recent works report the use of HPLC-DAD for the quantification of vitamin C in diverse fruits[Citation53] of water-soluble vitamins in edible plants[Citation54] and of water- and fat-soluble vitamins in fruits,[Citation55,Citation56] berries and leaves.[Citation57] The numerous above-mentioned advantages and applications of HPLC made this technique a tool of choice for the determination of vitamins in food.
In the present study, three extraction procedures for α-tocopherol were compared and two rapid, stable, and sensitive HPLC methods used to determine AsA and α-tocopherol in 24 accurately identified apple cultivars after storage and shelf life. For six cultivars, the vitamin content was analyzed over three consecutive years.
Materials and methods
Reagents
AsA (≥99%), α-tocopherol (≥96%), formic acid (98%), potassium hydroxide (≥85%), n-hexane (for HPLC), butylated hydroxytoluene (BHT, ≥99%), sodium chloride (≥99.5%), ethyl acetate (for HPLC, ≥99.7%), and ethanol (96%) were purchased from Sigma Aldrich (St. Louis, MO, USA); acetic acid (96%) was obtained from Merck KGaA (Darmstadt, Germany), acetonitrile (HPLC-grade) and methanol (HPLC-grade) were purchased from VWR Chemicals (Milan, Italy), and meta-phosphoric acid (≥99%) and monopotassium phosphate KH2PO4 (≥99%) from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Samples, standard solutions, and aqueous mobile phases were prepared using ultrapure water (18. M * cm, 25°C) purified by a MilliQ-System (Merck Millipore, Vimodrone, MI).
Fruit material
All apple cultivars used in this study were from the Laimburg Research Center varietal collection. The genetic identity of all accessions was certified to be ‘true to type’ with microsatellite markers.[Citation58–Citation60] Trees were grown on M9 rootstocks in the experimental fields of the Laimburg Research Center in Ora/Auer at 220 m a.s.l. (South Tyrol, Italy) and cultivated following the guidelines for integrated fruit production in South Tyrol.[Citation8] Apples were harvested at the optimal harvest time for each cultivar. Samples (~50 apples per cultivar) were randomly taken from the central part of five trees. In order to reduce the biological variability, only fruits of average size and color were collected according to King et al.[Citation61]
Storage and sampling
Apples were stored for 60 ± 10 days (30 ± 5 days for the three early ripening varieties ‘Roter Herbstkalvill,’ ‘Gravensteiner,’ and ‘Cellini’) in cold storage (normal atmosphere, 2°C, 90% humidity) and kept at room temperature for three days of shelf life. Ten apples per cultivar, peeled, cored, and sliced were used as biological replicates. The slices were immediately frozen at −30°C before lyophilization. Freeze drying was carried out using a LABCONCO equipment (Kansas City, MO, USA). After freeze drying the slice was ground to a homogeneous powder and stored at −80°C until analysis.
Chromatographic conditions
Analyses were performed on an Agilent 1260 Infinity HPLC system (Santa Clara, CA, USA), provided with autosampler, degasser, quaternary pump, column oven, and a diode array detector (1260 DAD VL). For data acquisition, the software Agilent ChemStation™ (ver. C.01.03) (Agilent, Palo Alto, CA) was used. A Kinetex 5 µ C18 100 Å column (4.6 mm x 150 mm; Phenomenex Torrance, CA, USA) was used with a C18 4.6 mm SecurityGuard (Phenomenex, Torrance, CA, USA).
AsA determination
For the quantification of AsA, sample preparation and HPLC-DAD analysis were performed according to Bassi et al.[Citation2] with a few modifications. An aliquot (250 mg) of freeze-dried apple pulp was suspended in 5 mL extraction solution (3.5 mL deionized water containing 8% v/v acetic acid and 3% w/v metaphosphoric acid added with 1.5 mL of methanol). The suspension was then mixed at 3200 rpm for about 20 s at room temperature, sonicated for 10 minutes, and centrifuged for 5 minutes at 4000 rpm. The supernatant was filtered (0.45 µm PTFE filters) and analyzed with HPLC-DAD according to Bassi et al.[Citation2] The AsA content was expressed as mg/100 g of fresh weight (FW).
α-tocopherol determination
Extraction with methanol: An aliquot (200 mg) of freeze-dried apple pulp was suspended in 1 mL of methanol. The suspension was mixed at 3,200 rpm for about 20 s at room temperature, sonicated for 10 minutes, and centrifuged for 5 minutes at 14,000 rpm. The supernatant was filtered (0.45 µm PTFE filters) and analyzed.
Extraction with n-hexane: The extraction procedure was based on Feliciano et al.[Citation9] with few modifications. Fresh apple pulp was rapidly frozen in liquid nitrogen, homogenized using an analytical mill, and 2 g were extracted twice with n-hexane (3 + 2 mL) containing 0.1% BHT. After each step, extracts were centrifuged at 8,000 rpm g for 5 min. The combined organic layers were adjusted to 5 mL with n-hexane. 1 mL of resulting solution was filtered (0.45 µm PTFE filters), evaporated to dryness, and resuspended in 0.5 mL of methanol before analysis.
Extraction with saponification: The procedure of Chun et al.[Citation45] was used, with further modifications. Briefly, fresh apple pulp was rapidly frozen in liquid nitrogen, homogenized using an analytical mill. 5 g of apple pulp were suspended in 20 mL of ethanol containing 10% AsA. The slurry was agitated and sonicated for 10 min at room temperature, transferred in a round-bottom flask, and 5 mL of a freshly prepared 60% aqueous potassium hydroxide solution was added. The flask was flushed with nitrogen gas for 1 min and heated to reflux for 30 min. The mixture was allowed to cool to room temperature, diluted with 20 mL of a 2% aqueous sodium chloride, solution and extracted three times with 10 mL n-hexane:ethyl acetate 85:15 v/v containing 0.05% BHT. The volume of the combined organic layers was adjusted to 50 mL with the organic solvent mixture. 1.5 mL of the resulting extract was filtered (0.45 µm PTFE filters), evaporated to dryness and resuspended in 0.3 mL of methanol before analysis.
HPLC-DAD analysis: Column temperature was set at 45°C, the detection wavelength was 300 nm and the flow rate 1.5 mL/min. The autosampler temperature was kept at 4°C and injection volume was 30 µL. The mobile phases consisted of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). An isocratic elution was used with 98% B and 2% A for 7 minutes. α-tocopherol retention time was 4.5 minutes. As for AsA, the α-tocopherol content was expressed as mg/100 g FW.
Method validation for extraction with methanol: The method was validated for linearity, precision (intra-day and inter-day), recovery, and sensitivity (limit of detection, LOD, and limit of quantification, LOQ). A stock solution of α-tocopherol pure standard was prepared in methanol and used for further dilutions. Each one of the following calibration points was injected in triplicate: 0.25, 0.5, 1.0, 3.0, 5.0, 10.0 µg/mL. Intra-day precision was determined based on the relative standard deviation (RSD) between the α-tocopherol amount found in five technical replicates and inter-day precision was calculated in the same way over three consecutive days. Recovery was determined in two apple cultivars: ‘Cellini’ with a low α-tocopherol content (0.16 ± 0.03 mg/100 g FW), and ‘Brixner Plattling’ with a high α-tocopherol content (0.33 ± 0.04 mg/100 g FW). Three replicates for each sample were spiked at three different concentration levels and the recovery was calculated as the ratio between the amount found in the spiked sample (expressed as mg/100 g FW) and the sum of the value found in the non-spiked sample and the theoretical spiked amount (both expressed as mg/100 g FW). LOD and LOQ were calculated based on the standard deviation on 10 injections of the lowest calibration point (0.25 µg/mL) and the slope of the calibration curve.
Data analysis and statistics
Content of AsA and α-tocopherol was calculated from the standard calibration curve in 10 biological replicates for each cultivar. For the statistical analysis Microsoft® Excel® 2016 Version 16.0 and IBM® SPSS® Statistics Version 24 were used. A one-way analysis of variance (ANOVA) was applied to highlight statistical differences among cultivars and the effect of the harvest year on each cultivar. The posthoc Tukey HSD (Honest Significant Difference) test was used for pairwise comparisons. Nonparametric tests (Kruskal−Wallis) were applied whenever the groups included in the comparison did not have an equal number of samples or conditions to variance homogeneity were not satisfied. A two-way ANOVA was performed to examine the influence of the cultivar and of the harvest year on the content of vitamins, as well as the interaction between these two variables.
Results and discussion
Comparison of three extraction procedures for α-tocopherol
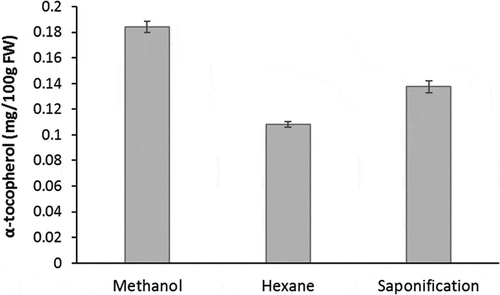
In the present work, three extraction procedures were tested: The first was based on a methanolic extraction of freeze-dried apple pulp, the second was an extraction with n-hexane of fresh apple pulp, and the third involved saponification of fresh material under alkaline conditions. The fresh pulp of three apples (‘Cripps Pink/Pink Lady®’) was homogenized and divided into three equal parts. One aliquot was freeze dried for subsequent extraction with methanol and the others were used as such. Each extraction was performed in triplicate. The direct extraction from freeze-dried apple powder with methanol led to higher yields than the extraction with n-hexane or with saponification (), confirming the positive effect of the freeze-drying on the stability of tocopherols reported by Knecht et al.[Citation49] In addition, the one-step was probably less prone to substance loss during extractions. Moreover, with water being removed, methanol proved to be sufficiently apolar for an efficient extraction of this lipophilic vitamin and was therefore chosen for the subsequent experiments.
Quantification of AsA and α-tocopherol in apple pulp
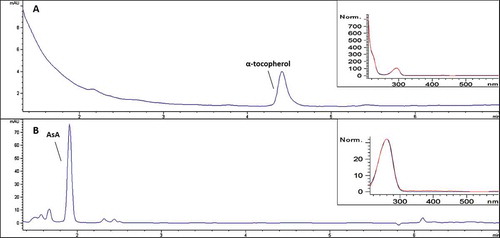
Two rapid, robust, and efficient HPLC-DAD methods were used to analyze AsA and α-tocopherol, respectively. The selectivity of the two methods was verified by comparing the retention time and UV/VIS spectra of the analyte peaks with those of a standard solution of AsA and α-tocopherol, respectively (). The HPLC-DAD method for α-tocopherol was validated with satisfactory results: the correlation coefficient (r2) of the calibration curve was 0.99, RSD between 5 sample replicates for the intra-day precision was 1.5%, while for the inter-day precision over three days did not exceed 2.0%. Recovery was between 94% and 103% (Table S1), on average 98% (). Validation parameters and results are summarized in , and detailed results of the recovery are reported in the supplementary information (Table S1). The performance of our HPLC-DAD method is in line with previously reported methods.[Citation48,Citation62,Citation63]
Table 1. Validation parameters of the HPLC-DAD method for the determination of α-tocopherol in apple pulp.
Vitamin content in apple pulp in 24 different varieties from the 2010 season
Twenty-four ‘true to type’ apple cultivars form the Laimburg varietal collection were harvested in the 2010 season. Current commercial cultivars, including the two most cultivated ones in South Tyrol (‘Golden Delicious’ and ‘Gala’), which account for more than 90% of the current South Tyrolean apple production,[Citation64] were selected along with old cultivars, including three old cultivars from South Tyrol (‘Brixner Plattling,’ ‘Boznerapfel,’ and ‘Weißer Rosmarin’). In addition, two pre-commercial hybrids from the Laimburg Research Center breeding program (Lb 04852 ‘Elstar’ x ‘Braeburn,’ and Lb 05090 ‘Cripps Pink’ x ‘Gala’) were included to obtain a diverse sample set. The average vitamin content of 10 apples per cultivar is given in , while box plots are reported in . The vitamin content ranged from a minimum of 0.43 ± 0.13 mg/100 g FW (‘Gala’) to a maximum of 6.22 ± 1.06 mg/100 g FW (‘Freiherr von Berlepsch’) for AsA, and from 0.13 ± 0.02 mg/100 g FW (‘Freiherr von Berlepsch’) to 0.33 ± 0.04 mg/100 g FW (‘Brixner Plattling’) for α-tocopherol, respectively. Average values of 2.86 mg/100 g FW for AsA and 0.25 mg/100 g for α-tocopherol were found among the 24 cultivars (). The RSD of both vitamins in 10 apples from the same cultivar was determined to estimate the biological variability. The RSD of AsA was found to be generally higher than the one of α-tocopherol. A minimum RSD of 8.4% (‘Golden Delicious’) and a maximum of 55% (‘Steinpepping’) were found for AsA; for α-tocopherol the RSD ranged from 3.3% (‘Champagner Renette’) to 18% (‘Cellini’) (). The high biological variability of AsA content is well documented: AsA has been shown to degrade after harvest and during storage.[Citation65,Citation66] Similarly, ripening, harvest date, and agronomical factors were linked to the variability of tocopherol.[Citation67] Bassi et al[Citation2] noted AsA variability in apple peel and pulp even though the same cultivation, storage, and sampling conditions were used for all cultivars. Therefore, we concluded that the biological variability noted in this and the other cited studies reflects the cultivar-specific content and stability of AsA in response to the external factors. Statistically relevant differences among the 24 cultivars harvested in 2010 were found for both AsA and α-tocopherol (p < .001). Three cultivars, two old ones, ‘Freiherr von Berlepsch’ and ‘Schweizer Orangenapfel,’ and Lb 04852 (‘Braeburn’ x ‘Elstar’), contained the highest average AsA amount with 6.22 ± 1.06, 6.07 ± 1.45, and 5.68 ± 0.94 mg/100 g FW, respectively. The pulp of one medium-sized apple (ca. 200 g) of one of these cultivars, covers approximately 13% of the recommended daily intake (RDA) of vitamin C of an adult man.[Citation68] The old cultivar ‘Freiherr von Berlepsch’ is known to be aromatic, suitable for raw consumption, and to maintain its organoleptic properties during months of storage. In addition, it is reported as a vitamin C-rich cultivar, confirming our results.[Citation69,Citation70] ‘Schweizer Orangenapfel,’ also described as a high-vitamin C variety,[Citation71] is a cross between ‘Ontarioapfel’ and ‘Cox Orangenrenette,’ a reportedly vitamin C-rich cultivar.[Citation69,Citation72] The Laimburg hybrid was obtained by crossing two commercial cultivars: ‘Braeburn,’ which is also known to be rich in vitamin C,[Citation73,Citation74] and ‘Elstar.’ According to Bassi et al.[Citation2] a high AsA content was found in other three commercial cultivars, ‘Cripps Pink/Pink Lady®,’ ‘Nicoter/Kanzi®,’ and ‘Scifresh/Jazz®,’ indicating their high nutritional value.
Table 2. Average content of L-ascorbic acid (AsA) and α-tocopherol (mg/100 g FW) in apple pulp and relative standard deviation (RSD in %) between the amount found in 10 apple samples for each of 24 cultivars from season 2010.
Figure 3. Box plots of the L-ascorbic acid (AsA) (a) and α-tocopherol (b) content (expressed as mg/100 g FW) in 24 apple cultivars from the 2010 season. Median values are reported (n = 10).
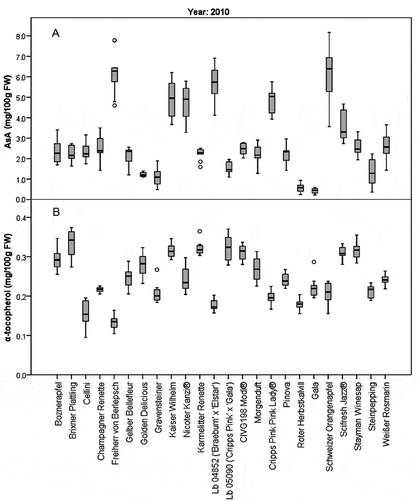
The average content of α-tocopherol (0.25 mg/100 g FW, ) was five times higher than the one reported by the USDA,[Citation13] but is consistent with other studies.[Citation45,Citation46] The RDA of α-tocopherol for both men and women is 15 mg/day.[Citation75] Considering the average amount of 0.25 mg/100 g FW found in the 24 cultivars, the pulp of one medium-sized apple (ca. 200 g) covers approximately 3.3% of the RDA.
Considering α-tocopherol, even though significant differences among the 24 varieties were found (p < .001), the variation range was much smaller compared to AsA (). Nine cultivars are notable for their high α-tocopherol content: ‘Brixner Plattling,’ ‘Karmelitter Renette,’ ‘Stayman Winesap,’ ‘Kaiser Wilhelm,’ Lb 05090 (‘Gala’ x ‘Cripps Pink’), ‘CIVG 198 Modí®,’ ‘Scifresh Jazz®,’ ‘Boznerapfel,’ ‘Golden Delicious’ ().
AsA and α-tocopherol content in apple pulp over three consecutive harvest years
The dataset included six old and commercial cultivars harvested over three consecutive years (2008, 2009, and 2010): the currently (2020) two most cultivated apple varieties in South Tyrol (‘Golden Delicious’ and ‘Gala’), the currently (2020) most cultivated managed variety (‘Cripps Pink/Pink Lady®’), and three old ones, including an autochthonous one (‘Gelber Bellefleur’, ‘Champagner Renette’ and the local ‘Weißer Rosmarin’).
The results of the two-way ANOVA are shown in , while shows a variable AsA and α-tocopherol content in the pulp over the three years. Average values of 10 biological replicates for each cultivar and year are reported in Table S2. The two-way ANOVA was performed to evaluate the influence of the cultivar and the harvest year on the content of vitamins. A significant effect of the cultivar and the harvest year on the content of both vitamins was observed (). According to the results, the content of AsA and α-tocopherol is a result of the interaction between cultivar and harvest year ().
Table 3. Two-way analysis of variance (ANOVA) for the effects of the cultivar and the harvest year on the content of L-ascorbic acid (AsA) and α-tocopherol.
Figure 4. Box plots of the L-ascorbic acid (AsA) (a) and α-tocopherol (b) content (expressed as mg/100 g FW) in six varieties harvested over three consecutive seasons (2008 to 2010). Median values are reported (n = 10). Significant differences between the content of vitamins in the apple pulp of the same cultivar collected over three years are indicated by different letters (p < .05).
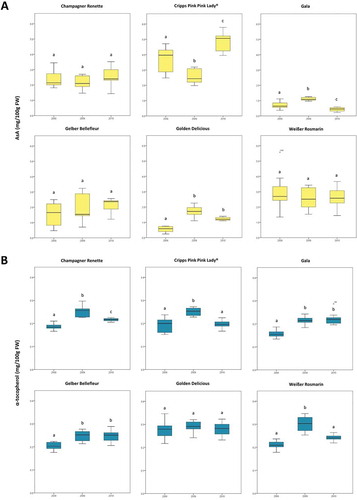
Some cultivars were found to be more affected by annual variability than others. Considering AsA, the three old cultivars ‘Champagner Renette,’ ‘Gelber Bellefleur,’ and ‘Weißer Rosmarin’ were least affected from annual variation, with no significant differences between the three harvest seasons (p > .05). The commercial cultivars (‘Golden Delicious,’ ‘Gala,’ and ‘Cripps Pink/Pink Lady®’), instead, showed statistically significant variations (p < .001). For example, the average AsA in ‘Cripps Pink Pink Lady®’ for 2008, 2009, and 2010 was 3.70, 2.60, and 4.90 mg/100 g FW, respectively, whereas the AsA contents in ‘Weißer Rosmarin’ were 2.92, 2.54, and 2.64 mg/100 g FW, respectively (Table S2). When comparing α-tocopherol contents, we noted generally less variable values between cultivars and years, as already observed for the 24 cultivars of 2010 ( and ). Unlike in the case of AsA, the influence of the harvest year 2008, 2009, and 2010 was significant for the three old cultivars ‘Champagner Renette,’ ‘Gelber Bellefleur,’ and ‘Weißer Rosmarin’ (p < .001). Statistical differences were also found for the commercial varieties ‘Gala’ and ‘Cripps Pink/Pink Lady®’ (p < .001), while no statistically significant annual effects (p = .57) were found in the commercial variety ‘Golden Delicious,’ with average values of 0.27, 0.29, and 0.28 mg/100 g FW for 2008, 2009, and 2010, respectively (Table S2). In accordance with previous reports on other quality parameters, including firmness, weight, acidity, total soluble solids, and dietary elements like nitrogen, phosphorus, potassium, a cultivar-dependent variation rather than a common trend was observed.[Citation1] We conclude that cultivars react differently to the external stimuli known to play a role in the final chemical composition of the ripe fruit like environmental and agricultural conditions.[Citation76–Citation78] Despite significant biological and inter-annual variability, cultivar-specific relative vitamin contents can be extracted from this study. The ranking of the AsA contents, for instance, remained constant from 2008 to 2010, with few exceptions (), confirming a cultivar-specific reaction to the environmental conditions in the three seasons. Similarly, the relative α-tocopherol contents showed a common pattern among the six cultivars, even though the small differences affect the ranking in some cases ().
Conclusion
The present study investigated two of the major apple vitamins, AsA and α-tocopherol, in the pulp of 24 accurately identified cultivars and three harvest seasons. Mild extraction conditions and rapid HPLC-DAD methods with excellent recovery, linearity, precision, and sensitivity enabled an efficient detection of the vitamins in all samples. Freeze-drying was found to be useful to avoid further extraction and concentration steps during sample preparation. Despite a high biological variation, some cultivars stood out for high vitamin contents, in particular for AsA. The vitamin C-rich ‘Freiherr von Berlepsch’, ‘Schweizer Orangenapfel’, ‘Kaiser Wilhelm’, ‘Nicoter Kanzi®’ as well as Lb 04852 (‘Braeburn’ x ‘Elstar’) could be interesting for a healthy diet or future crosses in breeding programs, highlighting the nutritional value of old cultivars and new varieties. This work adds valuable data to nutritional databases and previous studies on the chemical composition of apples. Importantly, all trees were grown on the same site and accurately characterized by molecular genetic methods. More work is required to describe the chemodiversity of apple genotypes and to dissect the complex interplay between them, the environment, and ultimately, the human microbiome. This research may lead to a deeper understanding of the nutritional value of fruit in general.
Supplemental Material
Download MS Word (18.9 KB)Acknowledgments
Josef Dalla Via, Sanja Baric, Alberto Storti, Irene Höller and Priska Steger are gratefully acknowledged for their contribution in the project “APFEL-FIT,” for helpful discussions and technical assistance (I.H. and P.S.).
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Agnolet, S.; Ciesa, F.; Soini, E.; Cassar, A.; Matteazzi, A.; Guerra, W.; Robatscher, P.; Storti, A.; Baric, S.; Dalla Via, J.; et al. Dietary Elements and Quality Parameters of 34 Old and Eight Commercial Apple Cultivars Grown at the Same Site in South Tyrol, Italy. Erwerbs-Obstbau. 2017, 59, 171–183.
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, S.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic Acid Content in Apple Pulp, Peel, and Monovarietal Cloudy Juices of 64 Different Cultivars. Int. J. Food Prop. 2018, 20(sup3), 2626–2634. DOI: 10.1080/10942912.2017.1381705.
- Boyer, J.; Liu, R. H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3(1), 5. DOI: 10.1186/1475-2891-3-5.
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a Source of Dietary Phytonutrients: Bioavailability and Evidence of Protective Effects against Human Cardiovascular Disease. Food Nutr. Sci. 2014, 5(13), 1234–1246.
- Hyson, D. A.;. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2(5), 408–420. DOI: 10.3945/an.111.000513.
- Jensen, E. N.; Buch-Andersen, T.; Ravn-Haren, G.; Dragsted, L. O. Mini-review: The Effects of Apples on Plasma Cholesterol Levels and Cardiovascular Risk - a Review of the Evidence. J. Horticultural Sci. Biotechnol. 2009, 84(6), 34–41. DOI: 10.1080/14620316.2009.11512592.
- Ciesa, F.; Höller, I.; Guerra, W.; Berger, J.; Dalla Via, J.; Oberhuber, M. Chemodiversity in the fingerprint analysis of volatile organic compounds (VOCs) of 35 old and 7 modern apple cultivars determined by proton-transfer-reaction mass spectrometry (PTR-MS) in two different seasons. Chem. Biodivers. 2015, 12, 800–812. DOI: 10.1002/cbdv.201400384.
- Dalla Via, J.; Mantinger, H. Agricultural Research in the Field of Fruit Growing in South Tyrol. Erwerb. Obstbau. 2012, 54(3), 83–115. DOI: 10.1007/s10341-012-0171-x.
- Feliciano, R. P.; Antunes, C.; Ramos, A.; Serra, A. T.; Figueira, M. E.; Duarte, C. M.; Carvalho, A. D.; Bronze, M. R. Characterization of Traditional and Exotic Apple Varieties from Portugal. Part 1 – Nutritional, Phytochemical and Sensory Evaluation. J. Funct. Foods. 2010, 2(1), 35–45. DOI: 10.1016/j.jff.2009.12.004.
- Décordé, K.; Teissèdre, P. L.; Auger, C.; Cristol, J.-P.; Rouanet, J.-M. Phenolics from Purple Grape, Apple, Purple Grape Juice and Apple Juice Prevent Early Atherosclerosis Induced by an Atherogenic Diet in Hamsters. Mol. Nutr. Food Res. 2008, 52(4), 400–407. DOI: 10.1002/mnfr.200700141.
- Conceição de Oliveira, M.; Sichieri, R.; Sanchez Moura, A. Weight Loss Associated with a Daily Intake of Three Apples or Three Pears among Overweight Women. Nutrition. 2003, 19(3), 253–256. DOI: 10.1016/S0899-9007(02)00850-X.
- Ferguson, L. R.; Philpott, M.; Karunasinghe, N. Dietary Cancer and Prevention Using Antimutagens. Toxicology. 2004, 198(1–3), 147–159. DOI: 10.1016/j.tox.2004.01.035.
- U.S. Department of Agriculture, Agricultural Research Service, 2016. USDA National Nutrient Database for Standard Reference Legacy Release, April 2018. https://ndb.nal.usda.gov/ndb/search/list. (Accessed April 03, 2019).
- Wang, X.;. Vitamin E and Its Function in Membranes. Prog. Lipid Res. 1999, 38(4), 309–336. DOI: 10.1016/S0163-7827(99)00008-9.
- Ulatowski, L. M.; Manor, D. Vitamin E and Neurodegeneration. Neurobiol. Disease. 2015, 84, 78–83.
- Amiri, M. E.; Fallahi, E.; Safi-Songhorabad, M. Influence of Rootstock on Mineral Uptake and Scion Growth of ‘Golden Delicious’ and ‘Royal Gala’ Apples. J. Plant Nutr. 2014, 37(1), 16–29. DOI: 10.1080/01904167.2013.792838.
- Ciesa, F.; Dalla Via, J.; Wisthaler, A.; Zanella, A.; Guerra, W.; Mikoviny, T.; Märk, T. D.; Oberhuber, M. Discrimination of Four Different Postharvest Treatments of ‘Red Delicious’ Apples Based on Their Volatile Organic Compound (VOC) Emissions during Shelf-life Measured by Proton Transfer Reaction Mass Spectrometry (PTRMS). Postharvest. Biol. Technol. 2013, 86, 329–336. DOI: 10.1016/j.postharvbio.2013.06.036.
- Dal Cin, V.; Danesin, M.; Botton, A.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene and Preharvest Drop: The Effect of AVG and NAA on Fruit Abscission in Apple (Malus Domestica L Borkh).. Plant Growth Regul. 2008, 56(3), 317–325. DOI: 10.1007/s10725-008-9312-5.
- Drogoudi, P. D.; Pantelidis, G. Effects of Position on Canopy and Harvest Time on Fruit Physico-chemical and Antioxidant Properties in Different Apple Cultivars. Sci. Hortic. 2011, 129(4), 752–760. DOI: 10.1016/j.scienta.2011.05.036.
- Durrani, Y.; Ayub, M.; Muhammad, A.; Ali, A. Physicochemical Response of Apple Pulp to Chemical Preservatives and Antioxidant during Storage. Internet. J. Food Saf. 2010, 12, 20–28.
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determination of Antioxidant Capacity, Total Phenolic Content and Mineral Composition of Different Fruit Tissue of Five Apple Cultivars Grown in Chile. Chil. J. Agric. Res. 2010, 70(4), 523–536. DOI: 10.4067/S0718-58392010000400001.
- Moor, U.; Karp, K.; Põldma, P.; Asafova, L.; Starast, M. Post-harvest Disorders and Mineral Composition of Apple Fruits as Affected by Pre-harvest Calcium Treatments. Acta Agric. Scand B Soil Plant Sci. 2006, 56(3), 179–185.
- Stopar, M.; Bolcina, U.; Vanzo, A.; Vrhovsek, U. Lower Crop Load for Cv. Jonagold Apples (Malus X Domestica Borkh.) Increases Polyphenol Content and Fruit Quality. J. Agric. Food Chem. 2002, 50(6), 1643–1646. DOI: 10.1021/jf011018b.
- Szalay, L.; Ordidge, M.; Ficzek, G.; Hadley, P.; Tóth, M.; Battey, N. H. Grouping of 24 Apple Cultivars on the Basis of Starch Degradation Rate and Their Fruit Pattern. Hortic. Sci. 2013, 40(3), 93–101. DOI: 10.17221/143/2012-HORTSCI.
- Veberic, R.; Schmitzer, V.; Petkovsek, M. M.; Stampar, F. Impact of Shelf Life on Content of Primary and Secondary Metabolites in Apple (Malus Domestica Borkh.). J. Food Sci. 2010, 75(9), 461–468. DOI: 10.1111/j.1750-3841.2010.01823.x.
- Jiang, H.; Ji, B.; Liang, J.; Zhou, F.; Yang, Z.; Zhang, G. Changes of Contents and Antioxidant Activities of Polyphenols during Fruit Development of Four Apple Cultivars. Eur. Food Res. Technol. 2006, 223(6), 743–748. DOI: 10.1007/s00217-006-0262-8.
- Kumar, P.; Sethi, S.; Sharma, R. R.; Singh, S.; Saha, S.; Sharma, V. K.; Verma, M. K.; Sharma, S. K. Nutritional Characterization of Apple as a Function of Genotype. J. Food Sci. Technol. 2018, 55(7), 2729–2738. DOI: 10.1007/s13197-018-3195-x.
- Łata, B.; Przeradzka, M.; Binkowska, M. Great Differences in Antioxidant Properties Exist between 56 Apple Cultivars and Vegetation Seasons. J. Agric. Food Chem. 2005, 53(23), 8970–8978. DOI: 10.1021/jf051503x.
- Planchon, V.; Lateur, M.; Dupont, P.; Lognay, G. Ascorbic Acid Level of Belgian Apple Genetic Resources. Sci. Hortic. 2004, 100(1), 51–61. DOI: 10.1016/j.scienta.2003.08.003.
- Van Der Sluis, A. A.; Dekker, M.; De Jager, A.; Jongen, W. M. Activity and Concentration of Polyphenolic Antioxidants in Apple: Effect of Cultivar, Harvest Year, and Storage Conditions. J. Agric. Food Chem. 2001, 49(8), 3606–3613. DOI: 10.1021/jf001493u.
- Varming, C.; Petersen, M. A.; Toldam-Andersen, T. B. Ascorbic Acid Contents in Danish Apple Cultivars and Commercial Apple Juices. LWT-Food Sci. Technol. 2013, 54(2), 597–599. DOI: 10.1016/j.lwt.2013.06.024.
- Baric, S.; Wagner, J.; Storti, A.; Dalla Via, J. Application of an Extended Set of Microsatellite DNA Markers for the Analysis of Presumed Synonym Cultivars of Apple. Acta Hortic. 2011, 918(918), 303–308. DOI: 10.17660/ActaHortic.2011.918.38.
- Łata, B.; Przeradzka, M.; Bińkowska, M. Great Differences in Antioxidant Properties Exist between 56 Apple Cultivars and Vegetation Seasons. J. Agric. Food Chem. 2005, 53(23), 8970–8978. DOI: 10.1021/jf051503x.
- Davey, M. W.; Keulemans, J. Nutritional Enhancement in Apple: Identification of QTL for Fruit Vitamin C Concentration and Their Stability over Different Production Years. Acta Hortic. 2009, 814(814), 591–598. DOI: 10.17660/ActaHortic.2009.814.100.
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of Biostimulants for Organic Apple Production: Effects on Tree Growth, Yield, and Fruit Quality at Harvest and during Storage. Front. Plant Sci. 2018, 9, 1342. DOI: 10.3389/fpls.2018.01342.
- Altisent, R.; Plaza, L.; Alegre, I.; Viñas, I.; Abadias, M. Comparative Study of Improved Vs. Traditional Apple Cultivars and Their Aptitude to Be Minimally Processed as ‘Ready to Eat’ Apple Wedges. LWT. Food Sci. Technol. 2014, 58, 541–549.
- Assunção, B. R.; Mercadante, A. Carotenoids and Ascorbic Acid Composition from Commercial Products of Cashew Apple (Anacardium Occidentale L.). J. Food Compost. Anal. 2003, 16(6), 647–657. DOI: 10.1016/S0889-1575(03)00098-X.
- Felicetti, E.; Mattheis, J. P. Quantification and Histochemical Localization of Ascorbic Acid in ‘Delicious,’ ‘Golden Delicious,’ and ‘Fuji’ Apple Fruit during On-tree Development and Cold Storage. Postharvest. Biol. Technol. 2010, 56(1), 56–63. DOI: 10.1016/j.postharvbio.2009.12.002.
- Nováková, L.; Solich, P.; Solichová, D. HPLC Methods for Simultaneous Determination of Ascorbic and Dehydroascorbic Acids. TrAC Trends Anal. Chem. 2008, 27(10), 942–958. DOI: 10.1016/j.trac.2008.08.006.
- Abe-Matsumoto, L. T.; Sampaio, G. R.; Bastos, D. H. M. Is Titration as Accurate as HPLC for Determination of Vitamin C in Supplements? Titration versus HPLC for Vitamin C Analysis. Am. J. Anal. Chem. 2020, 11(7), 269–279. DOI: 10.4236/ajac.2020.117021.
- Sarker, U.; Oba, S. Antioxidant Constituents of Three Selected Red and Green Color Amaranthus Leafy Vegetable. Sci. Rep. 2019, 9(1), 18233. DOI: 10.1038/s41598-019-52033-8.
- Quirós, A. R.-B.; Fernández-Arias, M.; López-Hernández, J. A. Screening Method for the Determination of Ascorbic Acid in Fruit Juices and Soft Drinks. Food Chem. 2009, 116(2), 509–512. DOI: 10.1016/j.foodchem.2009.03.013.
- Fatin Najwa, R.; Azrina, A. Comparison of Vitamin C Content in Citrus Fruits by Titration and High Performance Liquid Chromatography (HPLC) Methods. Int. Food Res. J. 2017, 24(2), 726–733.
- Spínola, V.; Llorent-Martínez, E. J.; Castilho, P. C. Determination of Vitamin C in Foods: Current State of Method Validation. J. Chromatogr. A. 2014, 1369, 2–17. DOI: 10.1016/j.chroma.2014.09.087.
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R. R. Tocopherol and Tocotrienol Contents of Raw and Processed Fruits and Vegetables in the United States Diet. J. Food Compost. Anal. 2006, 19(2–3), 196–204. DOI: 10.1016/j.jfca.2005.08.001.
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, Lycopene, and Alpha-tocopherol Contents of Selected Thai Fruits. Food Chem. 2009, 113(1), 202–207. DOI: 10.1016/j.foodchem.2008.07.074.
- Gentili, A.; Caretti, F. Evaluation of a Method Based on Liquid Chromatography-diode Array Detector-tandem Mass Spectrometry for a Rapid and Comprehensive Characterization of the Fat-soluble Vitamin and Carotenoid Profile of Selected Plant Foods. J. Chromatogr. A. 2011, 1218(5), 684–697. DOI: 10.1016/j.chroma.2010.12.001.
- Irakli, M. N.; Samanidou, V. F.; Papadoyannis, I. N. Development and Validation of an HPLC Method for the Simultaneous Determination of Tocopherols, Tocotrienols and Carotenoids in Cereals after Solid-phase Extraction. J. Sep. Sci. 2011, 34(12), 1375–1382. DOI: 10.1002/jssc.201100077.
- Knecht, K.; Sandfuchs, K.; Kulling, S. E.; Bunzel, D. Tocopherol and Tocotrienol Analysis in Raw and Cooked Vegetables: A Validated Method with Emphasis on Sample Preparation. Food Chem. 2015, 169, 20–27. DOI: 10.1016/j.foodchem.2014.07.099.
- Luque-Garcı́a, J. L.; Luque de Castro, M. D. Extraction of Fat-soluble Vitamins. J. Chromatogr. A. 2001, 935(1–2), 3–11. DOI: 10.1016/S0021-9673(01)01118-9.
- Quek S. Y.; Chu B. S.; Baharin, B.; Preedy V. R.; Watson R. R. (Eds.), The Encyclopedia of Vitamin E, CAB International, Wallingford (2007), 140–152. https://www.researchgate.net/publication/288516996_Commercial_extraction_of_vitamin_E_from_food_sources.
- Köseoğlu, K.; Ulusoy, H. İ.; Yilmaz, E.; Soylak, M. Simple and Sensitive Determination of Vitamin A and E in the Milk and Egg Yolk Samples by Using Dispersive Solid Phase Extraction with Newly Synthesized Polymeric Material. J. Food Compost. Anal. 2020, 90, 103482. DOI: 10.1016/j.jfca.2020.103482.
- Gîrd, C. E.; Nencu, I.; Duţu, L. E.; Popescu, M. L.; Costea, T.; Neagu, A. F. HPLC Evaluation of the Ascorbic Acid Content of Romanian Fruits and Vegetables from Retail Markets. Farmacia. 2018, 66(5), 5. DOI: 10.31925/farmacia.2018.5.21.
- Datta, S.; Sinha, B. K.; Bhattacharjee, S.; Seal, T. Nutritional Composition, Mineral Content, Antioxidant Activity and Quantitative Estimation of Water Soluble Vitamins and Phenolics by RP-HPLC in Some Lesser Used Wild Edible Plants. Heliyon. 2019, 5(3), e01431. DOI: 10.1016/j.heliyon.2019.e01431.
- Bakar, B.; Çakmak, M.; Ibrahim, M. S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of Amounts of Vitamins, Lycopene, and Elements in the Fruits of Opuntia Ficus-indica Subjected to Different Pretreatments. Biol. Trace Elem. Res. 2020. DOI: 10.1007/s12011-020-02050-w.
- Schmidt, H. O.; Rockett, F. C.; Pagno, C. H.; Possa, J.; Assis, R. Q.; de Oliveira, V. R.; da Silva, V. L.; Flôres, S. H.; de Oliveira Rios, A. Vitamines, Bioactive Compounds Diversity of Seven Fruit Species from South Brazil. J. Sci. Food Agric. 2019, 99(7), 3307–3317. DOI: 10.1002/jsfa.9544.
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, L.; Mišurcová, L. Impact of Phenolic Compounds and Vitamins C and E on Antioxidant Activity of Sea Buckthorn (Hippophaë Rhamnoides L.) Berries and Leaves of Diverse Ripening Times. Food Chem. 2020, 310, 125784. DOI: 10.1016/j.foodchem.2019.125784.
- Baric, S.; Monschein, S.; Hofer, M.; Grill, D.; Dalla Via, J. Comparability of Genotyping Data Obtained by Different Procedures an Inter-laboratory Survey. J. Hortic. Sci. Biotechnol. 2008, 83(2), 183–190. DOI: 10.1080/14620316.2008.11512368.
- Baric, S.; Storti, A.; Hofer, M.; Dalla Via, J. Molecular Genetic Characterization of Apple Cultivars from Different Germplasm Collections. Acta Hortic. 2009, 817(817), 347–353. DOI: 10.17660/ActaHortic.2009.817.37.
- Storti, A.; Dalla Via, J.; Baric, S. Comparative Molecular Genetic Analysis of Apple Genotypes Maintained in Germplasm Collections. Erwerb. Obstbau. 2012, 54(3), 137–141. DOI: 10.1007/s10341-012-0168-5.
- King, G. J.; Lynn, J. R.; Dover, C. J.; Evans, K. M.; Seymour, G. B. Resolution of Quantitative Trait Loci for Mechanical Measures Accounting for Genetic Variation in Fruit Texture of Apple (Malus Pumila Mill.). TAG Theoretical Appl. Gene. 2001, 102(8), 1227–1235. DOI: 10.1007/s001220000530.
- Gong, X.; Qi, N.; Wang, X.; Li, J.; Lin, L. A New Method for Determination of α-Tocopherol in Tropical Fruits by Ultra Performance Convergence Chromatography with Diode Array Detector. Food Anal. Methods. 2014, 7(8), 1572–1576. DOI: 10.1007/s12161-014-9789-7.
- Stinco, C. M.; Benítez-González, A. M.; Hernanz, D.; Vicario, I. M.; Meléndez-Martínez, A. J. Development and Validation of a Rapid Resolution Liquid Chromatography Method for the Screening of Dietary Plant Isoprenoids: Carotenoids, Tocopherols and Chlorophylls. J. Chromatography. A. 2014, 1370, 162–170. DOI: 10.1016/j.chroma.2014.10.044.
- Serni, E.; Venir, E.; Romano, G.; Guerra, W.; Robatscher, P. Determination of Major Phenolics Content in Dried Apples from Three New Cultivars (Malus Domestica Borkh.) Using HPLC-UV-FL with Pentafluorophenyl Stationary Phase. Food Anal. Methods. 2020, 13(4), 863–871. DOI: 10.1007/s12161-020-01703-9.
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J.-O.; Dommes, J. Influence of Cultivar, Harvest Time, Storage Conditions, and Peeling on the Antioxidant Capacity and Phenolic and Ascorbic Acid Contents of Apples and Pears. J. Agric. Food Chem. 2011, 59(11), 6165–6171. DOI: 10.1021/jf201013k.
- Łata, B.;. Relationship between Apple Peel and the Whole Fruit Antioxidant Content: Year and Cultivar Variation. J. Agric. Food Chem. 2007, 55(3), 663–671. DOI: 10.1021/jf062664j.
- Beltrán, G.; Jiménez, A.; Del Rio, C.; Sánchez, S.; Martínez, L.; Uceda, M.; Aguilera, M. P. Variability of Vitamin E in Virgin Olive Oil by Agronomical and Genetic Factors. J. Food Compost. Anal. 2010, 23(6), 633–639. DOI: 10.1016/j.jfca.2010.03.003.
- Otten, J. J.; Hellwig, J. P.; Meyers, L. D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, D.C., 2006.
- Hartmann, W.;. Farbatlas Alte Obstsorten, 5th ed.; Eugen Ulmer press: Stuttgart, Germany, 2015.
- Koloc, R.;. Wir zeigen Apfelsorten und werten deren Eigenschaften 5thNeumann,, Editor, 1972. Leipzig, Germany: Neumann Verlag.
- Mozafar, A. Plant Vitamins. Boca Raton, Florida, United States: CRC Press, 2018.
- Petzold, H.;. Apfelsorten; Neumann press: Radebeul, 1990.
- Davey, M. W.; Auwerkerken, A.; Keulemans, J. Relationship of Apple Vitamin C and Antioxidant Contents to Harvest Date and Postharvest Pathogen Infection. J. Sci. Food Agric. 2007, 87(5), 802–813. DOI: 10.1002/jsfa.2777.
- Powell, R. S.;. Apples of New England: A User’s Guide, First cloth ed.; The Countryman Press: Woodstock, Vermont, 2014.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds; National Academy Press: Washington, D.C., 2000.
- Barritt, B. H.; Konishi, B. S.; Drake, S. R.; Rom, C. R. Influence of Sunlight Level and Rootstock on Apple Fruit Quality. Acta Hortic. 1997, 451(451), 569–578. DOI: 10.17660/ActaHortic.1997.451.66.
- Sharples, R. O.;. The Influence of Orchard Nutrition on the Storage Quality of Apples and Pears Grown in the United Kingdom. Acta Hortic. 1980, 92(92), 17–28. DOI: 10.17660/ActaHortic.1980.92.3.
- Wagenmakers, P. S.; Callesen, O. Influence of Light Interception on Apple Yield and Fruit Quality Related to Arrangement and Tree Height. Acta Hortic. 1989, 243(243), 149–158. DOI: 10.17660/ActaHortic.1989.243.19.