ABSTRACT
Several articles have shown a positive effect of Inulin Type-Carbohydrates (ITC) on blood pressure (BP); nevertheless, these findings are controversial. Therefore, a systematic review and meta-analysis of randomized-controlled trials (RCTs) proposed to investigate the effects of ITC supplementation on BP. An online search was carried out in PubMed, Scopus, ISI Web of Science, Cochrane library, and Google Scholar up to December 2019. Weighted Mean difference (WMD) with 95% confidence intervals (CIs) were calculated using a Random-effects model. Statistical heterogeneity was evaluated using Q tests and the I2 statistic. Subgroup analysis was accomplished based on the clinical characteristics (Gender and health status) of the subjects to exclude heterogeneity. We used Begg’s rank correlation and Egger’s regression asymmetry tests to assess publication bias. The proportion of each study in the overall effect was evaluated by sensitivity analysis. The current meta-analysis of five trials (with six arms) RCTs with 233 participants indicated that supplementation with ITC cannot reduce both systolic BP (WMD: −5.83 mmHg; 95% CI: −12.49, 0.82, P = .086) and diastolic BP (WMD: −2.62 mmHg, 95% CI: −6.15 to 0.92, P = .147). Subgroup analysis based on gender revealed meaningful effects of ITC on SBP in subsets of females. Further well-designed RCTs in subjects with hypertension are required for a more robust assessment of the anti-hypertensive properties of this type of prebiotics.
Introduction
Hypertension (HTN), or high blood pressure, is described as a state in which the blood vessels have continuous elevated pressure. High blood pressure considered one of the major risk factors for stroke, cardiovascular, and kidney disease incidence. Approximately 1.13 billion people worldwide have HTN.[Citation1] C-1: References must be number format? The ongoing prevalence of HTN is due to population growth, aging, and unhealthy diet, lack of physical activity, excess weight, and exposure to persistent stress. Hypertension gives rise to elevated morbidity and mortality rates.[Citation2,Citation3]
Numerous evidences demonstrated that gut flora might have favorable on the health status, and microbiome imbalance may contribute to the pathogenesis of many metabolic diseases like high blood pressure. Gut dysbiosis has been noticed in animal models of HTN. It is influenced by several elements, such as dietary interventions.[Citation4] Today, prebiotics benefits go beyond gastrointestinal health, and studies are investigating their possible therapeutic effects on cardiovascular disease management such as HTN.[Citation5] Hence, modifying Gut microflora via prebiotics may be a promising approach to hypertension treatment.[Citation4] Prebiotics defined as a non-digestible fermented ingredient that stimulates the growth and/or activity of the gut microbiota and results in health benefits for the host.[Citation6,Citation7]
Inulin type carbohydrates (ITC) are the best prebiotics known as functional ingredients that have beneficial effects in intestine especially, by increasing the growth of bifidobacteria and lactobacillus species and reducing toxic substances.[Citation8,Citation9] Short Chain Fatty Acids (SCFA), including acetate, propionate, and butyrate, are the products of ITC fermentation in the gut, and most of the health benefits of ITCs related to them.[Citation10] ITCs include oligofructose, fructooligosaccharides, inulin, and fructan, which are soluble dietary fibers made up of fructose monomers linked by β (1–2) bonds.[Citation11]The postulated underlying mechanisms by which prebiotics reduce the risk of hypertension are 1) Lowering the lipid and cholesterol synthesis through SCFA production. 2) Reducing obesity via the development of endogenous glucagon-like peptide-1 (GLP-1) in the gut, hence an improvement of satiety and lessening food intake.3) Amelioration of insulin resistance through SCFA and improvement of hepatic insulin sensitivity.4) Improvement of the intake of minerals such as calcium in the gastrointestinal tract.[Citation3,Citation11]
The impact of ITC on systolic and diastolic blood pressure has been assessed in several clinical trials with different clinical conditions. In a randomized-controlled trial in elderly patients of Type2 diabetes, milk powder co-supplemented with inulin decreased systolic and diastolic BP significantly.[Citation12] Alarcon et al. reported that inulin supplementation in early-stage breast cancer patients resulted in lower systolic blood pressure, and also diastolic blood pressure non significantly decreased.[Citation8] However, Louis et al. demonstrated that fructooligosaccharides enriched cookie consumption in obese subjects did not alter the systolic or diastolic blood pressure significantly.[Citation13] The results of RCTs are inconsistent, and there is no systematic review and meta-analysis of RCTs that evaluate the effect of inulin-type carbohydrates’ role in blood pressure. Thus, this meta-analysis will summarize the evidence about the precise impact of ITC on systolic and diastolic blood pressure in various clinical states.
Methods
Literature search strategy
A literature search was conducted on PubMed, Scopus, ISI Web of Science, Cochrane library, and Google Scholar to obtain published or gray articles before December 2019. Search terms were inulin, fructan, oligofructose, fructooligosaccharides combination with “Intervention Studies” OR “intervention” OR “controlled trial” OR “randomized” OR “randomized” OR “random” OR “randomly” OR “placebo” OR “assignment.” Since several studies examined the impact of inulin supplementation on blood pressure as the secondary outcome we did not use blood pressure keywords. The search was performed by two authors separately (A. Gh and N.R) and the reference lists of all available papers were reviewed at the final step to finding relevant studies not seen from the computer-assisted search.
Eligible criteria
The following inclusion criteria were determined before study selection process:1) inulin intervention in pure form or supplement form; 2) randomized controlled trials (RCT) with either parallel or crossover design; 3) participants≥18 years of age; 4) minimum intervention period of three weeks;5) no other supplementation; 6) evaluation of the outcome of interest: markers of blood pressure (systolic blood pressure (SBP), diastolic blood pressure (DBP)); 7) report of post-intervention mean values (or if not available, change from baseline values were used instead) with standard deviation (or primary data which allow to calculate these parameters, i.e., standard errors, 95% confidence interval, p-values).
Data extraction
The following essential items were removed from each included RCT: study design, subjects, sample size, baseline BMI, baseline Age, Type, dose and duration of inulin supplementation and main results about blood pressure parameters. An attempt was made to e-mail article authors to collect data which are not shown in the published paper. All data were separately extracted by (N. M. A & M.A) and verified by (Sh.Sh & S.GH) regarding disagreements about acceptability. The removed items were resolved by consultation between all authors, and the corresponding author (A.Gh & N.R) ruled on disputes.
Statistical analysis
All examinations were carried out using STATA 11 software (Stata Corp, College Station, Texas, USA). The SD of the mean differences for studies not reported was calculated by the following formula: SD2 = [(SD baseline 2+ SD final 2) – (2 × R × SD baseline × SD final)] where correlation coefficient (R) was considered as 0.5.[Citation14] To confirm that our meta-analysis is not sensitive to the chosen correlation coefficient (R = 0.5), all analyses for SBP and DBP were reported using the correlation coefficient of 0.2 and 0.8. Data were merged to calculate the mean difference (MD) and 95% confidence interval (CI). STATA software-generated forest plots of the pooled MDs with 95% CIs for all results. Because of heterogeneity between the studies, the data were pooled using a random-effects model to promote the generalizability of results. Statistical heterogeneity was evaluated using Q tests and the I2 statistic. Subgroup analysis was accomplished based on the clinical characteristics (Gender and health status) of the subjects to exclude heterogeneity. We used Begg’s rank correlation and Egger’s regression asymmetry tests to assess publication bias. The proportion of each study in the overall effect was evaluated by sensitivity analysis.
Quality assessment
The Cochrane Collaboration’s tool for evaluating risk of bias was applied to elucidate the risk of bias of the involved studies attaching either low, unclear or high risk of bias to the six domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting) to each study.
Results
Literature search
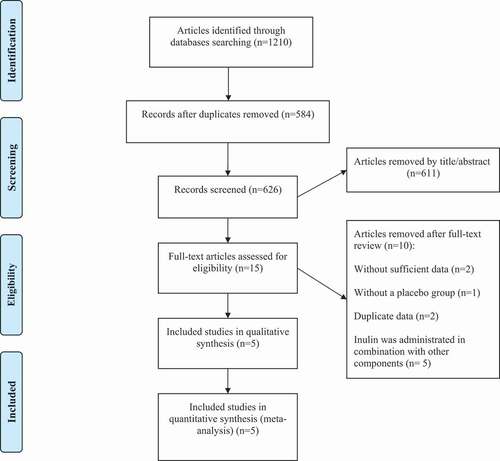
Selection criteria were performed by five studies (six arms)[Citation10,Citation13,Citation15,Citation16] with all 233 participants. The exact steps of the selection process are summarized in
Study characteristics
Whole 233 participants (121 as intervention group/112 as controls) were incorporated in the study, and the mean age of participants ranged from 40 to 51 years old. Mean BMI ranged from 26 to 37. The publication date of articles ranged from 2013 to 2019. Chosen studies were carried out in Iran[Citation10,Citation16] Spain[Citation13] and Mexico.[Citation15] A parallel design used for all the studies. The study duration varied between 3 and 8.5 weeks. Included trials enrolled participants with T2DM (n = 3)[Citation10,Citation16] and two other studies were conducted on women with breast cancer undergoing neoadjuvant chemotherapy[Citation15] and obese patients.[Citation13] The inulin dose ranged from 10 g/day to 15 g/day. Two studies carried out on both genders[Citation13,Citation16] and other studies conducted on females[Citation10,Citation15] General study characteristics are summarized in .
Table 1. Characteristic of included trials
Meta-analysis results
Effects of inulin on SBP
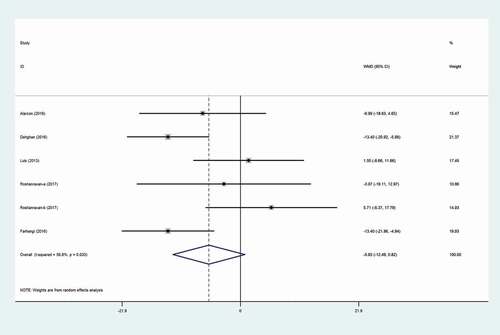
The assessment of the inulin supplementation on SBP effect in 5 trials was carried out (with six arms)[Citation10,Citation13,Citation15,Citation16] Including a total of 233 participants. Pooled effect size showed that SBP did not change following inulin supplementation (WMD: −5.83 mmHg; 95% CI: −12.49, 0.82, P = .086) with significant between-study heterogeneity (I2 = 58.8%, P = .033) (). Based on Gender (Both of gender and females) and health status (type 2 diabetes and other disease or health status) subgroup analysis was conducted. This heterogeneity might be explained by gender-based subgroup analysis. The results from subgroup analysis showed that SBP had a large significant decrease meant in female’s subset (WMD: −12.19 mmHg; 95% CI: −17.25, −7.13, P = .012). as outlined in
Table 2. Subgroup analysis to assess the effect of Inulin type fructan supplementation on systolic blood pressure and diastolic blood pressure
Effects of inulin on DBP
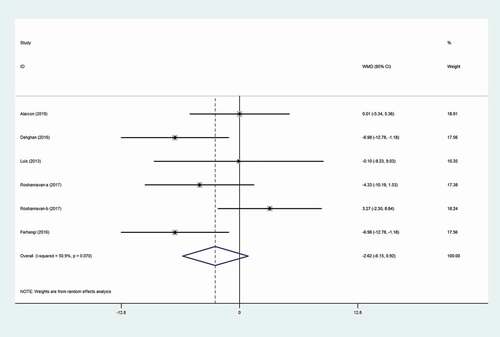
Overall, five eligible studies[Citation10,Citation13,Citation15,Citation16] (with six treatment arms), including a total of 233 participants, assessed the impact of inulin supplementation on DBP. Merging the random-effects model findings, we realized that DBP had a non-significant increment after inulin supplementation (WMD: −2.62 mmHg, 95% CI: −6.15 to 0.92, P = .147) compared to the control group with significant between-study heterogeneity (I2 = 50.9%, P = .070) (). The results from subgroup analysis as outlined in
Sensitivity analysis and publication bias
Following the exclusion of two clinical trials (WMD:-8.16 mmHg; 95% CI: −14.20, −2.11)[Citation16] and (WMD: −7.50 mmHg; 95% CI: −14.46, −0.54)[Citation13] sensitivity analysis revealed that the effect of inulin on the level of SBP was significant. Also, the abovementioned analysis for DBP showed an alteration in the overall effect (WMD:-3.98 mmHg; 95% CI: −7.02, −0.95) by exclusion one of treatment arms from Roshanravan study[Citation16] from the analysis altered. There were no trace of publication bias for SBP (p = .09, Begg’s test and p = .157, Egger’s test) and DBP (p = .707, Begg’s test and p = .878, Egger’s test).
Risk of bias assessment
Quality assessment of included studies based on the Cochrane collaboration‘s risk of the bias assessment tool is exhibited by .[Citation17] All of the included studies were described as random allocation and allocation concealment of participants. Low risk of bias concerning blinding of participants and personnel were observed in most of the trials except Dehghan’s study’s,[Citation10] which had an unclear risk of bias. In terms of incomplete outcome data and selective reporting, most studies had a low or unclear risk of bias.
Table 3. Risk of bias assessment for included randomized controlled clinical trails
Discussion
The present systematic review and meta-analysis revealed that inulin type-carbohydrates supplementation is not an effective complementary therapy in the improvement of blood pressure in all individuals. However, it can help reduce SBP in women. Due to limited evidence and the low quality of the included studies, more clinical trials are needed to clarify the effects of inulin on blood pressure. Although the beneficial effects of prebiotics have been identified on several cardiometabolic factors,[Citation18,Citation19] focusing on gut microbiota as a target for hypertension needs further research.[Citation20] As far as we are aware, no systematic review and meta-analysis has been conducted in this regard so far. Therefore, we cannot compare our results with the findings of the earlier studies.
Based on a narrative review by Upadrasta et al., an increasing number of clinical trials approve positive impacts of probiotic on cardiovascular health. However, the current knowledge regarding this is still premature.[Citation5] A systematic review and meta-analysis by Khalesi et al. also showed the modest effect of probiotics on blood pressure. Based on their findings, probiotics can reduce SBP by −3.56 mm Hg (95% CI, −6.46 to −0.66) and DBP by −2.38 mm Hg (95% CI, −2.38 to −0.93) compared to control groups. They also found that taking multiple species compared to single led to a more significant reduction in both SBP and DBP.[Citation21] Based on another systematic review and meta-analysis, probiotic fermented milk can decrease both SBP (−3.10 mmHg; 95% CI: – 4 · 64, – 1 · 56) and DBP (−1.09 mmHg; 95% CI – 2 · 11, – 0 · 06). They also reported that its effects on SBP in hypertensive subjects were more significant than normotensive ones (−3 · 98 v. – 2 · 09 mmHg). As probiotics and prebiotics can affect intestinal microflora and their ratios, it is expected that in similar to probiotic, the consumption of prebiotic as supplements or food also affect blood pressure. However, the findings of the included clinical trials are conflicting.
Differences in findings can be related to several factors, including the sources of inulin, the type of interventions, baseline levels of SBP and DBP, disease background, basal characteristics of participants, and genetic. However, due to limited studies, we cannot examine the effects of most mentioned factors, and the between-study heterogeneity remained high, even after stratifications by genders and health status. Also, the quality of most included publications was low that this factor could influence findings. It seems based on the present evidence we cannot still decide on considering inulin supplement as a complementary therapy in whom suffering from hypertension, mainly due to high risk of bias in most included clinical trials.
The precise pathways that prebiotics such as inulin affect hypertension have not been fully understood. However, several potential mechanisms have been suggested. Prebiotics can alter the populations of intestinal microbiomes and affect the renin-angiotensin system by microbial-derived metabolites.[Citation20,Citation22] Also, inulin can reduce body weight, particularly visceral fat and waist circumference. Through these pathways, it can indirectly affect blood pressure and other cardiometabolic risk factors.[Citation23] Prebiotic can restore an optimal balance among gut flora and improve host metabolism. The impact of various types of prebiotics on energy intake, body weight, satiety hormones, insulin resistance, gastric emptying time, and inflammatory parameters, is evident, albeit these findings are conflicting.[Citation24]
Our systematic review and meta-analysis had several limitations. Due to limited studies, we were not able to perform a subgroup analysis based on the types of inulin and other characteristics. Besides, we cannot find the main reason for heterogeneity, and it remained high even after subgroup analysis. As for strengths, we can point to do this issue that the present study is the first systematic review and meta-analysis in which the effects of inulin on blood pressure were examined. Also, the risk of bias and complementary analyses (publication bias, sensitivity analysis, subgroup analysis) were conducted to provide clear findings as much as possible.
Conclusion
We can conclude that inulin supplementation can reduce only SBP in women. However, no positive effects were observed in men. Also, inulin did not show considerable effects on controlling blood pressure, even in patients with diabetes. However, due to limited studies, findings must be interpreted by more caution. To decide on the effectiveness of inulin as adjuvant therapy in patients with hypertension or at risk for cardiovascular disorders, more clinical trials with a larger sample size and lower risk of bias are necessary.
Authors’ contribution
The authors’ responsibilities were as follows—Z.F, N.R and N.M.A directed and supervised the study; S.GH and M.A wrote the protocol, AR.M and F.KH conducted the electronic searches; N.M.A, M.A, and M.R.K independently selected the study and extracted relevant articles and tabulated data; Z.F, E.N.E, and Sh.Sh analyzed the data and interpretation of results; N.N and N.R and S.Gh wrote the first draft of the manuscript; and all authors read and approved the final version.
Acknowledgments
Authors thank the research council of Cardiovascular Research Center, Tabriz University of Medical Sciences for the scientific support of the present study. The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Diemer, F. S.; Snijder, M. B.; Agyemang, C.; Haan, Y. C.; Karamat, F. A.; van Montfrans, G. A.; Stronks, K.; Peters, R. J. G.; Brewster, L. M.; Stronks, K. Hypertension Prevalence, Awareness, Treatment, and Control in Surinamese Living in Suriname and the Netherlands: The HELISUR and HELIUS Studies. Intern. Emerg. Med. 2020, 15(6), 1041–1049. DOI: https://doi.org/10.1007/s11739-019-02269-z.
- Evans, C. E.; Greenwood, D. C.; Threapleton, D. E.; Cleghorn, C. L.; Nykjaer, C.; Woodhead, C. E.; Burley, V. J.; Burley, V. J. Effects of Dietary Fibre Type on Blood Pressure: A Systematic Review and Meta-analysis of Randomized Controlled Trials of Healthy Individuals. J. Hypertens. 2015, 33(5), 897–911. DOI: https://doi.org/10.1097/hjh.0000000000000515.
- Yeo, S. K.; Ooi, L. G.; Lim, T. J.; Liong, M. T. Antihypertensive Properties of Plant-based Prebiotics. Int. J. Mol. Sci. 2009, 10(8), 3517–3530. DOI: https://doi.org/10.3390/ijms10083517.
- Qi, Y.; Kim, S.; Richards, E. M.; Raizada, M. K.; Pepine, C. J. Gut Microbiota: Potential for a Unifying Hypothesis for Prevention and Treatment of Hypertension. Circ. Res. 2017, 120(11), 1724–1726. DOI: https://doi.org/10.1161/circresaha.117.310734.
- Upadrasta, A.; Madempudi, R. S. Probiotics and Blood Pressure: Current Insights. Integr. Blood Press Control 2016, 9, 33–42. DOI: https://doi.org/10.2147/ibpc.s73246.
- Gómez-Reyes, E.; Orea-Tejeda, A.; Castillo-Martínez, L.; Cassis-Nosthas, L.; Vargas-Vorácková, F. Prebiotics Consumption Modifies Diastolic Blood Pressure, but Does Not Affect Serum Lipids Concentration in Volunteers with Ischemic Heart Disease. Int. J. Probiotics Prebiotics. 2010, 5(3), 141.
- O’Connor, S.; Chouinard-Castonguay, S.; Gagnon, C.; Rudkowska, I. Prebiotics in the Management of Components of the Metabolic Syndrome. Maturitas. 2017, 104, 11–18. DOI: https://doi.org/10.1016/j.maturitas.2017.07.005.
- Becerril-Alarcon, Y.; Campos-Gomez, S.; Valdez-Andrade, J. J.; Campos-Gomez, K. A.; Reyes-Barretero, D. Y.; Benitez-Arciniega, A. D.; Soto-Pina, A. E.; Soto-Piña, A. E. Inulin Supplementation Reduces Systolic Blood Pressure in Women with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Cardiovasc. Ther. 2019, 2019, 5707150. DOI: https://doi.org/10.1155/2019/5707150.
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Xu, Y.; Xu, Y. Effect of Inulin-Type Carbohydrates on Insulin Resistance in Patients with Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 5101423. DOI: https://doi.org/10.1155/2019/5101423.
- Dehghan, P.; Farhangi, M. A.; Tavakoli, F.; Aliasgarzadeh, A.; Akbari, A. M. Impact of Prebiotic Supplementation on T-cell Subsets and Their Related Cytokines, Anthropometric Features and Blood Pressure in Patients with Type 2 Diabetes Mellitus: A Randomized Placebo-controlled Trial. Complement Ther. Med. 2016, 24, 96–102. DOI: https://doi.org/10.1016/j.ctim.2015.12.010.
- Miremadi, F.; Sherkat, F.; Stojanovska, L. Hypocholesterolaemic Effect and Anti-hypertensive Properties of Probiotics and Prebiotics: A Review. J. Funct. Foods. 2016, 25, 497–510. DOI: https://doi.org/10.1016/j.jff.2016.06.016.
- Cai, X.; Yu, H.; Liu, L.; Lu, T.; Li, J.; Ji, Y.; Yang, Y.; Bao, L.; Ma, W.; Xiao, R. Milk Powder Co-Supplemented with Inulin and Resistant Dextrin Improves Glycemic Control and Insulin Resistance in Elderly Type 2 Diabetes Mellitus: A 12-Week Randomized, Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2018, 62(24), e1800865. DOI: https://doi.org/10.1002/mnfr.201800865.
- De Luis, D.; De la Fuente, B.; Izaola, O.; Aller, R.; Gutiérrez, S.; Morillo, M. Double Blind Randomized Clinical Trial Controlled by Placebo with a Fos Enriched Cookie on Society and Cardiovascular Risk Factors in Obese Patients. Nutr. Hospitalaria. 2013, 28(1), 78–85.
- Borenstein, M.; Hedges, L. V.; Higgins, J. P.; Rothstein, H. R. Introduction to Meta-analysis; John Wiley & Sons, 2011.
- Becerril-Alarcón, Y.; Campos-Gómez, S.; Valdez-Andrade, J. J.; Campos-Gómez, K. A.; Reyes-Barretero, D. Y.; Benítez-Arciniega, A. D.; Soto-Piña, A. E.; Soto-Piña, A. E. Inulin Supplementation Reduces Systolic Blood Pressure in Women with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Cardiovasc. Ther. 2019, 2019, 1–10. DOI: https://doi.org/10.1155/2019/5707150.
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M. A.; Hedayati, M.; Ghavami, A.; Ostadrahimi, A.; Alamdari, N.; Barati, M.; Ostadrahimi, A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-blind, Placebo-controlled Trial. Horm. Metab. Res. 2017, 49(11), 886–891. DOI: https://doi.org/10.1055/s-0043-119089.
- Higgins, J. P.; Altman, D. G.; Gøtzsche, P. C.; Jüni, P.; Moher, D.; Oxman, A. D.; Sterne, J. A.; Schulz, K. F.; Weeks, L.; Sterne, J. A. C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Bmj. 2011, 343(oct18 2), d5928. DOI: https://doi.org/10.1136/bmj.d5928.
- Johnson, M. Diet and Nutrition: Implications to Cardiometabolic Health,J Cardiovasc Sciences. 2019; 3(2): 4-9.
- Schiattarella, G. G.; Sannino, A.; Esposito, G.; Perrino, C. Diagnostics and Therapeutic Implications of Gut Microbiota Alterations in Cardiometabolic Diseases. Trends. Cardiovascul. Med. 2019, 29(3), 141–147. DOI: https://doi.org/10.1016/j.tcm.2018.08.003.
- Hsu, C.-N.; Hou, C.-Y.; Chan, J. Y.; Lee, C.-T.; Tain, Y.-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients. 2019, 11(12), 2908. DOI: https://doi.org/10.3390/nu11122908.
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-analysis of Randomized, Controlled Trials. Hypertension. 2014, 64(4), 897–903. DOI: https://doi.org/10.1161/HYPERTENSIONAHA.114.03469.
- Ghavami, A.; Roshanravan, N.; Alipour, S.; Barati, M.; Mansoori, B.; Ghalichi, F.; Ostadrahimi, A.; Ostadrahimi, A. Assessing the Effect of High Performance Inulin Supplementation via KLF5 mRNA Expression in Adults with Type 2 Diabetes: A Randomized Placebo-Controlled Clinical Trail. Adv. Pharm. Bull. 2018, 8(1), 39–47. DOI: https://doi.org/10.15171/apb.2018.005.
- AlBishi, L. A.;. Does Inulin Ingestion Reduce Visceral Fat Adiposity? Mini Review. Biomed. J. 2018, 2, 4.
- Kellow, N. J.; Coughlan, M. T.; Reid, C. M. Metabolic Benefits of Dietary Prebiotics in Human Subjects: A Systematic Review of Randomised Controlled Trials. Br. J. Nutr. 2014, 111(7), 1147–1161. DOI: https://doi.org/10.1017/S0007114513003607.