?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Chemical modifications (citrification, acetylation and phosphorylation) of breadfruit (Artocarpus altilis) starch were performed using dual (microwave-assisted and chemical) processes, the physicochemical properties, degree of substitution and in-vitro starch hydrolysis of the modified starches were evaluated using standard methods while changes in molecular structure was assessed using Fourier transform-infrared (FTIR) spectroscopy. The results showed that bulk density ranged from 0.22 to 0.28 g/ml, swelling power (5.89 to 6.23), solubility (0.13 to 0.17 g), water absorption capacity (WAC) (0.5 to 0.9 mg/g), and oil absorption capacity (OAC), 0.8 to 1.0 ml/g. Starch and amylose contents varied between 122 and 206 mg/g and 16 to 36%. The degrees of citrification in chemically and microwave modified starches were 0.2 and 0.31% while chemical and microwave modified acetylation were and 0.24 and 0.36%, respectively. The infrared spectra of modified starches showed characteristics band for C = O at 1754 to 1375 cm−1 for acetylated starches and at 1240 cm−1 to 1417 cm−1 belonging to P = O stretching vibration for phosphorylated starches. The in-vitro starch hydrolysis showed that modified starches recorded decreased rate of hydrolysis and glycemic index compared to native starch. Microwave-assisted chemically modified starch recorded better functional properties and low glycemic index compared to chemically modified starch. The study revealed that modification of breadfruit starch could enhance efficient utilization of breadfruit for both industrial and nutritional purposes.
Introduction
Breadfruit is a large tropical fruit, which belongs to the family of Moraceae. Breadfruit trees are found growing wild in western Nigeria, its ability to produce several fruits is noteworthy and a tree could produce 100 to 600 green and matured fruits per year.[Citation1,Citation2] Breadfruit is gluten-free and can potentially be used for a wide range of food applications, despite its nutritive value and high starch content the fruit is underutilized and has found limited applications in the food industries.[Citation3]
Breadfruits, like yam, corn and wheat is a good source of starch and starch serves as a multifunctional ingredient in foods and pharmaceuticals. It is used to influence or control characteristics such as texture, moisture, consistency and shelf stability. It can be used to bind or to disintegrate; to expand or densify; to clarify or to opacify; to attract moisture or inhibit moisture; to produce smooth texture or pulpy texture; soft coatings or crisp coatings.[Citation4]
Native starches irrespective of their source are undesirable for many industrial applications because of their inability to withstand processing conditions such as extreme temperature, diverse pH and high shear rate.[Citation5] Native starches also yield pastes of poor stability which decreases its shelf life (storage stability), causes shrinkage and the release of water. In order to improve on the desirable functional properties and overcome its limitations, native starches are often modified and tailored to specific industrial applications.[Citation6]
The starch modification techniques are usually physical (e.g. pregelatinization), chemical (e.g. etherification, esterification, crosslinking and oxidation) and enzymatic (enzymatic hydrolysis)[Citation5] The presence of hydroxyl groups in glucose makes it susceptible to substitution reactions, in the presence of acetylating such as acetic anhydride substitution of hydroxyl group lead to formation of acetylated starch, while in the presence of acids such as citric acid, esterified starch is formed, also the starch is crosslinked when reacted with phosphorylating agents. The degree of substitution will determine the extent of modification of the physicochemical properties of the native starch.[Citation7]
In recent years, the use of microwave in the synthesis of organic compounds is on the increase[Citation8] and when compared with conventional heating, microwave-assisted heating (under-controlled conditions) has been shown to be an advanced technology in reducing reaction time besides increasing product yield and purity.[Citation9] Modification of starch using microwaves involves several interacting mechanisms such as irradiations, furnace dimensions and characteristics of starch. The parameters such as moisture and temperature influence the dielectric properties of the starch these causes rearrangement of the starch molecules which in turn affect the functional properties of starch.[Citation9]
A lot of research has been carried out on chemical composition and nutritive values of breadfruits[Citation1,Citation10] and Zhura et al.,[Citation11] had performed acetylation of breadfruits. However, information is scanty on dual modifications methods, that is, the use of combined physical and chemical process like acetylation using microwave. Dual modification process like chemical/chemical (acetylation and phophrylation) process has been reported to optimize modified starch functionality in industry.[Citation8]
This study aimed to employ dual modification (chemical - and microwave-assisted chemical) methods in the modification (citrification, acetylation, phosphorylation) of breadfruit starch The objectives were to determine the degree of substitution, physicochemical properties and in-vitro starch hydrolysis of the samples. The findings of this study would provide insight into best modification method that could help in the improvement of functional properties and the glycemic index of breadfruit starch. This study will provide information on the suitability or otherwise of modified breadfruit starches for food and industrial purposes.
Materials and methods
Sample collection and preparation
Matured breadfruit was harvested from a local farm in Ile-Ife, Osun State, Nigeria. The starch from breadfruit was isolated using the method described by Singh et al.[Citation6] The breadfruit was debarked and the fibrous layer was removed. The debarked breadfruit samples were cut into tiny pieces in a clean plastic container and 10 L of distilled water containing 5 g of sodium metabisulphite (to prevent fermentation) was added. The breadfruit was allowed to soak for 72 h. The solid shaft was separated from starch slurry using a plastic sieve and the shaft was washed twice with distilled water to remove starch and the combined starch slurry was drained using cheese cloth. The solid (white starch) obtained was spread on a stainless tray and dried overnight in a laboratory oven at 50°C to a constant weight, cooled and then ground into powder. The percentage yield of starch was calculated in relation to the weight of the breadfruit and the starch powder was then put in an air tight container and kept in the freezer for further analysis.
Preparation of starch citrate (esterification)
Preparation of starch citrate was based on the method of Singh et al[Citation6] with some modifications. Breadfruit starch (30 g) was dissolved in 120 mL of 0.12 M citric acid and 10 mL of 0.1 M NaOH solution was added. The slurry was magnetically stirred for 2 h at room temperature. The pH of the slurry was adjusted to 5.5 using 0.1 M NaOH solution. The slurry was centrifuged and washed three times with distilled water to remove the unreacted citric acid. The residue was dried in the oven at 45°C; ground to powder using a warring blender (National model MX-795 N, Matsushita, Malaysia) and the resulting powdered sample was packed in an air tight container and kept in the freezer for further analysis.
Preparation of starch acetate (acetylation)
Starch acetate was prepared by slight alteration of the method described by Sodhi et al.[Citation12] The starch sample (30 g) was dispersed in 100 mL distilled water and stirred for an hour on a magnetic stirrer. The pH was adjusted to 8.0 using 0.1 M NaOH. Acetic anhydride (2 mL) was added drop wise to the stirred slurry while maintaining the pH within the range 8.0–8.4 using 0.1 M NaOH. The reaction was allowed to proceed for 2 h and the pH of the slurry was adjusted to 4.50 with 0.1 M NaOH. The slurry was washed thrice with distilled water, filtered and oven dried at 45°C using a laboratory oven. The residue was then ground into powder using a warring blender (National model MX-795 N, Matsushita, Malaysia), stored in an air tight container and kept in the freezer for further analysis.
Preparation of phosphorylated starch
Starch phosphate was prepared by the method of Singh et al.[Citation13] with slight modifications. 2.0 g of Sodium dihydrogen orthophosphate (NaH2PO4) was dissolved in 100 mL distilled water and 30 g of starch was added to form slurry. The slurry was placed in a water bath at 30°C for an hour and the pH adjusted to 4.50 using 0.1 M HCl. The slurry was washed three times with distilled water and filtered. The residue was oven dried in a laboratory oven at 45°C, ground into powder using a warring blender (National model MX-795 N, Matsushita, Malaysia), stored in an air tight container and kept in the freezer for further analysis.
Microwave-assisted starch modifications
Preparation of modified starch by acetylation, citrification and phosphorylayion using microwave-assisted chemical method as outlined above were carried out in domestic microwave oven (Model BRE799GMSSE, 800 W power output, 20 liter capacities, Breville, UK). The pH of the slurry was adjusted and washed to remove residual reagents. The residues were dried in a laboratory oven at 45°C, ground into powder using a warring blender (National model MX-795 N, Matsushita, Malaysia), stored in an air tight container and kept in the freezer for further analysis.
Determination of physico-chemical properties of samples
The degrees of citrification and acetylation of the modified starch were analyzed by the method of Sodhi et al.[Citation12] The bulk density was determined by the method of Narayana and Narasinga,[Citation14] the swelling power and solubility were determined by method of Daramola and Osanyinlusi[Citation15] while method of Beuchat[Citation16] was used to determine water and oil absorption capacity.
Fourier transform infrared (FT-IR) spectroscopy of starch
The spectra of the starch samples were obtained using an FT- IR spectrophotometer (Shimadzu, Japan). A KBr pellet was made by accurately weighed 100 mg of potassium bromide (KBr) and 2 mg of the sample, (KBr) and pressed into a disc of ca. 1 mm thick. The FT-IR spectra was analyzed within the range of 4000–500 cm-1and the spectral was analyzed using chemstation computer software.
Determination of total starch and amylose content
The sample was extracted with 90% ethanol to remove sugar, the residue obtained (200 mg) was refluxed with 0.7 M HCl for 2.5 h. The acid hydrolyzate was neutralized to pH 7.0 using 5.0 M NaOH, pour into 500 mL standard flask and made up to volume with distilled water. The hydrolyzate was filtered through a Whatman no. 541 filter paper and the starch was determined as the reducing sugar using 3, 5-dinitrosalicyclic acid (DNSA) reagent.[Citation17] The glucose content was calculated using a glucose standard linear equation and then converted to starch by multiplying with 0.9. Amylose content of the samples were determined based on the Iodine-binding procedure as described by Thomas et al. [Citation18] Potato starch was used as standard and the amylose content was calculated from the standard curve of potato amylose using the linear equation (R2 = 0.899)
Determination of in-vitro starch hydrolysis
The in-vitro starch digestibility was determined by multi-enzyme procedure described by Deepa et al. [Citation19]The sample (250 mg) was gelatinized in 10 mL distilled water on a hot plate. The gelatinized sample was homogenized with 10 mL of HCl – KCl buffer (pH 1.5) using a basic homogenizer (KikaLabortechnik 725, Janke and Kukel GmbH & Co., Stanfen Germany) at 9500 rpm for 1 min and the homogenate was then digested with 20 mg of pepsin (Sigma; CAS 2001/75-6, code 10132561, 666 iu/mg, porcine gastric mucosa) solution (prepared by adding 1.0 g of pepsin/10 mL of HCl-KCl buffer) for 1 h in a shaking water bath at 37°C, to mimic to the condition of stomach. The pH of the digestate was adjusted to 6.9 and the volume made to 25 ml using Tris-maleate buffer (pH 6.9). Then 5.0 mL of α-amylase (2.6 IU in 5 ml buffer pH 6.9) was added to the digestate which was incubated at 37°C in a shaking water bath. One ml of sample aliquots was collected at intervals of 30 min for 180 min, the enzyme activity in the aliquot withdrawn was inactivated by immediately placing the tube in a boiling water bath maintained at 100°C for 5 min and then refrigerated till the end of the incubation period, To these aliquots, 3 ml of 0.4 M sodium acetate buffer (pH 4.75) and 60 µl amyloglucosidase (Sigma, No; 10105–5GF,70 ui/mg. Aspegiliusniger) were added and incubated at 60°C for 5 min to hydrolyze the starch to glucose.The glucose released was determined using 2, 3-dinitrosalicylic acid.[Citation17] The concentration of glucose was calculated from the linear equation of glucose standard (R2 = 0.980) and glucose was converted into starch by multiplying with 0.9. The starch classification based on its digestibility was: RDS as the starch that was hydrolyzed within 30 min of incubation, RS as the starch not hydrolyzed after 180 min, and SDS as the starch digested during the period between 30 and 180 min.
Estimated glycemic index (GI)
Using the hydrolysis curve (0 − 180 min), the hydrolysis index (HI) was calculated as the percentage of total glucose released from the modified sample compared to native (unmodified) starch and the glycemic index of the samples were estimated according to the equation described by Goni et al.,[Citation20]
Statistical analysis
Analyses were carried out in triplicate for each determination and the results were expressed as mean and standard deviation. Analysis of variance (ANOVA) was used to determine significant variations among the samples. The values were considered to be significantly different at P ≤ 0.05. Statistical analysis of data was performed using Microsoft Excel Statistical Package (Microsoft Corporations, USA) and Graph-Pad Instat-3 Package (Graph Pad software Inc, USA).
Results and discussion
Starch components
The total starch, starch fractions (RDS, SDS and RS), amylose and degree of substitution are presented in . The percentage extractable starch yield obtained from the breadfruit used in this study was 18% which is greater than that of 14.26% for breadfruit reported by Akanbi et al.[Citation2] and 15.40% for breadfruit reported by Onwueme.[Citation21] The higher value of extractable starch compared to other studies might be due to the quantity of breadfruit sample used and starch isolation process. In addition, it is also reasonable to expect variation in starch contents because breadfruits regardless of their origin have different maturity stage, variety, climatic and agronomic conditions.
Table 1. Starch Content, Rapidly digested starch (RDS), slowly digested starch (SDS), Resistant Starch (RS) and Amylose content of modified Breadfruit starch
The total starch content of the samples ranged from 122 ± 2.8% to 234 ± 0.9 mg/g, all modified starch except AC recorded values that were lower than NS. The starch fractions showed that rapidly digestible starch (RDS) ranged from 12 to 32%, slowly digestible starch (SDS) from 20 to 67% while the resistant starch (RS) varied between 21 and 53%. The variation of these parameters follow this order; for RDS, AC > AM, CC > CM and PC >PM, an indication that microwave-assisted method leads to production of starch with lower RDS and also the percentage RDS recorded for the modified starches were lower than that of NS. The observation agrees with the report of Deepa et al[Citation19] the low value observed for RDS could be due to the stearic hindrance by the newly cross-linked structure in modified starches.
The formation of new bonds in starch during the process of modification (acetylation, citrification and phosphorylation) could sterically inhibit the proper positioning of the substrate into the active site of digestive enzymes such as α-amylase and amyloglucosidase, and consequently, restrict enzyme hydrolysis. These results corroborate the results of previous studies.[Citation22] RS is defined as the sum of starch or the sum of starch degradation products that are not absorbed by the small intestine in healthy individuals. The RS is the substrate of fecal microflora which is fermented to produce short chain fatty acids with different physiological and probiotic effects.[Citation8]
The amylose content () ranged from16% in PC to 36% in AC, The range of 12.8 to 26% amylose was reported for some starchy foods consumed in Nigeria.[Citation23] Amylose in food samples are categorized into waxy (2%), very low (3–9%), intermediate (20–25%) and high (> 25%).[Citation24] Samples AC, CM and PC recorded high amylose content (≥ 27%), and as mentioned earlier, amylose content is an important determinant factor in WAC and OAC. High amylose starches are a source of resistant starch and have been associated with reduced susceptibility to enzymatic hydrolysis of cooked rice starches whereas starches with low amylose content digests easily.[Citation25]
In phosphorylated starch, the introduction of phosphate substitution on amylose or amylopectin prevents linearity of the molecular chain due to the steric hindrances. The phosphate diester starch has the phosphate esterified with two hydroxyl groups, very often from two neighbouring starch molecules which could lead to the formation of a covalent bridge or cross – linking, thus a situation will arise whereby individual chain segment can no longer establish inter or intra- molecular association and this could lead to a better functional properties.[Citation11]
Physico-chemical properties of native and modified starches
The degree of substitution (DS) () for acetylated and citrified starch was 0.19 and 0.31%, 0.24 and 0.36%, in conventional and microwave methods, respectively. Higher degrees of substitutions (0.85% acetylation and 0.73% citrification) have been reported for corn starch.[Citation26] Zhura et al[Citation11] reported the range of substitution between 0.74 and 1.23% for acetylated breadfruit starch and observed that preparation of starch acetatate is influenced by contact time between the starch and the reagent (acetic anhydride, more contact time offers the possibility of hydroxyl bonds in starch to weaken and thus accommodate more acetyl group. The degree of substitution essentially represents the average number of sites per glucose unit that possess a substituted acetyl group, the low value of DS in an acetylated starch has been attributed to such factors as lack of granular surface pore, which facilitates physical access of acetic anhydride to the interior of the starch granules.[Citation27] It was generally observed that dual modification process (chemical and microwave) lead to production of starch with higher degree of substitution.
The degree of acetylation in starch, according to Singh et al.,[Citation6] has been categorized into high (1.5–3.0), medium (0.2–1.5) and low (0.01–0.2).The maximum limit set for percentage of acetylation for food grade acetylated starch by the United States Food and Drug Administration is 2.5% acetyl content[Citation8] Acetylated starch with the degree of substitution range of 0.01 and 0.2 had been reported to have several applications in conforming films, adherents, thickeners, stabilizers and encapsulating agent.[Citation8] Therefore, substitution of hydroxyl group of breadfruit starch could enhance its applicability in food and industrial raw material production.
The bulk density () which is a reflection of the load the samples can carry if it rests directly on one another, ranged from 0.22 to 0.28 g/ml. The bulk density recorded by NS was significantly higher than modified starches except for phosphorylated starch. The values reported in this work for bulk density were lower than 0.54 reported for whole breadfruit flour.[Citation28] Flour with low bulk density is more desirable as the sample will pack better during storage without losing volume.
Table 2. Functional properties of native and modified breadfruit starches
Swelling power which shows variation in gelling properties of the flour when heated, ranged from 5.89 to 6.23 at 60°C, NS recorded swelling power higher than PC. The swelling power reported in this study compared favourably with 6.08 reported for bitter yam starch.[Citation29] There was no significant difference in the swelling power between microwave and chemically modified starch. Since gelation involves the swelling of starch granules on heating, the low swelling power of modified starch could be attributed to higher level of total available carbohydrate. A greater swelling power was observed in flour with low amylose content, which showed that amylose is responsible for reinforcement of internal network within the granule thus restricting swelling. The swelling power is of great significance in tablet and capsule formulation as it is believed that starch with higher swelling power would be expected to release active pharmaceutical ingredients at faster rate from its compact structure.[Citation24] The low swelling power reported for modified starch implied that the rate of starch hydrolysis could be very low because according to Chung et al.[Citation30]it would be difficult for digestive enzyme to access the interior of the starch granules.
The solubility of starch which is also a measure of the water-binding capacity of the starch was highest in CC and lowest in NS. The values recorded for starch solubility were lower than 0.66 g reported for cassava starch.[Citation4] The water absorption capacity (WAC) represents the ability of flour-water to associate under conditions where water is limiting, this parameter ranged from 0.50 to 0.90 mg/ml with the highest value reported for AM starch. The result obtained in this study was lower than 2.39 and 2.02 mg/ml reported for whole and core breadfruit flour.[Citation28]
Oil absorption capacity (OAC) which is the ability of the protein of flour to bind with oil, since starch does not possess non polar sites akin to those found in proteins, the measure of OAC in starch could be regarded as a measure of bound protein. The value varied from 1.00 to 0.80 mg/ml. When oil capacity is high, as recorded in AC and NS, it showed that the starch could be used as flavour retainer, since fat acts as flavour retainer and also increases mouth-feel of foods.[Citation24] The quantity and the quality of the vegetable oil affect the oil absorption capacity of the starch.[Citation28]
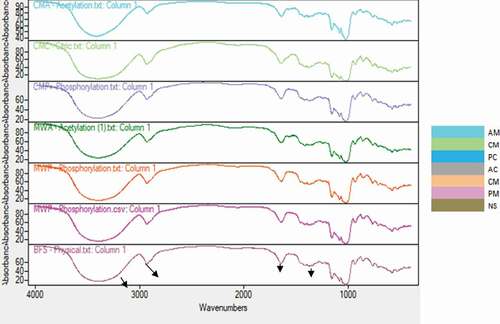
FT-IR spectroscopy analysis of modified starches
Fourier transform infra-red (FTIR) spectra are reported in and the diagnostic bands characteristic of the functional groups present in modified starch are reported in . The broad band at 3500–3200 cm−1 was attributed to hydrogen-bonded hydroxyl group.[Citation31] The strong peaks at 3000–2850 cm−1 assigned to the C-H stretching while peaks at 1030–990 cm−1 would be characteristic of the anhydroglucose ring O–C stretch. The peaks at around 1450 and 1200 cm−1 were characteristic of C–O–H. while peaks at 1600–1500 cm−1 attributed to the bending of water in starch.[Citation31] Comparison of the IR spectrum of NS with those of modified starch indicated that there are band shifts to a lower wave-numbers (red shifts), an indication of the substitution of the O-H bond with either acetyl or ester groups, or cross-linking as expected of phosphorylation.
Table 3. Diagnostic bands and intense peak wavelengths (cm−1) of IR spectral of native and modified breadfruits starch
In acetylated starches (AC and AM), some new absorption bands observed at 1754, 1435, 1375 and 1240 cm−1 which were assigned to carbonyl C = O, CH3 antisymmetry deformation vibration, and CH3 symmetry deformation vibration and carbonyl C–O stretch vibration, respectively, while the anhydroglucose unit moved toward a high wave number. In phosphorylated starches (PC and PM), new bands appeared at 1240 cm−1and 1417 cm−1 which has been reported to be characteristics of P = O stretching vibration.[Citation28]
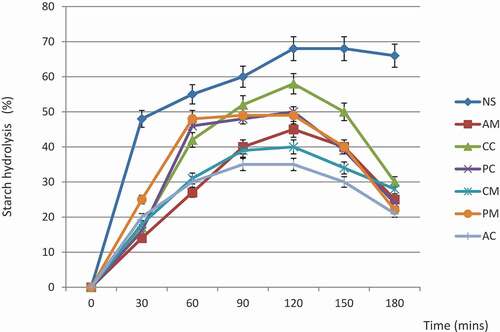
In-vitro starch digestibility
The starch hydrolysis curve of the starch is depicted in , while the kinetic constant, equilibrium concentration, hydrolysis index and glycemc index are summarized in . From , the percentage hydrolysis within the 30 min of hydrolysis was highest in NS, this observation conformed to the report of Adewusi et al[Citation1] that breadfruit meal digest within 30 min of consumption, therefore there is a general consensus among consumers of breadfruit that breadfruit diet in any form of preparation is a light diet. The high rate of digestion of breadfruit starch indicate the presence of high level of reducing sugar or low molecular weight dextrin and also the presence of appreciable amount of amylose. It implied that if breadfruit meal if is consumed regularly, it could raise blood glucose level and as such could pose health risk especially among diabetic patients.
Table 4. Hydrolysis constant, concentrations at infinity, area under curve, hydrolysis index and glycemic index of modified breadfruit starch
The kinetic parameters of starch hydrolysis are presented in . From the table, the kinetic constant (k) which indicates the digestion extent per unit time, and equilibrium concentration (C∞) which shows the concentration of substrate digested at infinity, ranged from 0.045 to 0.07 kmin−1, and 50.3 to 58.8%, respectively. The hydrolysis and glycemic indexes of NS were taken as 100% while those of modified starches varied between 74.4 to 78.8%, and 80.5 to 82.9%, respectively. The low hydrolysis and glycemic indexes recorded for modified starch is expected because chemical modifications of starch had long been known to render (α – 1 – 4) and (α – 1 – 6) glycosidic bonds resistant to hydrolysis, the acetyl, ester and phosphate groups of starch would prevent enzyme action, and thus leave a large portion of dextrin molecule in the hydrolyzate.[Citation32] Other factors that could affect starch digestibility include the structural characteristics of the starch such as the amylose/amylopectin ratio, degree of gelatinization, retrogradation and formation of amylose complexes.[Citation8] From the results obtained from starch digestibility study, it was observed that the rate of starch hydrolysis in modified starch is slow which implied that chemical modification could help produce high resistance starch, therefore chemical modification of breadfruit starch would be an advantage for formulation a low glycemic diets especially for people suffering from diabetes mellitus.
Conclusion
The findings of this study revealed that microwave-assisted chemical modification leads to formation of starch with higher degree of substitution compared to chemical method. Modified starch recorded improved functional properties and low glycemic index compared to native starch. Therefore, the study concluded that modification of breadfruit starch could enhance its efficient and effective utilization for both industrial and nutritional purposes.
Disclosure statement
There is no conflict of interest.
References
- Adewusi, S. R. A.; Orisadare, B. O.; Oke, O. L. Studies in Weaning Diet in Nigeria1.Carbohydrate Sources. Cereal Chem. 1991, 68(2), 165–169.
- Akanbi, T.; Nazamid, S.; Adebowale, A. Functional and Pasting Properties of a Tropicalbreadfruit Starch. Int. Food Res. J. 2009, 16, 151–157.
- Omobuwajo, T.;. Compositional Characteristics and Sensory Quality of Biscuits, Prawn Crackers and Fried Chips Produced from Breadfruit. Innovative Food Sci. Emerging Technol. 2003, 4(2), 219–225.
- Akpa, J. G.; Dadge, K. K. Modification of Cassava Starch for Industrial Uses. Int. J. Eng. Technol. 2012, 2(6), 913–919.
- Singh, J.; Kaur, L.; McCarthy, J. Factors Influencing the Physicochemical, Morphological, Thermal and Rheological Properties of Some Chemically Modified Starch for Food Applications- A Review. Food Hydrocoll. 2007, 21, 1–22.
- Cock, J.;. Cassava, a Basic Energy Source in the Tropics. Science. 1982, 218(4574), 755–762.
- Alcázar-Alay, S. C.; Meireles, M. A. A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. Campinas 2015, 35(2), 215–236.
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of Microwave Treatment on Thephysicochemical Properties of Potato Starch Granules. Chem. Cent. J. 2013, 7, 113. http://journal.chemistrycentral.com/content/7/1/113.
- da-cruz, D. R.; da-silva, W. S.; dos-Santos, I. P.; Zavareze, E. Structural and Technological Characteristics of Starch Isolated from Sorghum as a Function of Drying Temperature and Storage Time. Carbohydr. Polym. 2015, 133, 46–51. DOI: https://doi.org/10.1016/jcarbpol2015.07.003.
- Akpoborie, J. U.; Bolanle, A. O.; Muyiwa, O. F.; Adewusi, S. R. A. Chemical Analysis of Breadfruit (Artocarposcommunisforst) from Southwestern Nigeria. J. Food Technol. 2003, 1(2), 29–35.
- Zhura, C. F.; Gea, S.; Ginting, M.; Marponga, W.; Lenin, S. Acetylation of Breadfruit Starch Using Acetic Anhydride. J. Phys. 2018, 1116, 042047.
- Sodhi, N.; Singh, N.; Singh, Y. Characteristics of Acetylated Starches Prepared Using Starches Separated from Different Rice Cultivars. J. Food Eng. 2005, 70(1), 117–127.
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.; Gill, B. Morphological, Thermal and RheologicalProperties of Starches from Different Botanical Sources. Food Chem. 2003, 81(2), 219–231.
- Narayana, K.; Narasinga, R. Functional Properties of Raw and Heat Processed Winged Bean Flour. J. Food Sci. 1982, 47(1), 1534–1538.
- Daramola, B.; Osanyinlusi, A. Investigation on Modification of Cassava Starchusing Active Components of Ginger Roots. Afr. J. Biotechnol. 2006, 5(10), 917–920.
- Beuchat, L.;. Functional and Electrophoretic Characteristics of Succinylated Peanut Flour Protein. J. Agric. Food Chem. 1977, 25(1), 258–261.
- Grandfeidt, H. N.; Veenstra, J.; Hudson, G. J. Measurement of Rapidly Available Glucose (RAG) in Plant Foods: A Potential in In-vitro Predictor of the Glycemic Responses. Br. J. Nutr. 1992, 46, 649–660.
- Thomas, R.; Wan-Nadiah, W. A.; Rajeev, B. Physiochemical Properties, Proximate Composition, and Cooking Qualities of Locally Grown and Imported Rice Varieties Marketed in Penang, Malaysia. Int. Food Res. J. 2013, 20(3), 1345–1351.
- Deepa, G.; Singh, V.; Maidu, K. A. Comparative Study on Starch Digestibility Glycemic Index and Resistance Starch of Pigmented (“njavara”and “Jyothi”) and a Non Pigment (“IR 64”) Rice Varieties. J. Food Sci. Technol. 2010, 47(6), 644–649. DOI: https://doi.org/10.1007/s13197-010-0106-1.
- Goni, I.; Garcia-Alonsa, A.; Saura-Calixto, F. A Starch Hydrolysis Procedure to Estimate Glycemic Index. Nutr. Res. 1997, 17, 427–437. DOI: https://doi.org/10.1016/S0271-5317(97)00010-9.
- Onwuene, I.;. The Tropical Tuber Crops: Yams, Cassava, Sweet Potato and Cocoyam. Int. Food Res. J. 1978, 24(1), 154–157.
- Yadav, R. B.; Kumar, N.; Yadav, B. S. Characterization of Banana Potato and Rice Starch Blend for Their Physicochemical and Pasting Properties. Cogent Food Agric. 2015, 2(1), 1127873.
- Otemuyiwa, I. O.; Sanni, A. M.; OyewumiE., A. Comparative Study of Starch Characteristics, In-Vitro Starch Digestibility and Glycemic Index of Some Starchy Foods Consumed in Nigeria. Food Sc. Nutr. Stud. 2017, 1(2), 2017.
- Juliano, B. O.; Perez, C. M.; Blackney, A. B. International Cooperative Testing on the Amylose Content of Milled Rice. Starch. 1981, 33(5), 157–182. DOI: https://doi.org/10.1002/star.19810330504.
- Riley, C. K.; Bahado-Singh, P. S.; Wheatley, A. O.; Asemota, H. N. Physicochemical Properties of Low-amylose Yam (Dioscorea Spp.) Starches and Its Impact on α- Amylase Degradation in Vitro. Int. J. Nutr. Food Sci. 2014, 3(5), 448–454.
- Hui, C.; Kun, X.; Xuili, W.; Qiang, C. Effect of Acetylation on Properties of Corn Starch. Food Chem. 2008, 106(3), 923–928.
- Bolade, M. K.; Oni, O. J. Influence of Acetylation on the Physicochemical Properties of Composited Starches from Sweet Potato (Ipomeabatata L) and Water Yam (Dioscoreaalata L). Afr. J. Biotechnol. 2015, 74(51), 3340–3349.
- Rincon, A. M.; Padilla, F. C. Physicochemical Properties of Breadfruit (Artocarpusartilis) Starch from Margarita Island, Venezuela. ALAN. 2004, 54(4), 222–226.
- Olagunju, S.;. Physicochemical Properties of Some Selected Nigerian Yam. Carbohydr. Polym. 2016, 4(1), 35–36.
- Chung, H. J.; Dong, H. S.; Lim, S. T. In Vitro Starch Digestibility Ans Estimated Glycemic Index of Chemically Modified Corn Starches. Food Res. Int. 2008, 41(6), 579–585.
- Fang, J. M.; Fowler, P. A.; Tomkinso, J.; Hill, C. A. S. The Preparation and Characterization of a Series of Chemically Modified Potato Starches. Carbohydr. Polym. 2002, 47, 245–252.
- Goheen, S. M.; Wool, R. P. Degradation of Polyethylene Starch Blends in Soil. J. Appl. Polym. Sci. 1991, 42, 2691–2701.