ABSTRACT
The wild plant Campanumoea lancifolia (Roxb.) Merr (also called Hong Guo ginseng, HGG), is naturally distributed in a Hmong minority area in western Hunan Province, China. The objective of this study was to identify, quantify, and elucidate the polyphenols and anthocyanins, and their antioxidant activities (via 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and 2,2ʹ-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation scavenging abilities) tested in vitro in the whole fruit, fruit skin, fruit pulp, and leaves of the plant. Results showed that Total polyphenol content (TPC) was highest in leaves (97.76 mg/g), followed by whole fruit (18.18 mg/g), fruit skin (26.16 mg/g), and fruit pulp (8.99 mg/g). Total anthocyanin content (TAC) in fruit skin was the highest at 27.44 mg/g, compared with leaves (20.18 mg/g), whole fruit (12.94 mg/g), and fruit pulp (4.36 mg/g). DPPH scavenging ability and ABTS scavenging ability test followed a decreasing order with the highest ability in leaves, fruit skin, whole fruit, and fruit pulp. Main phenolic compounds (chlorogenic acid, rutin, luteolin-7-O-glucoside, and apigenin) were also highest in leaves. Phenolic compounds and anthocyanins in whole fruit and leaves were further identified using UPLC-QTOF-MS, resulting in the identification of six phenolic acids and their derivatives, seventeen flavonoids and their derivatives, and two anthocyanins observed. These results thus provide the phytochemical evidence to explain and understand the medical and health benefits of HGG.
Introduction
Wild plants, such as Vaccinium glaucoalbum,[Citation1] Malva verticillata L.,[Citation2] and Lonicera japonica Thunb. (Indongcho),[Citation3] may have excellent health benefits. The effects of these wild plants are mainly ascribed to their high antioxidant activities, which are related to their phytochemicals, especially polyphenols, flavonoids, and anthocyanins.[Citation4] Polyphenols can prevent cardiovascular disease, improve immunity, and fight cancer. Flavonoids can reduce blood lipids, blood sugar, and have antiviral properties. Anthocyanins can prevent heart disease, inhibit carcinogenesis, and inflammatory activity.[Citation5–8]
Campanumoea lancifolia (Roxb.) Merr. also called Hong Guo ginseng (HGG), is uncommonly known as a crop plant and is a perennial herb.[Citation9] It can grow at elevations ranging from 300 m to 1,500 m and is usually found in thickets, forests, and grasslands. It is a traditional Chinese medicinal plant that is found primarily in Hmong minority region of western Hunan Province, China, and has more recently been transplanted elsewhere in China, especially Southwest China. It is being developed as both a value-added nutritional food and a plant medicine. HGG fruit is tasty and nutritious, and can be a great source of vitamin C and anthocyanins. Its fresh leaves are consumed as a vegetable by local people. Its roots are used as a medicine that can remove blood stasis and have analgesic effects.[Citation10,Citation11] However, limited information is available about the phytochemical profiling of the edible parts of HGG. Therefore, in this study, the contents and profile of polyphenols and anthocyanins of the whole fruit (skin + pulp), fruit skin, fruit pulp, and leaves of the plant were comparatively analyzed and identified. The antioxidant activities of the extracts of these four plant parts were also tested. The obtained results provide insight on the nutritional health and medical benefits of HGG.
Materials and methods
Plant materials and sample preparations
Fresh HGG samples were collected from a valley (latitude 28°28ʹN, longitude 102°15′ E, and altitude 397 m) of Muchuan County, Sichuan Province, China on January 11, 2018. Collected fruit and leaf samples were free from disease and insects or pest. The species was identified again. The whole fruit of HGG was ground, homogenized, added to a Petri dish, then freeze dried. The fruit skin of HGG was prepared by peeling the fruit and then the peel was freeze dried. The pulp of HGG was homogenized, added to a Petri dish, and freeze dried. The leaves of HGG were also freeze dried. All whole fruit, skin, pulp, and leaves samples were ground, screened through a 200-mesh sieve, and stored in a glass desiccator for testing at room temperature.
Chemicals and reagents
Chlorogenic acid, rutin, luteolin-7-O-glucoside, luteolin, apigenin, and cyanidin-3-O-rutinoside were all purchased from Beijing Solarbio Science & Technology Co. Ltd. (Beijing, China). Analytical-grade formic acid, Folin-Ciocalteu phenol reagent, hydrochloric acid (HCl), methanol, and vitamin E were obtained from Chengdu Kelong chemical reagent Works (Chengdu, China). The 1,1-diphenyl-2-picrylhydrazyl (DPPH, >99.7%), 2-2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, > 99.7%), and chromatographic grade methanol and acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sample extraction
Approximately 0.5 g sample powder was transferred into 50 mL centrifuge tubes with 8 mL 85% methanol (containing 1% formic acid). Subsequently, the mixtures were shaken with a QL-861 vortexer (Kylinbell Lab Instruments, China) for 10 s. The mixtures were sonicated in the KQ5200DE ultrasonic cleaner (Kunshan Ultrasonic instrument co. LTD, China) at 40 °C for 30 min and centrifuged in a SIGMA 3K15 centrifuge (SIGMA centrifuge, Germany) at 8,000 rpm for 10 min. The supernatants were collected in 25 mL centrifuge tubes. The procedure was repeated twice, the extraction was combined, and then fixed to 25 mL. The sample solutions were then filtered with a 0.22 μm syringe filter, and the filtrates were collected and stored at 4°C. Each sample was prepared in triplicate.
Total phenolic content (TPC)
The TPC of each sample was determined spectrophotometrically using the Folin–Ciocalteu method with some modifications.[Citation12] Briefly, 20 μL of extract solution was mixed with 20 μL Folin–Ciocalteu and then the mixture was allowed to equilibrate for 5 min. Then, 160 μL aqueous Na2CO3 (10%, w/v) were added into the mixture and reacted for 1 h avoiding light at room temperature. The absorbance of the mixture was recorded at 765 nm. The standard curve was drawn with the mass concentration (μg/mL) of gallic acid as the x-coordinate (x) and the absorbance value as the y-coordinate (y). The calibration equation was y = 0.0078x + 0.0634 (R2 = 0.997), with a linear range of approximately 2.15–137.50 μg/mL.
Total anthocyanin content (TAC)
The TAC of each sample was determined by using spectroscopy with some modifications.[Citation13] The 50 μL extract solution was mixed with 150 μL 1% hydrochloric acid ethanol solution and the absorption value of the mixture was determined at 535 nm. The standard curve was drawn with the mass concentration (μg/mL) of cornflowery-3-glucosinolate solution as the x-coordinate (x) and the absorbance value as the y-coordinate (y). The regression equation was y = 0.0038x + 0.0385 (R2 = 0.9999), with a linear range of approximately 7.65–245.00 μg/mL.
Antioxidant activities
DPPH radical scavenging activity: The DPPH free radical scavenging activity of each extract was evaluated according to the procedure described by Loizzo et al.[Citation14] An aliquot of 50 μL extract was added into the prepared 100 μL of DPPH solution and 50 μL 85% methanol (contained 1% formic acid). Then, the mixture was reacted for 30 min at room temperature in the dark. The absorbance was measured at 517 nm. A blank control sample was used as a reference. The results of scavenging were expressed by the following equation: DPPH inhibition (%) = [(Acontrol – (Ai – Aj))/Acontrol] × 100%, where Acontrol is the absorbance of the control (without sample); Ai is the absorbance of the sample; and Aj is the absorbance of the reagent blank (without DPPH solution). The IC50 value is the concentration (mg/mL) required to reach 50% of the DPPH radical scavenging activity.
ABTS radical cation scavenging activity: The scavenging activity of the ABTS radical cation (ABTS•+) was determined according to the method described by Cheng et al.[Citation15] with slight modifications. Specifically, 400 μL of extract was added to 160 μL of the fresh ABTS+ solution and mixed. The reaction mixture was kept at room temperature in the dark for 6 min, and the absorbance at 734 nm was subsequently recorded. A blank control sample was used as a reference. The results of the scavenging effect were expressed by the following equation: ABTS inhibition (%) = [(Acontrol – (Ai – Aj))/Acontrol] × 100%, where Acontrol is the absorbance of the control (without sample); Ai is the absorbance of the sample; Aj is the absorbance of the reagent blank (without ABTS solution). The IC50 value is the concentration (mg/mL) required to reach 50% of the ABTS•+ scavenging activity.
Determination of major phenolic compounds
The contents of major phenolic compounds in HGG were performed using an HPLC instrument (Agilent LC1260 series), equipped with a diode array detector (DAD; Agilent Technologies, USA). A Poroshell 120 PFP column (100 mm × 4.6 mm, 2.7 μm particle size, Agilent Technologies, USA) was used. The sample was analyzed after filtration with a 0.22 μm microporous filter membrane. The mobile phase consisted of 0.1% methanoic acid in water (solvent A) and acetonitrile (solvent B). The gradient program was set to 0–10 min (5–10% B), 10–20 min (10–20% B), and 20–35 min (20–40% B) using a flow rate of 0.8 mL/min. The column temperature was 35°C, the injection volume was 5 μL, and the detection wavelengths were 320 nm, 350 nm, and 525 nm. The linearity was assessed by analyzing six different concentrations of the standard solutions of the six compounds in triplicate. Their concentrations are shown in Supplementary Table 1.
Identification of HGG by UPLC-QTOT-MS
The identification of phenolic compounds was determined by an Ultra High-Performance Liquid Chromatography system (Waters Corporation; Milford, MA, USA) with a PDA detector coupled to Waters Xevo G2-XS QTOF micro-mass spectrometer (Waters; Manchester, UK) fitted with an electrospray ionization (ESI) source acting in negative and positive modes. The Waters BEH C18 (2.1 × 100 mm, 1.7 µm; Waters Corporation; Milford, MA, USA) was kept at 30 °C. The injection volume was 1 µL, and the elution was completed in 18 min with a flow rate of 0.3 mL/min. Solvents A (water + 0.1% formic acid) and B (acetonitrile) were used in the following gradients: 0–2 min (5–10% B), 2–10 min (10–20% B), 10–15 min (20–40% B), 15–17 min (40–70% B), and 17–18 min (70–100% B). The PDA spectra for phenolic compounds were measured at 320 nm and 350 nm. For MS analysis, anthocyanins were analyzed in the positive ion (PI) mode; other phenolic compounds were analyzed in the negative ion (NI) modes. The MS parameters were as follows: source temperature of 120 °C, desolvation temperature of 250 °C (400°C in the positive ion), cone gas flow 50 L/h, desolvation gas flow 600 L/h (800 L/h in the positive ion), source capillary of 3.0 kV. The MS analysis was performed using mass scanning from m/z 50 to 1,500.
Statistical analysis
All tests were performed in triplicate unless otherwise specified. The data were evaluated by an analysis of the variance (ANOVA), and a Duncan’s test was conducted for a comparison of means. Differences were considered to be significant at P < 0.05. Statistical computation and analyses were conducted using SPSS software (version17.0, SPSS Inc., Chicago, Illinois, USA).
Results and discussion
Quantification of total polyphenols and anthocyanins
shows the total polyphenol content (TPC) and total anthocyanin content (TAC) of whole fruit, fruit skin, fruit pulp, and leaves. The TPC of leaves (97.76 mg/g) was significantly higher than that of whole fruit (18.18 mg/g), fruit skin (26.16 mg/g), and fruit pulp (8.99 mg/g), i.e., the leaf had a polyphenol content 3.7 times greater than that of skin, and the skin was 2.9 times greater than that of pulps. A greater polyphenol content has often been found in leaf tissue, the epidermis, and bark layers.[Citation16–18] This could be because leaves and bark are more prone to undergo hostile conditions that may promote the synthesis and accumulation of polyphenols.[Citation19,Citation20]
Table 1. Total polyphenol content (TPC) and total anthocyanin content (TAC) of the whole fruit, fruit skin, fruit pulp, and leaves
The TAC had a different order than the TPC. Specifically TAC followed the order: fruit skin > leaves > whole fruit > fruit pulp (P < 0.05). The TAC of skin was 27.44 mg/g, which was higher than leaves (20.18 mg/g) and far higher than pulp (4.36 mg/g). The findings for TAC indicated that anthocyanin existed in the skin of HGG. In general, research indicated that the dark color of the skin confirmed its high TAC.[Citation21] In addition, the anthocyanin content of extracts was consistent with their colors.[Citation22] According to above results, leaves might be an alternative and better resource of polyphenols and anthocyanins than skin and pulp, and the whole fruit was still between skin and pulp, suggesting that whole fruit was more nutritious than the pulp.
Quantification of major phenolic compounds
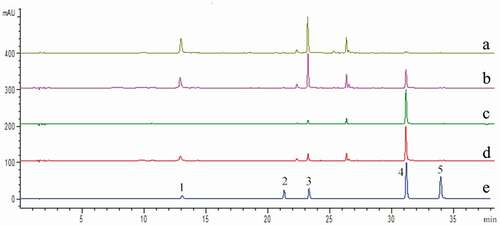
Because the TPC does not quantify the phenolic compounds, the content of six main phenolic compounds from HGG, including chlorogenic acid, rutin, luteolin-7-O-glucoside, luteolin, apigenin, and cyanidin-3-O-rutinoside, were determined by HPLC method (). Chromatograms are shown in . The contents of chlorogenic acid (41.20 mg/g), rutin (0.16 mg/g), luteolin-7-O-glucoside (34.20 mg/g), and apigenin (0.09 mg/g) in leaves were significantly higher than those in whole fruit, skin, and pulp, which indicated greater bioactivity for leaves. Phenolics can defend plants against herbivores or pathogens by reducing their palatability, which may be related to the greater phenolic content in leaves than fruits.[Citation23] Luteolin and cyaniding-3-O-rutinoside were higher in whole fruit, and skin, respectively. Luteolin is one of the main members of the flavonoid family, exists in numerous plant species, and has a variety of pharmacological activities, such as anti-inflammatory, anti-allergy, and reducing uric acid level.[Citation24] In our study, 4.14 mg/g luteolin was determined in whole fruit, which indicated that this fruit may have more medicinal effects, making it valuable for development and application.
Table 2. The concentrations of the major phenolic compounds of the whole fruit, fruit skin, fruit pulp, and leaves
Antioxidant activity
In this research, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging ability and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation scavenging ability assays were used to evaluate in vitro antioxidant activity of the extracts. The results are shown in . In general, antioxidant activity increased with the increase of the concentration of the extracts. In terms of DPPH scavenging ability, the half inhibitory concentration (IC50) value varied from 6.17 to 0.69 mg/mL with the following order: fruit pulp (IC50 = 6.17 mg/mL) > whole fruit (IC50 = 2.65 mg/mL) > fruit skin (IC50 = 1.93 mg/mL) > leaves (IC50 = 0.69 mg/mL). Leaves had the highest radical scavenging capacity while pulp showed the lowest antiradical capacity. When the DPPH scavenging ability was related to the various functional ingredients, Pearson’s correlation coefficients (R) of 0.709 (P > 0.05), −0.900 (P > 0.05), 0.641 (P > 0.05), 0.825 (P > 0.05), 0.642 (P > 0.05), −0.820 (P > 0.05), 0.302 (P > 0.05), and 0.919 (P >0.05) were obtained for the TPC, TAC, chlorogenic acid, rutin, luteolin-7-O-glucoside, luteolin, apigenin, and cyanidin-3-O-glucoside chloride, respectively, which indicated their similar contribution.
Figure 3. The DPPH scavenging ability (a) and ABTS scavenging ability (b) of extract of the whole fruit, fruit skin, fruit pulp and leaves
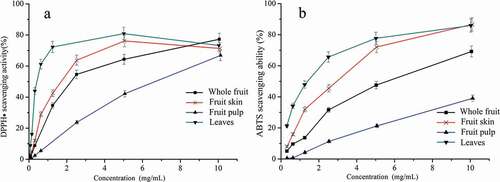
Similarly, ABTS scavenging ability was observed in leaves extract with an IC50 value of 1.36 mg/mL, and the following order: fruit skin (IC50 = 2.54 mg/mL) < whole fruit (IC50 = 2.95 mg/mL) < fruit pulp (IC50 = 14.03 mg/mL). In addition, Pearson’s correlation coefficients (R) of 0.725 (P > 0.05), 0.923 (P > 0.05), 0.658 (P > 0.05), 0.840 (P > 0.05), 0.664 (P > 0.05), 0.664 (P > 0.05), −0.877 (P > 0.05), 0.294 (P > 0.05), and 0.950 (P > 0.05), were obtained for the TPC, TAC, chlorogenic acid, rutin, luteolin-7-O-glucoside, luteolin, apigenin, and cyanidin-3-O-glucoside, respectively, which indicated their similar contribution. The results indicated that the antioxidant capacity of HGG may also be related to the structure and composition of polyphenols.[Citation25]
Identification of phenolics and anthocyanins in whole fruit and leaves
Because whole fruit and leaves are more suitable for daily consumption than skin and pulp, the phenolic and anthocyanin compounds contained in whole fruit and leaves were further identified by UPLC-QTOF-MS. As shown in , a total of twenty-three different phenolic compounds were identified according to commercial standards and the data reported above. Among those twenty-three phenolics, compounds 1–6 were categorized as phenolic acids and their derivatives; compounds 7–23 were likely flavonoids and their derivatives.
Table 3. Phenolic compounds identified from the whole fruit and leaves of Hong Guo Ginseng
In terms of identification of phenolic acids, the fragmentation pathways, as well as retention times and PDA spectrum of compounds 1 and 4 were identified as a chlorogenic acid and cryptochlorogenic acid based on the deprotonated molecule [M-H]− at m/z 353.0854 and tentatively based on the fragmented ion at m/z 191, which was determined by Wojdyło and Nowicka.[Citation26] Compound 2 was tentatively identified as caffeic acid-O-rutinoside. Compound 3 corresponded to feruloyl-O-glucoside, in which the MS spectra exhibited a deprotonated molecule [M-H]− at m/z 355.1046 and the peak was suggested as a feruloyl glucoside. The fragment ion at m/z 193 indicated ferulic acid. Compounds 5 and 6 were tentatively identified as p-coumaroylquinic acid and feruloyl-O-quinic acid, respectively, according to their exact mass and fragmentation. Compound 5 had a deprotonated precursor ion [M-H]− at m/z 337.0904 and fragmented ion at m/z 163 corresponding to coumaroyl moiety, a major fragment ion at m/z 191 corresponding to quinic acid moiety, and major fragment ion at m/z 173 corresponding to a loss of H2O from quinic acid moiety. Compound 6 was determined by the molecule at m/z 367.1012 and fragment ions at m/z 191 [M-H-feruloyl group]−.
Flavonoids and their glycosides were major classes of compounds present in HGG. Three quercetin glycosides, six kaempferol glycosides, four apigenin glycosides, and four luteolin glycosides were identified from HGG, in which kaempferol glycosides were clearly higher than that of quercetin, apigenin, and luteolin glycosides. Compounds 7 and 9 had the same [M-H]− at m/z 609.1476. The [M-162-H]− at m/z 447 and [M-162-162-H]− ion at m/z 285 as observed in MS2. In addition, their UV was similar to that of kaempferol and the compounds were identified as kaempferol-di-O-hexoside. Compounds 8 and 14 produced a diagnostic ion at 301 [M-162-H]−, thus were tentatively identified as quercetin-O-hexoside. By comparing with standards, compound 10, 22, and 23 were identified as quercetin-3-O-rutinoside, luteolin, and apigenin, respectively. Compound 11 was identified as luteolin-7-O-rutinoside, because of the data showing m/z 593.1516 that fragmented in m/z 285 with the loss of a 308 Da (rutinose) residue. Compound 12 was identified as luteolin-7-O-glucoside based on its [M-H]− ions at m/z 447.0940 and the corresponding loss of 162 Da, indicating the presence of a hexose unit. Compound 13 showed an ion fragment at m/z 285 corresponding to kaempferol with a [M-H]− ion at m/z 593.1516 as kaempferol-3-O-rutinoside. For compounds 16 and 19, fragmented ions at [M-H]− 285 corresponded to the successive loss of hexose; compound 19 was identified as kaempferol-3-O-glucoside by comparing with a standard, and compound 16 was suggested as kaempferol-3-O-galactoside due to the shorter retention time than kaempferol-3-O-glucoside.[Citation27] Compounds 18, 20, and 21 lost a fragment of (162+42) Da indicative of an (acetyl)-hexose moiety as suggested Kolniak-Ostek[Citation28] and they produced a main fragment at m/z 489.1023, 489.1023, and 473.1081, respectively.
shows the identified anthocyanins. The molecular ion of compound 1 (Rt 3.56 min) was detected at m/z 611.1614, and fragmented into m/z 303 corresponding to a derivative due to the elimination of a glucoside and a rhamnose ([M-162-146+H]+). Thus, peak 1 was tentatively identified as delphinidin-3-O-rutinoside. Compound 2 (Rt 4.46 min) showed a molecular ion [M+H]+ at m/z 595.1702 and a fragmented ion at m/z 287 ([M-162-146+H]+) was identified as cyanid-3-O-inrutinoside by its authentic reference standard. Overall, phenolics were more abundant in the leaves of HGG than in the whole fruit of HGG, and phenolic compounds in HGG were mainly manifested in the form of depsides and kaempferol glycosides. Previous studies have reported similar results.[Citation1,Citation29]
Table 4. Anthocyanin compounds identified from the whole fruit and leaves of Hong Guo Ginseng
Conclusion
Phytochemical profiling of Hong Guo ginseng (HGG) has been firstly comprehensively identified, quantified, and elucidated, including polyphenols and anthocyanins compositions, and their antioxidant activities in the whole fruit, fruit skin, fruit pulp, and leaves of the plant. It discovered that both leaves and whole fruit were with high content of total polyphenol and total anthocyanin. The strongest DPPH scavenging ability and ABTS scavenging ability were also found in leaves extract and whole fruit extract. Twenty-five compounds were tentatively characterized with HPLC-QTOF-MS/MS, including six phenolic acids, sevnteen flavonoids, two anthocyanins, in which indicated abundant phenolics and flavonoids in leaves and whole fruit. The result herein shows that both HGG plant parts were better resources of polyphenols and anthocyanin, and were also great potential in medical and health benefits as food for daily consumption, and deserves further investigation.
Conflict of Interest
The authors declare no conflict of interest.
Supplemental Material
Download MS Word (12.9 KB)Acknowledgments
This work was supported by the Innovation Team Project of Sichuan Province Authentic Chinese Medicine (Grant number SCCXTD-2020-19), China and the fund of the Innovation Team Project of Sichuan Province Authentic Chinese Medicine (Grant number SCCXTD-2020-19), China in the acknowledgments.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Feng, C. Y.; Wang,W. W.; Ye, J. F.; Li,S. S.; Wu, Q.; Yin,D. D.; Li, B.; Xu, Y. J.; Wang, L. S. Polyphenol Profile and Antioxidant Activity of the Fruit and Leaf of Vaccinium Glaucoalbum from the Tibetan Himalayas. Food Chem. 2017, 219, 490–495. DOI: https://doi.org/10.1016/j.foodchem.2016.09.083.
- Bao, L. M.; Bao, X. H.; Li, P.; Wang, X. L.; Ao, W. L. J. Chemical Profiling of Malva Verticillata L. By UPLC-Q-TOF-MS E and Their Antioxidant Activity in Vitro. J. Pharm. Biomed. Anal. 2018, 150, 420–426. DOI: https://doi.org/10.1016/j.jpba.2017.12.044.
- Seo, O. N.; Kim, G.S.; Park, S.; Lee, J. H.; Kim, Y. H.; Lee, W. S.; Lee, S. J.; Kim, C. Y.; Jin, J. S.; Choi, S. K.; et al. Determination of Polyphenol Components of Lonicera Japonica Thunb. Using Liquid Chromatography–tandem Mass Spectrometry: Contribution to the Overall Antioxidant Activity. Food Chem. 2012, 134(1), 572–577. DOI: https://doi.org/10.1016/j.foodchem.2012.02.124.
- Prior, R. L.; Cao, G. H.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46(7), 2686–2693. DOI: https://doi.org/10.1021/jf980145d.
- Liu, R. H.;. Whole Grain Phytochemicals and Health. J. Cereal Sci. 2007, 46(3), 207–219. DOI: https://doi.org/10.1016/j.jcs.2007.06.010.
- Anson, N. M.; Berg, R. V. D.; Havenaar, R.; Bast, A.; Haenen, G. R. M. M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49(2), 296–300. DOI: https://doi.org/10.1016/j.jcs.2008.12.001.
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306.
- Tsang, C.; Higgins, S.; Duthie, S. J.; Howie, M.; Mullen, W.; Lean, M. E.; Crozier, A. The Influence of Moderate Red Wine Consumption on Antioxidant Status and Indices of Oxidative Stress Associated with CHD in Healthy Volunteers. Br. J. Nutr. 2005, 93, 233–240.
- Chinese pharmacopoeia committee. Chinese Pharmacopoeia; China medical science and technology press: Beijing, 2010; pp 42–43.
- Zhang, A.; Wang, H. Z.; Shao, Q. S.; Xu, M. J.; Zhang, W. S.; Li, M. Y. Large Scale in Vitro Propagation of Anoectochilus Roxburghii for Commercial Application: Pharmaceutically Important and Ornamental Plant. Ind. Crop. Prod. 2015, 70, 158–162.
- Zhang, Y. H.; Cai, J. Y.; Ruan, H. L.; Pi, H. F.; Wu, J. Z. Antihyperglycemic Activity of Kinsenoside, a High Yielding Constituent from Anoectochilus Roxburghii in Streptozotocin Diabetic Rats. J. Ethnopharmacol. 2007, 114(2), 141–145.
- Chu, Y. F.; Sun, J.; Wu, X. Z.; Liu, R. H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50(23), 6910–6916.
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of Anthocyanins in Various Cultivars of Highbush and Rabbiteye Blueberries. Food Chem. 2008, 111(1), 249–254.
- Loizzo, M. R.; Pugliese, A.; Bonesi, M.; Tenuta, M. C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers a Rich Source of Phytochemicals with Antioxidant and Hypoglycemic Properties. J. Agric. Food Chem. 2016, 64(12), 2467–2474.
- Cheng, H.; Feng, S. L.; Jia, X. J.; Li, Q. Q.; Zhou, Y. H.; Ding, C. B. Structural Characterization and Antioxidant Activities of Polysaccharides Extracted from Epimedium Acuminatum. Carbohydr. Polym. 2013, 92(1), 63–68.
- Yancheva, S.; Mavromatis, P.; Georgieva, L. Polyphenol Profile and Antioxidant Activity of Extracts from Olive Leaves. J. Centr. Euro. Agric.. 2016, 17(1), 154–163.
- Hättenschwiler, S.; Vitousek, P. M. The Role of Polyphenols in Terrestrial Ecosystem Nutrient Cycling. Trends Ecol. Evol. 2000, 15(6), 238–243.
- Kumar, V.; Mohamed, M. S.; Veeranarayanan, S. Differential Distribution of Polyphenols in Plants Using Multivariate Techniques. Biotechnol. Res. Inno. 2019, 2, 1119–1130.
- Feng, S.; Luo, Z.; Zhang, Y.; Zhong, Z.; Lu, B. Phytochemical Contents and Antioxidant Capacities of Different Parts of Two Sugarcane (Saccharum Officinarum L.) Cultivars. Food Chem. 2014, 151, 452–458.
- Lopes, M. M.; Silva, E. O.; Canuto, K. M.; Silva, L. M.; Gallão, M. I.; Urban, L.; Miranda, M. R. A. Low Fluence Pulsed Light Enhanced Phytochemical Content and Antioxidant Potential of ‘Tommy Atkins’ Mango Peel and Pulp. Inno. Food Sci. Emer. Technol. 2016, 33, 216–224.
- Tuan, P. A.; Bai, S. L.; Yaegaki, H.; Tamura, T.; Hihara, S.; Moriguchi, T.; Oda, K. The Crucial Role of PpMYB10.1in Anthocyanin Accumulation in Peach and Relationships between Its Allelic Type and Skin Color Phenotype. BMC Plant Biol. 2015, 15(1), 280.
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of Phenolic Compounds Content and Antioxidant Activity in Skin, Pulp, Seed, Cane and Leaf of Five Native Grape Cultivars in West Azerbaijan Province, Iran. Food Chem. 2016, 199, 847–855.
- Sundqvist, M. K.; Wardle, D.; Olofsson, E.; Giesler, R.; Gundale, M. J. Chemical Properties of Plant Litter in Response to Elevation: Subarctic Vegetation Challenges Phenolic Allocation Theories. Funct. Ecol. 2012, 26(5), 1090–1099.
- Lin, Y.; Shi, R. X.; Wang, X.; Shen, H. M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Tar. 2008, 8(7), 634–646.
- Zheng, W.; Wang, S. Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51(2), 502–509.
- Wojdyło, A.; Nowicka, P. Anticholinergic Effects of Actinidia Arguta Fruits and Their Polyphenol Content Determined by Liquid Chromatography-photodiode Array Detector-quadrupole/time of Flight-mass Spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223.
- Ye, M.; Yan, Y.; Guo, D. A. Characterization of Phenolic Compounds in the Chinese Herbal Drug Tu-Si-Zi by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Sp. 2005, 19(11), 1469–1484.
- Kolniak-Ostek, J.;. Identification and Quantification of Polyphenolic Compounds in Ten Pear Cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77.
- Di Lecce, G.; Arranz, S.; Jauregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R. M. Phenolic Profiling of the Skin, Pulp and Seeds of Albarifio Grapes Using Hybrid Quadrupole Time-of-flight and Triple-quadrupole Mass Spectrometry. Food Chem. 2014, 145, 874–882.