?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Owing to its nontoxicity and better efficiency, low-temperature plasma (LTP) has been proposed to be a strong antibacterial agent against multidrug-resistant bactericidal pathogens. On the other hand, previously several food-borne pathogens were treated by chemically hazardous and least-efficient irradiation techniques. Because of its superior efficiency, in the current study, we implemented LTP protective mechanism against Escherichia coli (E.coli). In particular, we investigated the sterilization effects of plasma on the degradation of pure samples of E.coli strain ( colony forming units) in the presence and absence of
fumes. Interestingly, the proposed mechanism improved considerably the microbial reduction, inactivation efficiency and kill rate of the plasma-treated E.coli samples. The decrease in bactericidal cell viability was confirmed by deoxyribonucleic acid (DNA) quantification and membrane damage by protein leakage test. The reactive oxidants species (ROS) were explored via optical emission spectroscopy (OES). For the investigation of the surface characteristics, we carried out the scanning electron microscopy (SEM) analysis of the plasma-treated nutrient media containing E.coli colonies. The reported results confirmed that low pressure and low temperature plasma can be a potential alternative for the degradation of multidrug-resistant E. coli.
KEYWORDS:
INTRODUCTION
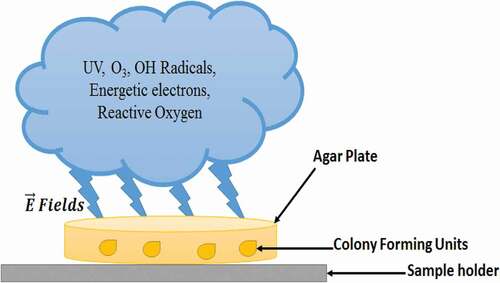
A low-temperature plasma (LTP) is partially ionized gas comprising energetic electrons, diversity of ions, active molecules, intense ultraviolet radiations and energetic electric fields .[Citation1] LTP, cold plasma or non-thermal plasma, is mainly classified as ‘low pressure glow discharge’ and ‘high pressure (atmospheric pressure) plasma.[Citation2] Owing to nontoxic and environment-friendly characteristics of LTP, it is widely used for biological sterilization and disinfection in food industry, especially to increase the shelf life of food and to keep the natural food ingredients active and durable.[Citation3,Citation4] In the recent past, conventional approaches involving non-plasma-based methods were adopted for the sterilization purposes; however, they proved to be least effective due to the germicidal properties of agents used for the sterilization. On the other hand, plasma-based gases that become biocide after plasma ignition possess no such harmful aspects .[Citation5–7] The effects of LTP on biological matter in diversity of plasma-based systems were addressed in recent studies.[Citation8–12] The studies concluded that after plasma discharge occurrence, a complex mixture of rich active components of ionic radicals was created that caused diversity of chemical reactions. It also produced ROS, highly reactive nitrogen species (RNS), ultraviolet (UV) radiations and active molecules. The electric fields originated in the plasma were found to be effective for degradation of bactericidal effects.[Citation13] Plasma discharge outcomes play a vital role in bactericidal disinfection process as illustrated in .[Citation14] Low-pressure plasma results in a uniform broader glow discharge, which yields high ROS density of radicals, oxygen (O), and UV radiations which may prove to be highly useful for antibacterial applications .[Citation15–17]
In the current study, we aimed to investigate the effects of low-temperature DC induced air plasma on E.coli disinfection. It is expected that LTP treatment may play a vital role in the breaching of the cell membranes and cell walls, DNA strands, and may affect the survival rate of E.coli colonies in the agar plates. The investigations have also been extended to observe the cell content leakage, reproduction and self-duplication of E.coli. As the plasma treatment affects all the metabolism of cell, so the same may influence E.coli inactivation considerably. A thorough study has been carried out by using SEM, OES, DNA quantification, protein leakage test and visual analysis. In the study, we investigated rigorously and reported an extensive impact of all reactive species provoked by low temperature and low pressure air plasma discharge on E. coli inactivation. The expected outcome of the study may be referred as a guideline for a large scale solid food sterilizations processes.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The multidrug-resistant strains isolated from the clinical samples E.coli (190CRC) were resistant to Ampicillin, erythromycin, ciprofloxacin, nalidixic acid, streptomycin and sulfamethoxazole–trimethoprim, gentamycin. The samples were collected from Microbiology and Public Health Lab, COMSATS University, Pakistan. E.coli was revived on Luria-Bertani (LB) Broth (Sigma-Aldrich, Ireland Ltd.) agar plates. For each set of experiment, overnight (16 to 18 hrs) bacterial cultures grown in Lauria broth at 37°C were used.
Bacterial cell survival assay
Overnight culture of each bacterial strain (~ CFU) was used to inoculate autoclaved distilled water to make a water suspension. Later, these suspensions were used to prepare different groups of experimental design (with and without treatment) and were then exposed to plasma subjected to different treatment times. Each sample was then serially diluted, spot plated on to LB agar plates which were incubated at 37°C for 24 hours. The number of colonies on each plate was counted and the colony forming units per ml (CFU/ml) were calculated using the following formula.[Citation18]
The killing percentage of overall inactivation efficiency of plasma was calculated using the following formula .[19]
Experiment
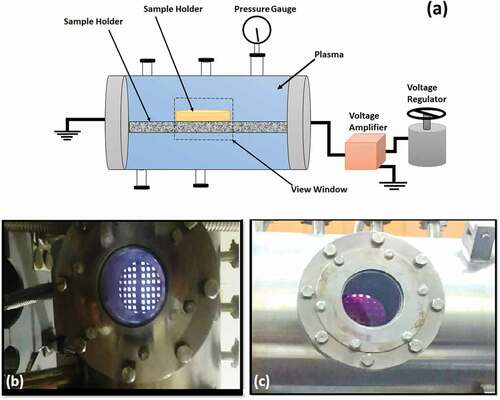
A stainless-steel made sterilization chamber was used for the E.coli degradation as schematically illustrated in . A visual view of discharge for 0 mbar and 0.1 mbar air pressure inside the chamber is illustrated in . Initially, a DC voltage (2.5 kV for 0 mbar pressure and 4 kV for 0.1 mbar pressure) was applied. The strain containing plates were treated for different time intervals of 10, 20, 30 and 40 seconds. Initially, when no spray was introduced the pressures prevailing inside the chamber was 0 mbar which rose to 0.1 mbar when 5.9 sccm of
was introduced inside the chamber. The untreated as well as plasma-treated samples were characterized by visual counting of the colony forming units (CFU) on agar plates. DNA, protein leakage tests and SEM analysis were performed to study degradation of E.coli, cell viability and surface morphology of the plasma treated samples.
RESULTS AND DISCUSSION
The OES, statistical technique, visual counting, and SEM analyses were adopted to investigate and report on the outcomes of low-pressure plasma-treated E.coli. Stepwise analytical processes were performed and their findings are illustrated and discussed in the following sections.
OES
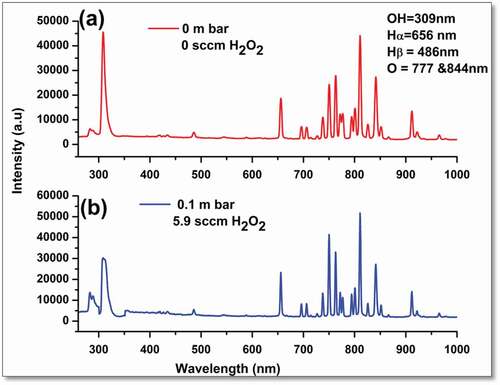
The plasma-generated ROS, responsible for bactericidal inactivation, were observed and recorded by emission spectroscopy. The emission spectrum of plasma generated inside the sterilization chamber, in the wavelength range of 250–900 nm, was numerically analyzed to measure the normalized density of the reactive oxidizing species (OH radicals, reactive oxygen, and reactive hydrogen). The wavelengths versus intensity spectral peaks are shown in . It is obvious that the OH root peak appears at 309 nm, Balmer α and β peaks of Hydrogen at 656 nm and 486 nm, respectively. A highly reactive oxygen peaks appeared at 777 nm and 844 nm. The normalized ROS density was obtained via Gaussian de-convolution approach .[Citation20]
where
is full width at half of maximum (nm) and
(x) dx = 1. Calculations were performed for
radicals, reactive oxygen and reactive hydrogen species for various treatments times, with and without
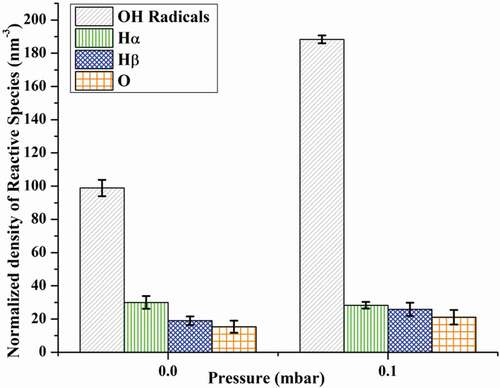
fumes. The calculated normalized density of examined reactive species (,
,
& O) is illustrated in . The graph indicates an increasing trend in the yield of ROS in the presence of
-generated air plasma in contrast to the absence of
.
Antibacterial analysis by CFU’s
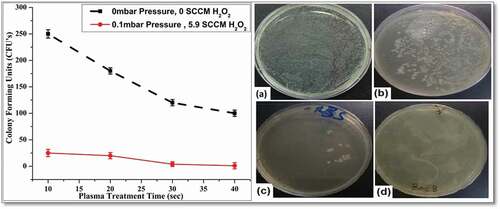
The feasible cell counts of CFUs were developed after spreading bacteria over the surface of a nutrient medium solidified with agar, and were contained in a petri dish. The inhibition of E.coli growth and cell colonies on agar-plates were detected by viable cell counts. Investigation of the inactivation of the nutrient medium and degradation of E.coli after plasma treatment was targeted under different experimental conditions. represents typical images of E.coli containing LB broth agar plates, with counted number of colonies illustrated graphically as well. It has been noticed that increase in plasma treatment time reduced the E.coli growth rate. The plasma treatment up-to 40 seconds, in presence of vapors inside the sterilization chamber, resulted in complete degradation of E.coli. At high input voltage, the occurrence of plasma discharge, inside the sterilization chamber caused generation of ROS, RNS and UV radiations, energetic electrons and intense electric fields. Depending upon the level of sensitivity of bacterial species, intense electric fields, circulating current, massive ions, duration of exposure and energetic electrons, the plasma treatment caused damaging of cells irreversibly and prevent yield of bacteria. The bacteria containing media exposed to an electric field became charged and induced a potential resulting in cellular death. These plasma outcomes caused the rupturing of cytoplasm material inside the cell wall, which damaged the integrity of cell wall and surface morphology of E.coli cell inside colonies. The nutrient medium which is responsible for bacterial growth neutralized after plasma treatment and resulted in prevention of E.coli growth.
Figure 5. (Color online) Graphical and visual representation of E.coli colonies under different experimental conditions. (a) Pure strain of E.coli cultured Agar plates with 107CFU’s
Degradation efficiency and eradication rate
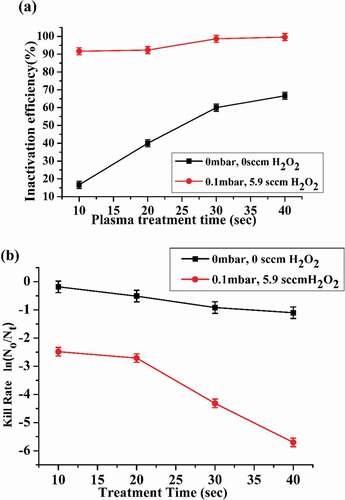
The plasma treatment caused splitting of the E.coli cell wall and cell membrane. The cell content leakage occurred and E.coli lost the ability of reproduction and self-duplication. Plasma treatment affected all the metabolism of cell which resulted in E.coli inactivation. (a) illustrates the inactivation efficiency of low-pressure plasma subjected to various conditions calculated by relation[Citation19Citation20]:
Figure 6. (a) Inactivation efficiency of plasma on E.coli cells. (b) Kill rate for different plasma treatment times
No is the number of untreated colonies and Nt is the number of colonies after plasma treatment.
The inactivation efficiency is mainly determined by the ROS densities of O, , H, etc., radicals, generated inside the chamber. ROS caused cell wall/cell membrane etching, cell wall perforation, electro-perforation, oxidation of proteins and DNA distortion. Moderate reactive species exerted effective bactericidal activity by destroying bacterial cells membrane, which caused intracellular leakage and ultimately led to cells lyses. (b) demonstrates the kill rate calculated by logarithmic ratio
The inactivation efficiency and kill rate was observed maximum (99.9%) at 0.1 mbar air pressure and 5.9 sccm
inside the sterilization chamber for 40 sec plasma exposure time.
DNA and protein quantification
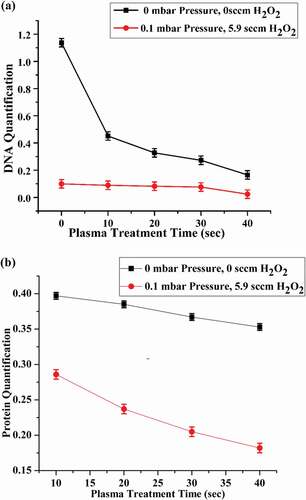
Leakage of DNA concentration with respect to plasma treatment under various experimental conditions is illustrated in (a). DNA leakage is minimum for the case of (0 mbar, 0 sccm ) and maximum for the case of (0.1 mbar, 5.9 sccm
) for 40 seconds plasma exposure. Due to the disruption of DNA strands, the integrity of DNA got damaged which resulted in the breaching of cell membrane and affected the survival rate of E.coli colonies in the agar plates. ROS produced by plasma have been found to be a cause of DNA residue damage and DNA itself. The generation of
radicals, increased significantly, due to energetic electrons produced by plasma in presence of
vapors, inside sterilization chamber, by the reaction .[Citation21,Citation22]
Figure 7. (a) DNA quantification of plasma treated E.coli samples. (b) Protein leakage tests of plasma treated E.coli samples for different durations
+2e−➔2
These radicals are highly reactive, capable of strands breaking of DNA, and caused the segregation of DNA strands near the cell wall of the E.coli. Other ROS like reactive oxygen (O2) generated by plasma discharge oxidized the base guanine and led-up to the oxidation process by formation of 8-oxy guanine, which broke the DNA strands. The highly reactive ozone (O3) also damaged the DNA strand directly and also preceded the oxidation process by the formation of O2 and
radicals. Proteins are the important structural component of the cell membrane and cytoplasm. The results of protein concentration are shown in (b) under different experimental conditions, showing maximum protein leakage for the case of 0.1 mbar pressure and 5.9 sccm
addition. The bacteria which are responsible for protein degradation releases enzymes for protein degradation, which are itself proteins. These protein leakages demonstrate the cell wall damage and affect the normal cell growth. Also the release of such proteins indicates the cell membrane integrity.
SEM Analysis
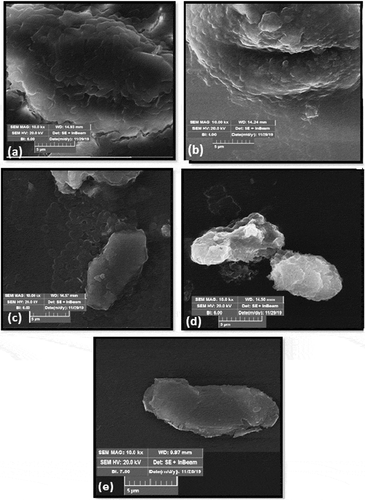
SEM utilizes the beam of electrons which was focused on the agar plates that were cultured with bacteria to investigate the mutation occurrence in the physical structure of bactericidal DNA. It is also helpful in determining the media activity where the bacterial colonies formulate. In this research SEM analysis (TESCON-SEM at Institute of Space Technology, Islamabad) of agar plates, containing E.coli colonies, treated by low-pressure plasma under different experimental conditions, were performed and presented to examine the ultra-structural change of plasma-treated E.coli. (a-e) represents the typical SEM images of E.coli containing nutrient medium treated for 10, 20, 30 and 40 seconds plasma exposure at 0.1 mbar pressure and 5.9 sccm of injection. Plasma treatment caused the shrinkage of cell wall suggesting the inactivation of media. During plasma treatment some cells were completely destroyed which ceased the growth rate of bacteria and this bactericidal effect is noted. With the increase in plasma treatment time from (10 to 40 sec) and in presence of vaporized
in the sterilization chamber, the disruption and erosion of the cell wall of E.coli colonies occurred and resulted in higher bactericidal inactivation. The bacterial cytoplasm has electric charges that caused for nutrition purposes. Under the exposure of intense electric field, the distribution of voltage between the cell membrane structure and the cell membrane surface charges got disturbed. Highly energetic and penetrated electrons caused the disintegration and separation of cell wall which resulted in the release of cytoplasm material which neutralized E.coli.
CONCLUSION
In conclusion, our experimental results suggest that LTP is a highly useful technique for food sterilization and bactericidal degradation, especially for E.coli. In particular, -injected low-temperature air plasma discharge is responsible to generate ROS, UV radiations, reactive oxygen (O), reactive hydrogen (
), energetic electrons and intense electric fields, which effectively disinfected E.coli. The normalized ROS density calculated by applying Gaussian distribution function on OES spectrum resulted in its higher yield rate in presence of
fumes. Higher plasma treatment time in presence of
vapors has positively influenced the inactivation efficiency, kill rate, DNA strands damage, protein leakage and reduced re-generation of E.coli. SEM and visual analysis confirm the reduction in colonial bacterial size and its number density. Higher plasma exposure time in presence of
fumes reduced the DNA quantification and protein leakage. The findings of current investigations may be beneficial and referred as a guideline for the sterilization of food, surgical equipment and solid materials having bactericidal infection.
References
- Report of the Department of Energy Office of Fusion Energy Sciences Workshop on Low Temperature Plasmas, (2008), March 25-27, pp. 8–12, University of California, USA.
- Parvulescu, V. I.; Magureanu, M.; Lukes, P., (2012), Plasma Chemistry and Catalysis in Gases and Liquids, ch.1, pp. 1–34, First Edition. Wiley-VCH Verlag GmbH & Co. KGaA. DOI: https://doi.org/10.1002/9783527649525.
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using PlasmaTechnology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20(20), 5216. DOI: https://doi.org/10.3390/ijms20205216.
- Kim, H. J.; Yong, H. I.; Park, S.; Kim, K.; Kim, T. H.; Choe, W.; Jo, C. Effect of Atmospheric Pressure Dielectric Barrier Discharge Plasma on the Biological Activity of Naringin. Food Chem. 2014, 160, 241–245. DOI: https://doi.org/10.1016/j.foodchem.2014.03.101.
- Chu, P. K.; Lu, X. Low Temperature Plasma Technology Methods and Applications: CRC press, 1st Edition. 2013. 1–100. doi:https://doi.org/10.1201/b15153
- Jacobs, P. T.; Lin, S. M. (1987), Hydrogen Peroxide Plasma Sterilization System, US patentNo. 4643876, pp. 1–45, Surgikos, Inc., Arlington, Tex.
- Bol’shakov, A. A.; Cruden, B. A.; Mogul, R.; Rao, M. V. V. S.; Sharma, S. P.; Khare, B. N.; Meyyappan, M. Radio-Frequency Oxygen Plasma as a Sterilization Source. AIAA Journal. 2004, 42(4), 823–832. DOI: https://doi.org/10.2514/1.9562.
- Moreau, S.; Moisan, M.; Ricard, A.; Ricard, A.; Ricard, A. Using the Flowing Afterglow of a Plasma to Inactivate Bacillus Subtilis Spores: Influence of the Operating Conditions. J. Appl. Phys. 2000, 88(2), 1166. DOI: https://doi.org/10.1063/1.373792.
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier,M, J.; Tabrizian,; Yahia, L. H. Low-temperature Sterilization Using Gas Plasmas: A Review of the Experiments and an Analysis of the Inactivation Mechanisms. Int. (2001),, J. Pharm., 226, 1–21, DOI: https://doi.org/10.1016/s0378-5173(01)00752-9 .
- Abd, R.; Jalil. A Review of Low Temperature Plasma Treatment of Textile Materials. J.mater.sci. 2015, 50, 5913–5943. DOI: https://doi.org/10.1007/s10853-015-9152-4.
- Park, B. J.; Lee, D. H.; Park, J. C.; Sterilization Using Microwave-induced Argon Plasma Systemat Atmospheric Pressure. Phys. Plasmas. 2003, 1011, 4539. DOI:https://doi.org/10.1063/1.1613655.
- Eto, H.; Ono, Y.; Ogino, A.; Nagatsu, M., (2008), Low Temperature Sterilization of Wrapped Materials Using Flexible-Sheet Type Dielectric Barrier Discharge, 93, pp. 221502. DOI: https://doi.org/10.1063/1.3039808.
- Laroussi, M.; Leipold, F. Evaluation of the Roles of Reactive Species, Heat, and UVradiation in the Inactivation of Bacterial Cells by Air Plasmas at Atmospheric Pressure. Int. J. Mass Spectrom. 2004, 233(1–3), 81–86. DOI: https://doi.org/10.1016/j.ijms.2003.11.016.
- Perinban, S.; Orsat, V.; Raghavan, V. Non-thermal Plasma Liquid Interactions in Food Processing. COMPR. REV. FOOD SCI. F. 2019, 18(6), 1985–2008. DOI: https://doi.org/10.1111/1541-4337.12503.
- Jin –Pyo Lim’s, Han S. Uhm’s. Influence of Oxygen in Atmospheric-pressure Argon Plasma Jet on Sterilization of Bacillus Atrophaeous Spores. Phys. Plasmas. 2007, 14 (9), 093504. DOI: https://doi.org/10.1111/1541-4337.12503.
- Kelly-Wintenberg, K.; Hodge, A.; Montie, T. C.; Use of a One Atmosphere Uniform Glow Discharge Plasma to Kill a Broad Spectrum of Microorganisms. J. VAC. SCI. TECHNOL A. 1999, 174, 1539. DOI:https://doi.org/10.1116/1.581849.
- Gaunt, L. F.; Beggs, C. B.; Georghiou, G. E.; Bactericidal Action of the Reactive Species Produced by Gas-Discharge Nonthermal Plasma at Atmospheric Pressure: A Review, IEEE. trans. plasma sci. 2006, 344, 1257–1269. DOI:https://doi.org/10.1109/TPS.2006.878381.
- http://technologyinscience.blogspot.kr.
- Zhang, R.; Wang, L.; Wu, Y.; Guan, Z.; Jia, Z.; Bacterial Decontamination of Water by Bipolar Pulsed Discharge in a Gas–Liquid–Solid Three-Phase Discharge Reactor,IEEE. Trans. Plasma Sci. 2006, 344, 1370–1374. DOI:https://doi.org/10.1109/TPS.2006.878383.
- Ahmed, M. W.; Yang, J. K.; Mok, Y. S.; Lee, H. J.; Underwater Capillary Discharge with Air and Oxygen Addition. J. Korean Phys. Soc. 2014, 659, 1404–1413. DOI:https://doi.org/10.3938/jkps.65.1404.
- Arjunan, K. P.; Sharma, V. K.; Ptasinska, S.; Effects of Atmospheric Pressure Plasma on Isolated and Cellular DNA- a Review. Int. J. Mol.. 2015, 162, 2971–3016. DOI:https://doi.org/10.3390/ijms16022971.
- Sharma, V. K.;. Oxidation of Amino, Peptides, and Proteins; Wiley: New Jersey, NJ, USA, 2013.