 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In order to improve the color and functional properties of sunflower seed protein, low-temperature defatted sunflower seed meal was used as the raw material to study the effects of limited enzyme hydrolysis combined with macroporous resin adsorption decolorization technology on the color and functional properties of sunflower seed protein. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), circular dichroism (CD), particle size, intrinsic fluorescence intensity, surface hydrophobicity (H0) and scanning electron microscopy (SEM) were used to analyze the influence on its structure. The results showed that the molecular weight of sunflower seed protein was reduced after limited enzyme hydrolysis combined with macroporous resin decolorization; the content of α-helix and β-turn was significantly reduced, and the content of β-sheets and random coils was significantly increased; the average particle size was reduced; the fluorescence intensity was increased and the red shift phenomenon occurred. H0 also increased, SEM results showed that the surface of sunflower seed protein changed from a smooth large sheet-like structure to an irregular fragmented fast-like structure. Solubility, foamability, emulsification and emulsification stability were all significantly improved (p < .05), while foam stability and oil retention were reduced. The above results indicate that limited enzyme hydrolysis combined with macroporous resin adsorption and decolorization changes the structure of sunflower seed protein, and then improve its functional properties.
Introduction
Sunflower seeds are an important oil crop. The by-product of extracting oil from sunflower seeds is sunflower seed meal (cake), which is rich in protein, dietary fiber and biologically active substances, and has a relatively balanced amino acid composition. The crude protein content is 30%-50%.[Citation1] Additionally, the antinutritional substances and toxic cyanogens in sunflower protein are very low.[Citation2] It is a high-quality protein source very suitable for human ingestion. Sunflower seed meal contains 1–4% of polyphenols (mainly chlorogenic acid).[Citation3] These polyphenols are easily oxidized under alkaline conditions, causing browning, resulting in the extracted sunflower seed protein being dark gray or ink. It is green and brings bitterness and astringency to sunflower seed protein.[Citation2,Citation4,Citation5] It is usually underutilized or used as animal feed.[Citation6] At present, the commonly used decolorization methods for plant proteins mainly include activated carbon adsorption, hydrogen peroxide oxidation, organic solvent extraction and macroporous resin adsorption. Malik et al.[Citation7] Repeated extraction with methanol 4 times to remove polyphenols, found that the color of sunflower seed protein was significantly improved after methanol extraction, and its Lightness value (L*) increased from 40.87 to 62.53, and solubility, foaming, emulsification and gelation significantly improved. Macroporous adsorption resin is a kind of organic polymer adsorbent, which has the advantages of fast adsorption speed, large specific surface area, simple resin regeneration and high purification ability. Therefore, it is also widely used in the decolorization of plant proteins. Pickardt et al.[Citation8] Used XAD-16 HP macroporous resin to extract sunflower seed protein with low phenolic compounds under acidic conditions. They found that the initial phenolic compound content was reduced by 85.7% after adsorption by the macroporous resin. The whiteness of the protein is increased by 26%, and its L* value is 77.3, which has a significant decolorization effect.
Sunflower seed protein has poor functional properties, especially solubility.[Citation7] Modification methods are needed to improve the functional properties of proteins and then be used in the food industry. At present, the commonly used modification methods are: physical modification, chemical modification and enzymatic modification (limited enzymatic hydrolysis). Many research reports claim that enzymatic hydrolysis can actively modify proteins, such as soybean, pumpkin seed and rice proteins.[Citation9–11] Enzymatic hydrolysis may destroy specific peptide bonds, thereby changing the structure of the protein.[Citation11] Limited enzymatic hydrolysis usually refers to mild enzymatic hydrolysis, and the degree of hydrolysis is generally 1%-10%. The advantages over traditional enzymatic hydrolysis are: limited enzymatic hydrolysis has the advantages of milder reaction conditions, shorter action time and lower cost, and does not reduce the nutritional value of the original protein, and can efficiently improve the hydrolyzate.[Citation12,Citation13] For example, JIN et al.[Citation14] Used trypsin to carry out limited enzymatic hydrolysis of walnut protein (the degree of hydrolysis was 1%, 3% and 7.1% respectively), and found that the secondary and tertiary structure of walnut protein occurred after enzymatic hydrolysis. Significant changes, solubility, foaming and emulsifying properties are all significantly improved.
Although there have been some studies on the decolorization and modification of sunflower seed protein, so far, It is rarely reported that the combination of decolorization technology and modification technology is applied to sunflower seed protein. Qin et al.[Citation15] Used limited enzymatic hydrolysis combined with macroporous resin decolorization technology to extract light-colored sunflower seed protein, optimized the limited enzymatic hydrolysis and macroporous resin resin decolorization process, and achieved significant decolorization effects. At present, the theoretical and technical knowledge about the effect of limited enzymatic hydrolysis and macroporous resin adsorption treatment on the functional properties and structure of sunflower seed protein is still insufficient. Understanding the functional and structural properties of sunflower seed protein and its hydrolyzates is essential to control/improve its properties during processing and/or utilization. Therefore, this study aimed to explore the effect of limited enzymatic hydrolysis combined with macroporous resin adsorption and decolorization treatment on the color, function and structural properties of sunflower seed protein.
Materials and methods
Materials and reagents
Low-temperature defatted sunflower seed meal (protein content 39.03%) was purchased from Luhua Sunflower Seed Oil Co., Ltd. (Inner Mongolia, China). AB-8 macroporous resin was obtained from Zhengzhou Hecheng New Material Technology Co., Ltd. (Henan, China). Alcalase protease (1 × 104 U/g) was provided by Novozymes Biotechnology Co., Ltd. (Tianjin, China). Test reagents were of analytical purity, and ultra-pure water was used to prepare solutions.
Preparation of sunflower seed protein
Sunflowr seed protein was prepared according to the procedure used by alkali-soluble acid precipitation method. Weigh 50 g of low-temperature defatted sunflower seed powder, add 500 mL of absolute ethanol 1:10 (g/mL) to mix and stir evenly, stir in a constant temperature water bath at 25°C for 1 h and centrifuged at 4000 rpm for15min, take the precipitate and add Stir 750 mL distilled water evenly, adjust the pH to 7.0 with 0.1 mol/L NaOH, stir for 1 h in a constant temperature water bath at 50°C, centrifuge at 4000 rpm for15 min, take the supernatant and adjust the pH to 0.1 mol/L HCl 4.0. Carry out protein acid precipitation, centrifuged at 4000 rpm for15 min to take the precipitate, wash the precipitate 3 times, collect the precipitate and adjust the pH to 7.0 with 0.1 mol/L NaOH, freeze-dry and store for later use. The protein content of sunflower seed protein was 76.96% (dry basis) as determined by the Kjeldahl method (N%×5.30).
Preparation of decolorized sunflower seed protein adsorbed by macroporous resin
Weigh 50 g of low-temperature defatted sunflower seed powder, add 500 mL 1.3 mol/L sodium chloride solution (1:10, g/mL), mix and stir evenly, adjust the pH of the solution to 6.0 with 0.1 mol/L NaOH, and then place it in a constant temperature water bath at 25°C Stir in medium for 1 h, collect the supernatant by centrifugation 4000 rpm for15 min, add AB-8 macroporous resin to the crude protein extract and react for 120 min at 25°C. macroporous resin is added at a ratio of 1:500 (macroporous resin: crude protein extract), filter the resin to collect the supernatant, adjust the pH to 3.8 with 0.1 mol/L HCl, take the precipitate by centrifugation 4000 rpm for15 min, wash the precipitate with distilled water 3 times, adjust the pH of the solution to 7.0 with 0.1 mol/L NaOH, freeze-dried and stored for later use.
Preparation of limited enzyme hydrolysis combined with macroporous resin decolorized of sunflower seed protein
Weigh 50 g of low-temperature defatted sunflower seed powder, add 500 mL 1.3 mol/L sodium chloride solution (1:10, g/mL), mix and stir evenly, adjust the pH of the solution to 6.0 with 0.1 mol/L NaOH, and then place it in a constant temperature water bath at 25°C Stir in medium for 1 h, centrifuge at 4000 rpm for15min to collect the supernatant, adjust the pH value of the crude sunflower seed protein extract to 7.0 with 0.1 mol/L NaOH, and incubate the crude protein extract at 55°C for 10 minutes. Alkaline protease is added at a ratio of 1:500 (enzyme: protein). Inactivate the alkaline protease by heat treatment at 100°C for 10 minutes to stop the reaction, and cool the sample to room temperature (25 ± 1°C), add AB-8 macroporous resin to the crude protein extract and react for 120 min at 25°C. Macroporous resin was added at a ratio of 1:500 (macroporous resin: crude protein extract), filtered the resin to collect the supernatant, and adjust the pH to 3.8 with 0.1 mol/L HCl for acid precipitation, centrifuge at 4000 rpm for15min to take the precipitate, wash the precipitate with distilled water 3 times, adjust the pH of the solution to 7.0 with 0.1 mol/L NaOH, and freeze-dry it for later use.
Determination of the degree of hydrolysis (DH)
The degree of hydrolysis (DH) was determined using the pH-stat method and calculated based on the following formula:
where C, concentration of NaOH, mol/L; V, volume of NaOH consumed, mL; htot, the number of millimoles of peptide bonds per gram of raw protein,7.5 mmol/g. Mp, Protein quality, g; α, represents the average degree of dissociation of the α-amino acid groups and expressed as:
where pH and pK are values at which the hydrolysis was done.
Color measurement
The color of sunflower seed protein isolate was measured in the CIE-L* value color system using a CR-10 colorimeter (Konica Minolta Holdings Co., Ltd, Japan) as the average of 3 measurements.
Polyphenol content measurement
According to the method of the International Organization for Standardization (ISO) 14502–1 (ISO, 2005). The content of polyphenols was measured at 325 nm by ultraviolet spectrophotometry, with chlorogenic acid as the standard. The content of polyphenols is expressed as chlorogenic acid equivalent (CAE) in g/100 g.
SDS-PAGE
Samples were dissolved in 10 mM phosphate buffer sodium (PBS) pH 7.2 at a concentration of 5 mg/mL. The protein solution and loading buffer containing β-mercaptoethanol were mixed. Proteins were characterized by SDS-PAGE using a 5% stacking gel and 12% separating gel. Electrophoresis was carried out using an electrophoresis apparatus (HT-300, Beijing Hongtao Foundation Technology Development Co., Ltd., China). The gel was stained with 1% (w/v) Coomassie Blue R-250 for 8 h. Molecular weight (MW) standard (14–95 kDa, Blue Plus Protein Marker, TransGen Biotech, Beijing, China) was used. decolor with methanol-glacial acetic acid decolorizing solution, and take pictures after decolorization.[Citation16]
Circular dichroism (CD)
A far-ultraviolet circular dichromatograph (CD J-815, JASCO company, Japan) was used for the determination. The sample is configured as a 0.1 mg/mL protein solution (pH = 7.0, 10 mmol/LPBS), poured into a 1 mm quartz colorimetric cell, and scanned in the range of 190–250 nm.The average value of six scans was taken as spectrogram. CD-pro software was used to analyze secondary structures, namely, α-helix, β-sheet, β-turn, and random coil.[Citation17]
Particle size distribution
The laser particle size analyzer (Nano-ZS90, Malvern Instruments Ltd., Malvern, UK) was preheated for 30 min. The system was turned on, followed by the blender and ultrasonic switch. The agitator circulated in sequence. The particle size meter was cleaned until the shading rate was 0.00%. The sunflower protein was slowly added to the sample tank until the software showed normal concentration and more than 5% shading rate. Data were collected when the fluctuation range of the shading rate was very small.
Intrinsic fluorescence
Use fluorescence spectrometer for analysis (FL-6500, Hitachi, Tokyo, Japan). The 0.1 mg/mL sample was dissolved in 10 mmol/L PBS pH7.0. The excitation wavelength was 280 nm, the emission wavelength was 300–420 nm, and the slit width was 2.5 nm. At the same time, make a blank control of the corresponding buffer.[Citation18]
Surface hydrophobicity (H0)
Using 1-anilino-8 – naphthalene sulfonate (ANS) as a hydrophobic fluorescence probe. Take a certain amount of protein sample and disperse it in 10 mmol/L PBS (pH 7.0) to prepare a concentration of 0.01 mg/mL-0.1 mg/mL. Take 4 mL of solutions of different concentrations of samples, add 8 mmol/L ANS solution 20 μL, vortex for 5 s, and let stand for 10 min in the dark, Shake well, and then measure the fluorescence of the sample. In this experiment, the excitation wavelength was 390 nm and the emission wavelength was 470 nm. The fluorescence intensity was plotted against the protein concentration, and the slope of the initial segment was the surface hydrophobicity index H0 of the protein molecule.[Citation19]
Scanning electron microscopy (SEM)
The morphology of sunflower seed protein was observed using a TM-4000 Plus scanning electron microscope (Hitachi, Tokyo, Japan) at an accelerating voltage of 10 kV. The freeze-dried samples were deposited using double-faced adhesive tape and then sputter-coated with a gold layer (MC-1000, Hitachi, Tokyo, Japan). The SEM images of the samples were captured with magnification of 500 times.
Solubility
Prepare 30 mL of 1% (w/v) protein solution (10 mmol/L PBS, pH 7.0), adjust the pH to 7 with 0.1 mol/L HCl or NaOH, After stirring at room temperature for 1 h and centrifuged at 4000 rpm for 15 min. The protein content was determined by Kjeldahl nitrogen analyzer (ATN-100, Shanghai Hongji Instrument Equipment Co., Ltd.).[Citation20] Calculated according to the following formula:
Foaming capacity (FC) and foam stability (FS)
Prepare 50 mL of 1% (w/v) protein solution (10 mmol/L PBS, pH 7.0), used a homogenizer(IKA-T10, Sai de Instrument Equipment Co., Ltd., Zhejiang, China) to disperse at 10000 rpm for 3 minutes, and then quickly transfer to a 100 mL graduated cylinder. Record the foaming volume V0 and V30 at 0 min and 30 min.[Citation21] The formula was as follows:
Emulsifying activity index (EAI) and emulsifying stability index (ESI)
Prepare 15 mL of 1% (w/v) protein solution (10 mmol/L PBS, pH 7.0), add 5 mL of soybean oil, and use a disperser to disperse at 12000 rpm for 3 minutes. Immediately measure 50 μL of the emulsion from the bottom of the solution and disperse it in 5 mL of 0.1% SDS (w/v), vortex to mix, and measure the absorbance values A0 and A10 after 0 min and 10 min at a wavelength of 500 nm.[Citation22] The calculation formula was as follows:
where DF, dilution factor, 100; c weight of protein as BASE/volume, g/mL; Φ optical path, 0.01; θ, oil volume fraction of the emulsion, 0.25.
Oil absorption capacity (OAC)
The sample was weighed into a centrifuge tube, and the weight was recorded. The sample was added with soybean oil and mixed in vortex until it was saturated with oil. The mixture was allowed to stand for 30 min and the centrifuged at 4500 rpm for 15 min. Oil was removed from the supernatant and weighed in a centrifuge tube. oil absorption capacity was expressed as mL of oil/g of sample.[Citation23]
Statistical analysis
The experiment was repeated three times, and the results were expressed as mean ± standard deviation. The test data were analyzed by ANOVA in SPSS 16.0 statistical software. Origin 2017 was used for mapping.
Results and discussion
Degree of hydrolysis, Lightness value, polyphenol and protein content
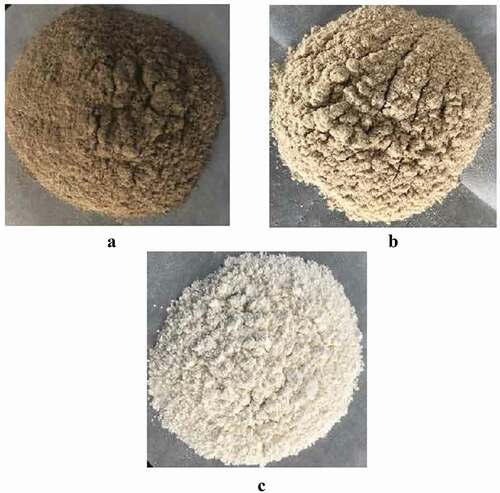
showed the degree of hydrolysis, L* value, polyphenol, protein content and changes of sunflower seed protein. It can be seen from the table that the degree of hydrolysis of sunflower seed protein after enzymatic hydrolysis with alkaline protease reaches 3%. Compared with natural sunflower seed protein, the sunflower seed protein was more decolorized by macroporous resin and limited enzymatic hydrolysis combined with macroporous resin. The phenol content decreased, and its polyphenol content decreased from 1.27 g/100 g to 0.28 g/100 g and 0.16 g/100 g, respectively. After the polyphenols were removed, the brightness increased, and the L* value increased from 55.7 to 72.1 and 86.3, respectively. The protein content also increased after macroporous resin decolorization and limited enzyme hydrolysis. In addition, it can be seen from that the color of natural sunflower seed protein was darker and dark gray. After macroporous resin decolorization and limited enzyme hydrolysis combined with macroporous resin decolorization, the color of sunflower seed protein was significantly improved, and the color becomes shallower. Previous reported also pointed out that the L* value of the protein extracted after the polyphenols in sunflower seed meal was removed was significantly increased, and the protein content also increased.[Citation7,Citation24]
Table 1. Lightness value, degree of hydrolysis, protein and polyphenol content of sunflower seed protein. (A) sunflower seed protein, (B) macroporous resin adsorption decolorization of sunflower seed protein, (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein. The data represents the mean ± standard deviation of three experiments (n = 3). Different lowercase letters in each row indicate significant differences (p < .05)
Figure 1. The color of sunflower seed protein. (A)sunflower seed protein, (B) macroporous resin adsorption decolorization of sunflower seed protein, (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein
SDS-PAGE
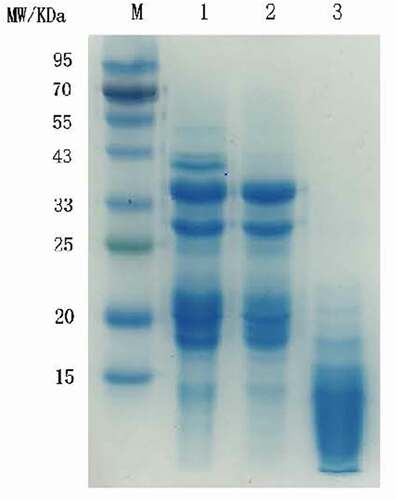
The SDS-PAGE spectrum of sunflower seed protein were showed in . Lane 1 was the main bands of untreated sunflower seed protein: 50, 33 ~ 43, 29, 18 ~ 22 kDa. The subunit bands near 50 kDa and 40 kDa of macroporous resin decolorized sunflower seed protein disappeared. After limited enzyme hydrolysis combined with macroporous resin adsorption, the molecular weight of decolorized sunflower seed protein has changed, and the macromolecular subunit bands gradually disappeared. The subunit bands near 50, 40, and 30 kDa disappear, mainly concentrated below 23 kDa limited enzyme hydrolysis changes the protein subunit composition, there by reduces the molecular weight.[Citation25] This result was consistent with previously reported results.[Citation15,Citation26]
Figure 2. SDS-PAGE profile of sunflower seed protein. Lane M: the marker proteins, Lane 1: sunflower seed protein, Lane 2: macroporous resin adsorption decolorization of sunflower seed protein, Lane3: limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein
CD
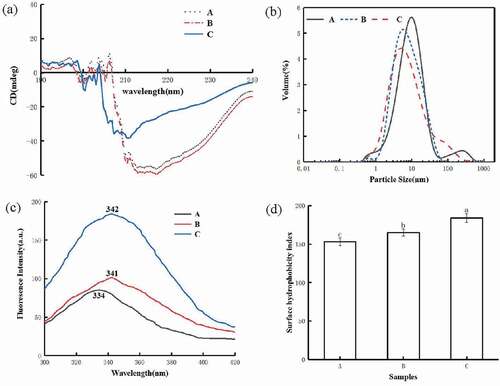
The peak change of the circular dichroic spectrum of sunflower seed protein were showd in . The CD spectrum of sunflower seed protein showed two negative absorption bands at 208 and 216 nm, indicating a high proportion of α-helix and β-sheet. A small negative peak was observed at 208 nm, but the intensity decreased significantly. The negative peak at 216 nm basically disappeared, and a negative absorption band appeared at 200 nm, indicating that the content of α-helix was reduced, and it turned into a β-turn, β-sheet or random coil structure. In addition, the secondary structure changes of sunflower seed protein are shown in . As can be seen from the table, the content of α-helix decreased from 19.71% to 16.50%, the content of β-turns decreased from 25.40% to 21.14%, and the content of β-helix decreased-sheets and random coils increased from 24.29% to 26.00% sum increased from 30.59% to 36.36%. α-helix and β-sheet are ordered secondary structures of proteins with high stability, while β-turn and random coils were relatively flexible disordered structures.[Citation27] After limited enzymatic hydrolysis, the secondary structure of sunflower seed protein became more flexible and extended.[Citation12]
Table 2. Secondary structure of sunflower seed protein. (A) sunflower seed protein, (B) macroporous resin adsorption decolorization of sunflower seed protein, (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein. Data are represents the mean ± the standard deviation of three experiments (n = 3). Different lowercase letters in each column indicate a significant difference (p < .05)
Figure 3. Circular dichroism (a), particle size (b), intrinsic fluorescence intensity (c) and surface hydrophobicity (d) of sunflower seed protein. (A) sunflower seed protein, (B) macroporous resin adsorption decolorization of sunflower seed protein, (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein
Particle size distribution
The number or quality of particles of different sizes was called the particle size distribution, and the average particle size is expressed as the surface average particle size (D32) and the volume average particle size (D43). The average particle size and particle size distribution of sunflower seed protein are shown in and . The particle size of sunflower seed protein decolorized by macroporous resin and limited enzymatic hydrolysis combined with macroporous resin decolorization was lower than that of natural sunflower seed protein (). The D32 and D43 of unbleached sunflower seed protein were 6.04 μm and 24.30 μm, respectively. The particle size of sunflower seed protein decolorized by macroporous resin adsorption and limited enzymolysis combined with macroporous adsorption resin was reduced to 5.85 μm, 22.16 μm and 4.78 μm, 11.00 μm, respectively. On the one hand, the large-molecule polyphenols may be adsorbed and removed by the pore resin, resulting in the reduction of the protein particle size. On the other hand, the peptide bond was cleaved by the protease after the limited enzymatic hydrolysis to form a polypeptide with a smaller molecular weight, and the degree of protein aggregation Decrease, the particle size decreases. Similar results have been observed in previous reports.[Citation28]
Table 3. Average particle size of sunflower seed protein. (A) sunflower seed protein, (B) macroporous resin adsorption decolorization of sunflower seed protein, (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein. Data are represents the mean ± the standard deviation of three experiments (n = 3). Different lowercase letters in each row indicate a significant difference (p < .05)
Intrinsic fluorescence
The measurement of endogenous fluorescence intensity can determine the degree of exposure of amino acids to water, thereby characterizing changes in the tertiary structure of proteins.[Citation29] The endogenous fluorescence intensity changes of sunflower seed protein before and after limited hydrolysis are shown in . It can be seen from the figure that compared with the sunflower seed protein decolorized by natural sunflower seed protein and macroporous resin, the fluorescence intensity of sunflower seed protein increased after limited enzyme hydrolysis, which indicates that more aromatic groups were exposed to water and can emit fluorescence.[Citation12] The maximum emission fluorescence wavelength (λ max) of sunflower seed protein is 334, the sunflower seed protein decolorized by macroporous resin and the sunflower seed protein decolorized by the limited enzyme combined with macroporous resin (λ max) are 341 nm and 342 nm, respectively. The red shift of after limited enzymatic hydrolysis indicates that the protein structure becomes looser and more aromatic amino acids are exposed after enzymatic hydrolysis.[Citation14,Citation15,Citation30]
Surface hydrophobicity (H0)
The change of the surface hydrophobicity of sunflower seed protein were showed in . As can be seen from the figure, compared with natural sunflower seed protein. Macroporous resin adsorption and decolorization and limited enzymatic hydrolysis enhanced the H0 of sunflower seed protein. The increase in surface hydrophobicity may be related to the changes in the surface structure of the protein caused by the adsorption and removal of polyphenols by the macroporous resin.[Citation7] The limited enzyme hydrolysis leads to the unfolding of the protein structure and exposing the hydrophobic groups previously hidden inside the protein, thereby increasing H0.[Citation31,Citation32]
Morphology
The SEM image of sunflower seed protein were showed in . The surface of sunflower seed protein presents a smooth and regular large sheet structure. The sunflower seed protein adsorbed and decolorized by the macroporous resin has a porous honeycomb structure. It may be related to the adsorption and removal of polyphenols (chlorogenic acid) by the macroporous resin, and the hydrolyzate is fragmented, The structure was loosed and there were many folds. The changes of SEM indicate that alkaline protease can effectively act on protein structure. Studies have shown that these changes increase the contact between protein and water molecules, thereby increasing solubility.[Citation33]
Solubility
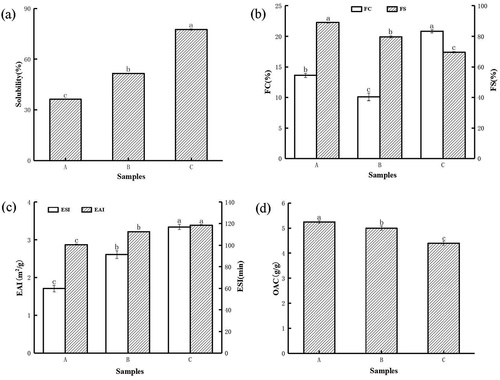
Solubility was one of the important functional properties of protein. Prerequisites for other functional properties such as foaming, gelling and emulsifying properties. Proteins need high solubility to have good functional properties. showed that the change in solubility of sunflower seed protein. It can be seen from the figure that compared with the natural sunflower seed protein, the solubility of sunflower seed protein increased by 14.96% and 38.96% after macroporous resin decolorization and limited enzymatic hydrolysis combined with macroporous resin decolorization, respectively. This may be because the polyphenols present in sunflower seed protein have a significant negative impact on the solubility of the protein. After the protein interacts with phenolic compounds, it was denatured to produce phenol, which reduces the solubility of the protein. The removal of polyphenols was also one of the reasons for the increased solubility.[Citation7,Citation34,Citation35] After limited enzyme hydrolysis, the particle size of the protein was reduced, the specific surface area was increased, the interaction between the protein and water was enhanced, and the solubility was improved.[Citation14] The results of this study also show that the smaller the particle size, the greater the solubility. In addition, the peptide bond in the protein has broken after limited enzyme hydrolysis, which enhances the interaction between the protein molecule and water, and the soluble protein was released from insoluble aggregates and precipitates, thereby increasing the solubility of the protein.[Citation36,Citation37]
Figure 5. Solubility (a), foaming ability (b), emulsifying capacity (c), and oil absorption capacity (d) of sunflower seed protein. (A)sunflower seed protein; (B) macroporous resin adsorption decolorization of sunflower seed protein; (C) limited enzyme hydrolysis combined with macroporous resin decolorize sunflower seed protein
FC and FS
In the processing of beverages and baked goods, protein foaming properties are important. It can be seen from that compared with the untreated sunflower seed protein, the foamability of the protein was slightly reduced after the macroporous resin was decolorized. This result was inconsistent with the previously reported results, which may be related to polyphenols, raw materials and high-speed dispersion. Machines have a certain relationship, which needs to be further explored. After limited enzymatic hydrolysis combined with macroporous resin adsorption and decolorization, the foamability of the protein is significantly improved, but the foam stability shows the opposite trend. The foam stability of untreated sunflower seed protein was 89.17%, and the foam stability of the protein treated by macroporous resin adsorption decolorization and limited enzymatic hydrolysis combined with macroporous resin adsorption decreased to 79.72% and 69.62%, respectively. This finding may be due to the decrease in the molecular weight of the protein after limited enzyme hydrolysis, which makes it easier for the protein to diffuse at the interface, thereby reducing the interfacial tension and increasing the foaming ability of sunflower seed protein.[Citation22,Citation33] The foam stability of sunflower seed protein with limited enzymatic hydrolysis combined with macroporous resin decreased significantly, which may be due to the decrease in the molecular weight of the protein after treatment. In addition, the small-molecule proteins or peptides produced are not enough to maintain the stability of the foam.[Citation38]
EAI and ESI
Emulsification was also one of the important functional properties of protein, which was widely used in food processing. It can be seen from that the emulsification of natural sunflower seed protein is 1.71 m2/g, and the emulsification of sunflower seed protein was increased to 2.61 m2/g and 3.34 m2/g after macroporous resin decolorization and limited enzymatic hydrolysis combined with macroporous resin decolorization. The emulsification stability also showed the same trend. Compared with natural sunflower seed protein, the emulsification stability of sunflower seed protein was also significantly improved after macroporous resin decolorization and limited enzymatic hydrolysis combined with macroporous resin adsorption decolorization (p < .05). The low emulsification and emulsification stability of natural sunflower seed protein were due to the presence of polyphenols. Phenols will reduce the solubility of proteins. Good emulsifying ability must have high solubility. The low solubility prevents the peptide from quickly moving to the oil-water interface. Phenolic components can enhance protein aggregation and reduce hydrophobicity. Therefore, the removal of phenols by the macroporous resin was also the reason for the improved emulsification.[Citation7,Citation24] In addition, the molecular weight of the protein becomes smaller after limited enzymatic hydrolysis, and the presence of small peptides and hydrophobic residues may rapidly diffuse and adsorb on the surface of newly formed oil droplets, thereby improving emulsification and emulsification stability.[Citation22,Citation37]
OAC
The OAC value of sunflower seed protein was showed in . Compared with the natural sunflower seed protein, the oil retention of sunflower seed protein after decolorization by macroporous resin and limited enzymatic hydrolysis combined with macroporous resin decolorization decreased by 0.24 g/g and 0.84 g/g, respectively. It has been reported that oil retention is affected by many factors such as protein content, particle size, surface properties, and hydrophobicity.[Citation39] The decrease in oil retention of sunflower seed protein after limited enzymatic hydrolysis may be related to the change in the ratio of hydrophobic and hydrophilic groups within the protein molecule. The specific reason was still unclear and needs to be further explored. This was consistent with the previously reported results.[Citation38]
Conclusion
This study showed that limited enzyme hydrolysis combined with macroporous resin decolorization technology could enhance the functional properties of sunflower seed protein. The solubility, foamability, emulsification and emulsification stability of protein were all significantly improved. The results of SDS-PAGE showed that limited enzyme hydrolysis would reduce the molecular weight of the protein. The results of CD and intrinsic fluorescence showed that the secondary and tertiary structure of the protein changed, the structure became more flexible, and the H0 increased. This may be related to the buried protein molecule. When the internal hydrophilic groups are exposed, the increase in the number of hydrophilic groups and the decrease in the number of hydrophobic groups are related. The particle size and SEM analysis results showed that the reduction of protein particle size was beneficial to the improvement of its solubility. In conclusion, the current research shows that limited enzyme hydrolysis combined with macroporous resin decolorization technology may be a limited good method for industrial use of sunflower seed protein. Sunflower seed protein hydrolyzate with improved color and functional properties can be used as an effective emulsifier and foaming agent.
Additional information
Funding
References
- Dorrell, D. G.; Vick, B. A. Properties and Processing of Oilseed Sunflower; technology and production: Sunflower, 1997.
- González-Pérez, S.; Vereijken, J. M. Sunflower Proteins: Overview of Their Physicochemical, Structural and Functional Properties. J. Sci. Food Agric. 2007, 87(12), 2173–2191. DOI: https://doi.org/10.1002/jsfa.2971.
- Pedrosa, M. M.; Muzquiz, M.; Garcia-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L. M. Determination of Caffeic and Chlorogenic Acids and Their Derivatives in Different Sunflower Seeds. J. Sci. Food Agric. 2000, 80(4), 459–464. DOI: https://doi.org/10.1002/(SICI)1097-0010(200003)80:43.0.CO;2-O.
- Sinisi, V.; Forzato, C.; Cefarin, N.; Navarina, L.; Berti, F. Interaction of Chlorogenic Acids and Quinides from Coffee with Human Serum Albumin. Food Chem. 2015, 168(2), 332–340. DOI: https://doi.org/10.1016/j.foodchem.2014.07.080
- Budryn, G.; Palecz, B.; Rachwal-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Belica, S.; Navarro-González, I., Meseguer, J.M.V., Pérez-Sánchez, H. Effect of Inclusion of Hydroxycinnamic and Chlorogenic Acids from Green Coffee Bean in β-cyclodextrin on Their Interactions with Whey, Egg White and Soy Protein Isolates. Food Chem. 2015, 168(2), 276–287. DOI: https://doi.org/10.1016/j.foodchem.2014.07.056
- Dabbour, M. I.; Xiang, J.; Mintah, B.; He, R.; Ma, H. Localized Enzymolysis and Sonochemically Modified Sunflower Protein: Physical, Functional and Structure Attributes. Ultrason. Sonochem. 2020, 63, 104957. DOI: https://doi.org/10.1016/j.ultsonch.2019.104957.
- Malik, M. A.; Saini, S. C. Polyphenol Removal from Sunflower Seed and Kernel: Effect on Functional and Rheological Properties of Protein Isolates. Food Hydrocoll. 2017, 63(2), 705–715. DOI: https://doi.org/10.1016/j.foodhyd.2016.10.026.
- Pickardt, C.; Weisz, G. M.; Eisner, P.; Kammerer, D. R.; Neidhart, S.; Carle, R. Processing of Low Polyphenol Protein Isolates from Residues of Sunflower Seed Oil Production. Procedia Food Sci. 2011, 1(9), 1417–1424. DOI: https://doi.org/10.1016/j.profoo.2011.09.210
- Barca, A. M. C. D. L.; Ruiz-Salazar, R. A.; Jara-Marini, M. E. Enzymatic Hydrolysis and Synthesis of Soy Protein to Improve Its Amino Acid Composition and Functional Properties. J. Food Sci. 2010, 65(2), 246–253. DOI: https://doi.org/10.1111/j.1365-2621.2000.tb15988.x.
- Buˇcko, S.; Katona, J.; Popovic, L.; Petrovic, L.; Milinkovi, J. Influence of Enzymatic Hydrolysis on Solubility, Interfacial and Emulsifying Properties of Pumpkin (Cucurbita Pepo) Seed Protein Isolate. Food Hydrocoll. 2016, 60, 271–278. DOI: https://doi.org/10.1016/j.foodhyd.2016.04.005.
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D. J.; Wu, L. Effect of Limited Enzymatic Hydrolysis on Structure and Emulsifying Properties of Rice Glutelin. Food Hydrocoll. 2016, 61, 251–260. DOI: https://doi.org/10.1016/j.foodhyd.2016.05.023
- Zhao, G. L.; Liu, Y.; Zhao, M. M.; Ren, J. Y.; Yang, B. Enzymatic Hydrolysis and Their Effects on Conformational and Functional Properties of Peanut Protein Isolate. Food Chem. 2011, 127(4), 1438–1443. DOI: https://doi.org/10.1016/j.foodchem.2011.01.046.
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of Limited Enzymatic Hydrolysis on the Structure and Emulsifying Properties of Rice Bran Protein. J. Cereal Sci. 2018, 85(1), 168–174. DOI: https://doi.org/10.1016/j.jcs.2018.09.001.
- Jin, F.; Wang, Y.; Tang, H.; Regenstein, J. M.; Wang, F. Limited Hydrolysis of Dehulled Walnut (Juglans Regia L.) Proteins Using Trypsin: Functional Properties and Structural Characteristics. LWT - Food Sci. Technol. 2020, 133, 110035. DOI: https://doi.org/10.1016/j.lwt.2020.110035.
- Qin, N. R.; Bao, X. L.; Wu, J. L. Research on the Decolorization Technology of Sunflower Seed Protein. China Oils Fats. 2021, 46(4), 47–51. DOI: https://doi.org/10.19902/j.cnki.zgyz.1003-7969.2021.04.010.
- Lamsal, B. P.; Jung, S.; Johnson, L. A. Rheological Properties of Soy Protein Hydrolysates Obtained from Limited Enzymatic Hydrolysis. LWT - Food Sci. Technol. 2007, 40(7), 1215–1223. DOI: https://doi.org/10.1016/j.lwt.2006.08.021.
- Du, Y.; Shi, S.; Yan, J.; Xiong, H.; Sun, W. Physicochemical Properties and Emulsion Stabilization of Rice Dreg Glutelin Conjugated with κ-carrageenan through Maillard Reaction. J. Sci. Food Agric. 2012, 93(1), 125–133. DOI: https://doi.org/10.1002/jsfa.5739.
- Spontonc, O. E.; Perez, A. A.; Carrara, C.; Santiago, L. G. Effect of Limited Enzymatic Hydrolysis on Linoleic Acid Binding Properties of β-lactoglobulin. Food Chem. 2014, 146(3), 577–582. DOI: https://doi.org/10.1016/j.foodchem.2013.09.089.
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Modifications of Soy Protein Isolates Using Combined Extrusion Pre-treatment and Controlled Enzymatic Hydrolysis for Improved Emulsifying Properties. Food Hydrocoll. 2011, 25(5), 887–897. DOI: https://doi.org/10.1016/j.foodhyd.2010.08.013.
- Bera, M. B.; Mukherjee, R. K. Solubility, Emulsifying, and Foaming Properties of Rice Bran Protein Concentrates. J. Food Sci. 2010, 54(1), 142–145. DOI: https://doi.org/10.1111/j.1365-2621.1989.tb08587.x.
- Motoi, H.; Fukudome, S.; Urabe, I. Continuous Production of Wheat Gluten Peptide with Foaming Properties Using Immobilized Enzymes. Eur. Food Res.Technol. 2004, 219(5), 522–528. DOI: https://doi.org/10.1007/s00217-004-0986-2.
- Yust, M. M.; Pedroche, J.; Millan-Linares, M. C.; Alcaide-Hidalgo, J. M.; Millan, F. Improvement of Functional Properties of Chickpea Proteins by Hydrolysis with Immobilised Alcalase. Food Chem. 2010, 122(4), 1212–1217. DOI: https://doi.org/10.1016/j.foodchem.2010.03.121.
- Wani, I. A.; Sogi, D. S.; Shivhare, U. S.; Gill, B. S. Physico-chemical and Functional Properties of Native and Hydrolyzed Kidney Bean (Phaseolus Vulgaris L.) Protein Isolates. Food Res. Int. 2015, 76, 11–18. DOI: https://doi.org/10.1016/j.foodres.2014.08.027.
- Salgado, P. R.; Ortiz, S. E. M.; Petruccelli, S.; Mauri, A. N. Sunflower Protein Concentrates and Isolates Prepared from Oil Cakes Have High Water Solubility and Antioxidant Capacity. J. Am. Oil Chem. Soc. 2011, 88(3), 351–360. DOI: https://doi.org/10.1007/s11746-010-1673-z.
- Mao, X. Y.; Hua, Y. F.; Chen, G. G. Amino Acid Composition, Molecular Weight Distribution and Gel Electrophoresis of Walnut (Juglans Regia L.) Proteins and Protein Fractionations. Int. J. Mol. Sci. 2014, 13(2), 2003–2014. DOI: https://doi.org/10.3390/ijms15022003.
- Avramenko, N. A.; Low, N. H.; Nickerson, M. T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013, 51, 162–169. DOI: https://doi.org/10.1016/j.foodres.2012.11.020.
- Yong, Y. H.; Yamaguchi, S.; Matsumura, Y. Effects of Enzymatic Deamidation by Protein-glutaminase on Structure and Functional Properties of Wheat Gluten. J. Agric. Food Chem. 2006, 54(16), 6034–6040. DOI: https://doi.org/10.1021/jf060344u.
- Gao, Y.; Li, J.; Chang, C.; Wang, C.; Su, Y. Effect of Enzymatic Hydrolysis on Heat Stability and Emulsifying Properties of Egg Yolk. Food Hydrocoll. 2019, 97, 105224. DOI: https://doi.org/10.1016/j.foodhyd.2019.105224.
- Cui, C.; Zhao, M.; Yuan, B.; Zhang, Y.; Ren, J. Effect of Ph and Pepsin Limited Hydrolysis on the Structure and Functional Properties of Soybean Protein Hydrolysates. J. Food Sci. 2003, 78(10-11-12), C1871–C1877. DOI: https://doi.org/10.1111/1750-3841.12309.
- Chao, D. F.; He, R.; Jung, S.; Aluko, R. E. Effect of Pressure or Temperature Pretreatment of Isolated Pea Protein on Properties of the Enzymatic Hydrolysates. Food Res. Int. 2013, 54(2), 1528–1534. DOI: https://doi.org/10.1016/j.foodres.2013.09.020.
- Xiao, G.; Yao, H.; Chen, Z.; Liang, S.; Zhang, M. Some Functional Properties of Oat Bran Protein Concentrate Modified by Trypsin. Food Chem. 2007, 101(1), 163–170. DOI: https://doi.org/10.1016/j.foodchem.2006.01.011.
- Jung, S.; Murphy, P. A.; Johnson, L. A. Physicochemical and Functional Properties of Soy Protein Substrates Modified by Low Levels of Protease Hydrolysis. J. Food Sci. 2005, 70(2), 180–187. DOI: https://doi.org/10.1111/j.1365-2621.2005.tb07080.x.
- Bao, Z. J.; Zhao, Y.; Wang, X. Y.; Chi, Y. J. Effects of Degree of Hydrolysis (DH) on the Functional Properties of Egg Yolk Hydrolysate with Alcalase. J. Sci. Food Sci Technol. 2017, 543, 669–678. DOI:https://doi.org/10.1007/s13197-017-2504-0
- Ali, M.; Homann, T.; Khalil, M.; Kruse, H. P.; Rawel, H. Milk Whey Protein Modification by Coffee-Specific Phenolics: Effect on Structural and Functional Properties. J. Agric. Food Chem. 2013, 61(28), 6911–6920. DOI: https://doi.org/10.1021/jf402221m.
- Rawel, H. M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of Different Phenolic Acids and Flavonoids with Soy Proteins. Int. J. Biol. Macromol. 2002, 30(3–4), 137–150. DOI: https://doi.org/10.1016/S0141-8130(02)00016-8.
- Yin, S. W.; Tang, C. H.; Cao, J. S.; Hu, E. K.; Wen, Q. B.; Yang, X. Q. Effects of Limited Enzymatic Hydrolysis with Trypsin on the Functional Properties of Hemp (Cannabis Sativa L.) Protein Isolate. Food Chem. 2008, 106(3), 1004–1013. DOI: https://doi.org/10.1016/j.foodchem.2007.07.030.
- Ghribi, A. M.; Gafsi, I. M.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of Enzymatic Hydrolysis on Conformational and Functional Properties of Chickpea Protein Isolate. Food Chem. 2015, 187, 322–330. DOI: https://doi.org/10.1016/j.foodchem.2015.04.109
- Zhang, H. Y.;. Effect of Alkaline Protease Modification on the Structure and Functional Properties of Sunflower Protein Isolate. Qiqihar University. 2015.
- Jiang, L. Z.;. Plant Protein Technology. Science Press. 2011.

